Abstract
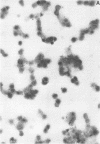
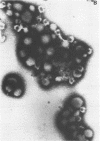
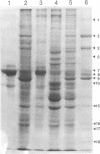
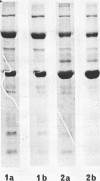
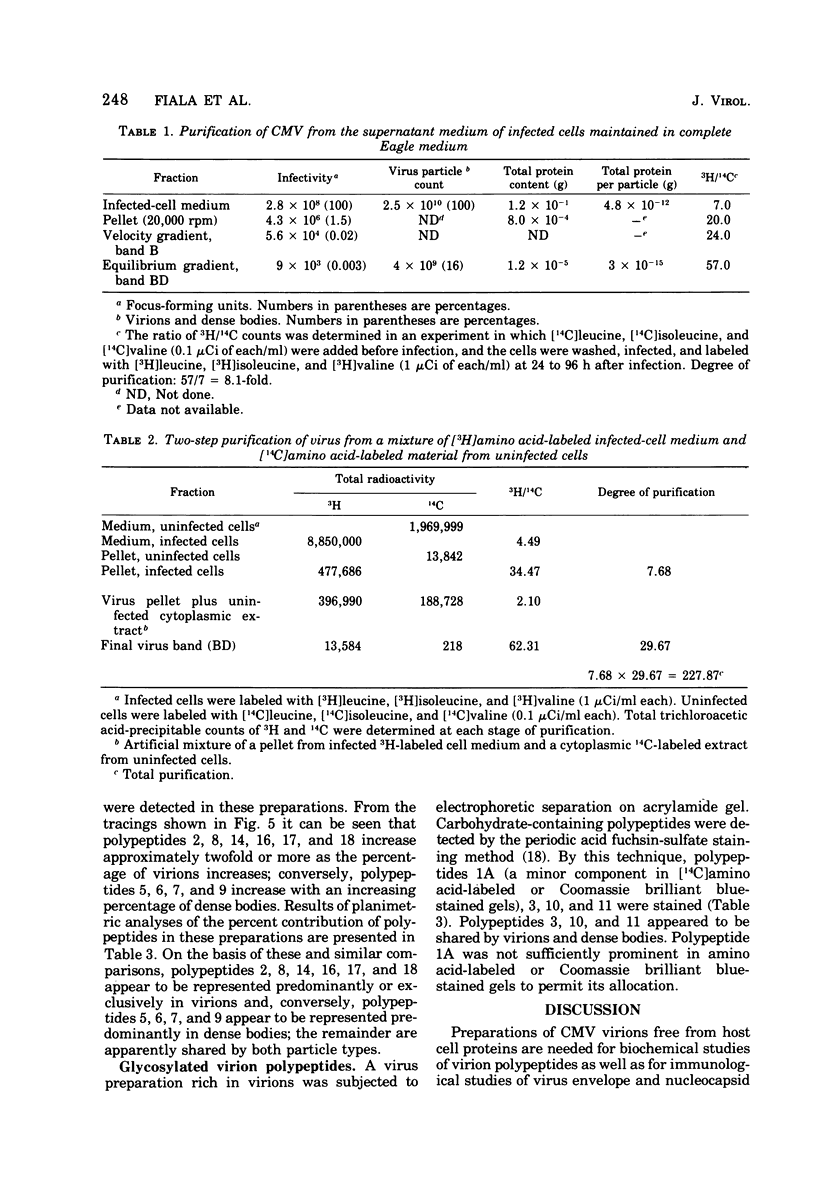
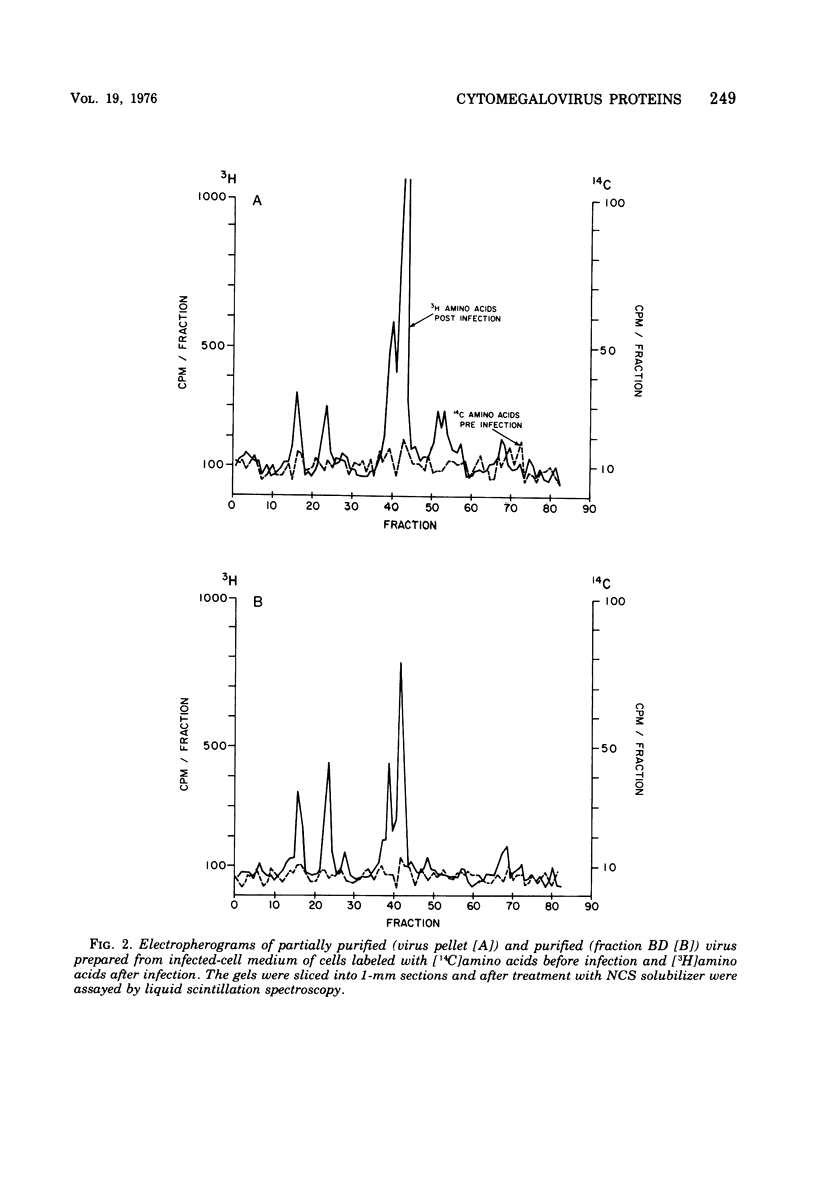
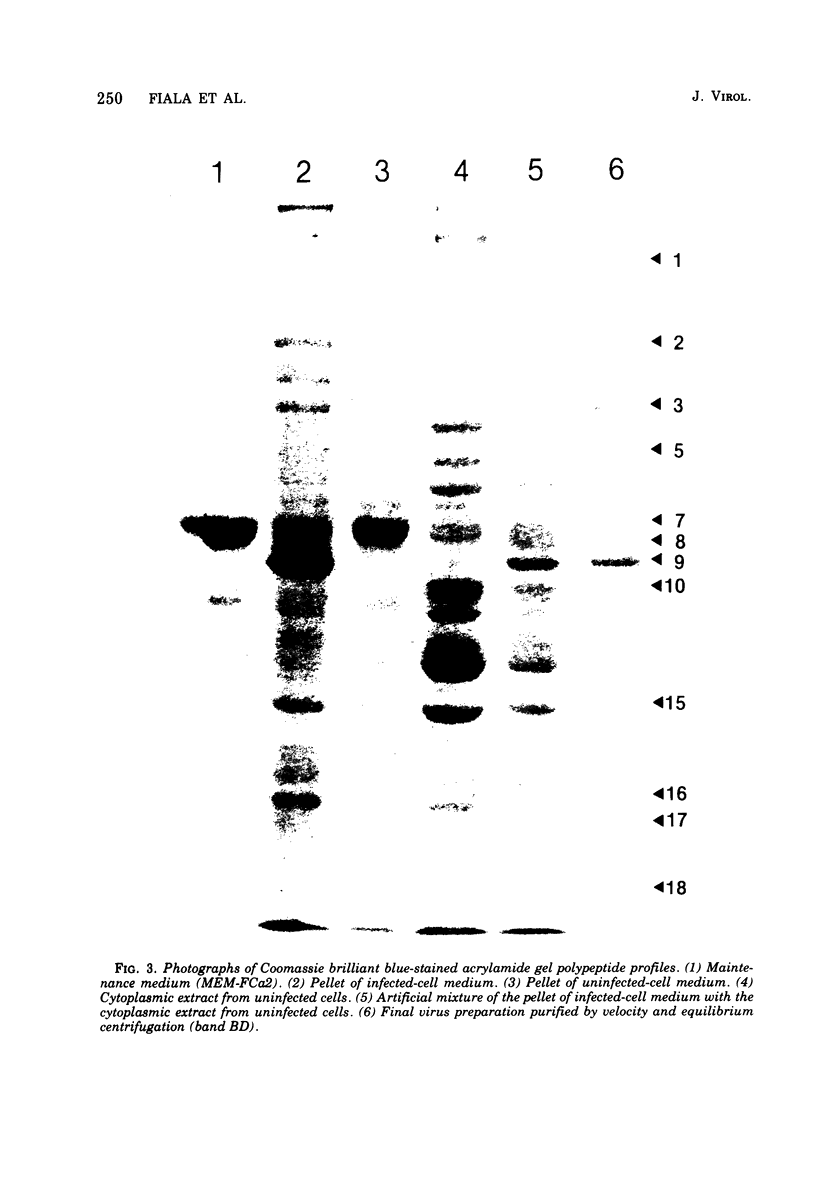
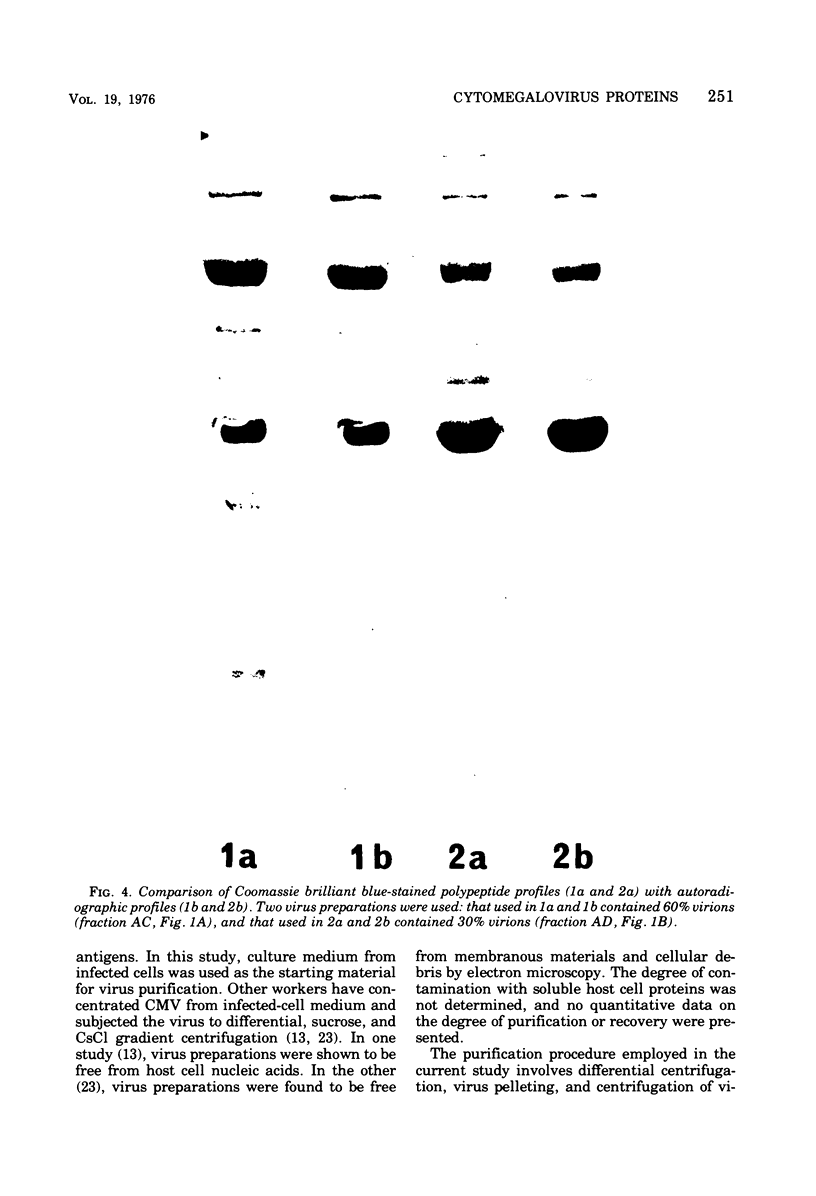
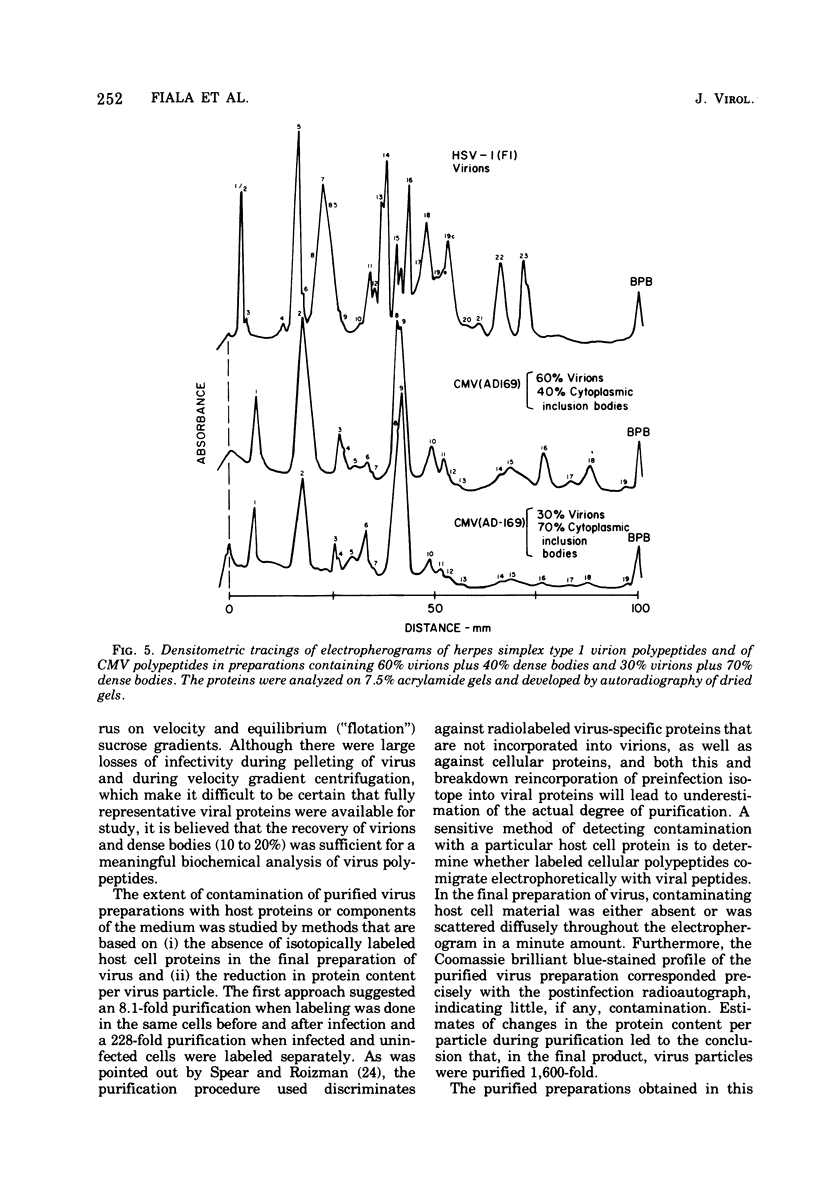
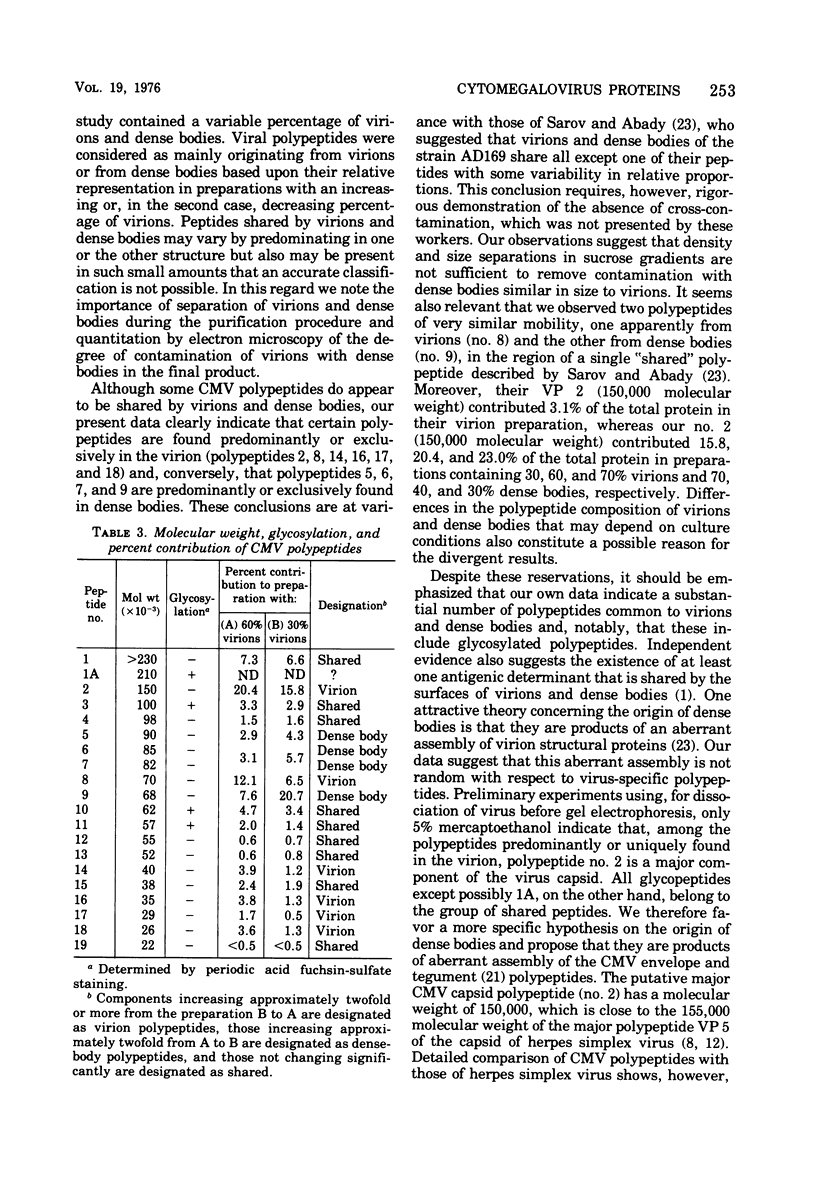
Cytomegalovirus virions and dense bodies were purified by sucrose velocity and equilibrium centrifugation from the medium of fibroblasts infected with the strain AD169. The final virus preparations were purified more than 228-fold with respect to cellular proteins