Abstract
We report the case of a patient who was submitted to coronary artery bypass graft surgery (CABG) during heart transplant as, during bench exploration, the donor heart presented a palpable atherosclerotic lesion in the anterior descending artery, not detected before harvesting. The patent internal thoracic artery from a previous CABG was used.
Keywords: Heart transplantation, Coronary artery bypass graft surgery, Organ donor management
INTRODUCTION
Based on its good long-term results, heart transplantation is an established therapy for patients with end-stage heart failure. The number of patients presented for heart transplantation has been rising, while the number of the so-called ‘ideal’ or ‘optimal’ donors available has remained low and inadequate to answer this demand, resulting in the death of many patients in the waiting list [1]. However, there is some evidence, today, that some of the standard donor criteria can be liberalized, accepting the so-called ‘marginal donors’, who would otherwise be declined [2].
Another possible solution is the optimization of the donor hearts, performing surgical correction of minor pathologies detected in the preparation for donation or during bench exploration.
We report the case of a donor heart suspected of isolated left anterior descending artery (LAD) lesion, grafted with the patent left internal mammary artery (LIMA) used in a previous revascularization procedure.
CASE REPORT
A 64-year old man was accepted for coronary artery bypass graft surgery (CABG) in 1988 after an acute inferior and posterior myocardial infarction and unsuccessful angioplasty attempt. His risk factors for coronary artery disease were dyslipidaemia, obesity and sleep apnoea. After surgery, he maintained some sporadic fatigue complaints with moderate exertion. In 2000, he presented the first manifestations of cardiac insufficiency, when severe left ventricle dysfunction was detected in the follow-up cardiology consult. His clinical status progressively deteriorated, needing the implantation of a cardiodefibrillator in 2003. In June 2009, he presented in New York Heart Association (NYHA) class IV, having had multiple admissions in intensive care units for the treatment of heart failure, the latest with inotropic support for a long period, complicated by progressive renal deterioration. Hence he was referred for cardiac transplantation.