as determined by double-isotopic labeling and at least 1,600-fold on the basis of changes in the ratio of total protein to virus particles. The protein content of purified particles approximated that found for purified preparations of other herpesviruses. Twenty polypeptides ranging from 22,000 to greater than 230,000 molecular weight were detected in purified virus preparations by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Polypeptides of virions and dense bodies were allocated on the basis of analyses of preparations containing differing percentages of virions and dense bodies. Six polypeptides were represented predominantly or exclusively in virions, and four polypeptides were represented predominantly or exclusively in dense bodies, whereas the remainder appeared to be shared by both types of particles. Four polypeptides were glycosylated, and at least three of these appeared to be shared by both particles. Four polypeptides were glycosylated, and at least three of these appeared to be shared by both particle types. The protein composition of cytomegalovirus differs profoundly from that of herpes simplex virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAIG J. M., MACAULEY J. C., WELLER T. H., WIRTH P. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc Soc Exp Biol Med. 1957 Jan;94(1):4–12. doi: 10.3181/00379727-94-22841. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Kanich R. E., Almeida J. D. Nonviral microbodies with viral antigenicity produced in cytomegalovirus-infected cells. J Virol. 1972 Oct;10(4):766–775. doi: 10.1128/jvi.10.4.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Farmer G. W., Vincent M. M., Fuccillo D. A., Horta-Barbosa L., Ritman S., Sever J. L., Gitnick G. L. Viral investigations in ulcerative colitis and regional enteritis. Gastroenterology. 1973 Jul;65(1):8–18. [PubMed] [Google Scholar]
- Fiala M., Chow A. W., Miyasaki K., Guze L. B. Susceptibility of herpesviruses to three nucleoside analogues and their combinations and enhancement of the antiviral effect of acid pH. J Infect Dis. 1974 Jan;129(1):82–85. doi: 10.1093/infdis/129.1.82. [DOI] [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL R. B., Jr, ROWLANDS D. T., Jr, RIFKIND D. INFECTIOUS PULMONARY DISEASE IN PATIENTS RECEIVING IMMUNOSUPPRESSIVE THERAPY FOR ORGAN TRANSPLANTATION. N Engl J Med. 1964 Nov 12;271:1021–1027. doi: 10.1056/NEJM196411122712001. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson D. E., Grimley P. M., Strano A. J. Postnatal cytomegalovirus hepatitis. An autopsy and liver biopsy study. Hum Pathol. 1974 Jan;5(1):93–103. doi: 10.1016/s0046-8177(74)80103-6. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemola E., Käriäinen L. Cytomegalovirus as a possible cause of a disease resembling infectious mononucleosis. Br Med J. 1965 Nov 6;2(5470):1099–1102. doi: 10.1136/bmj.2.5470.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGUCKIN W. F., McKENZIE B. F. An improved periodic acid fuchsin sulfite staining method for evaluation of glycoproteins. Clin Chem. 1958 Dec;4(6):476–483. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Sarov I., Abady I. The morphogenesis of human cytomegalovirus. Isolation and polypeptide characterization of cytomegalovirions and dense bodies. Virology. 1975 Aug;66(2):464–473. doi: 10.1016/0042-6822(75)90218-4. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]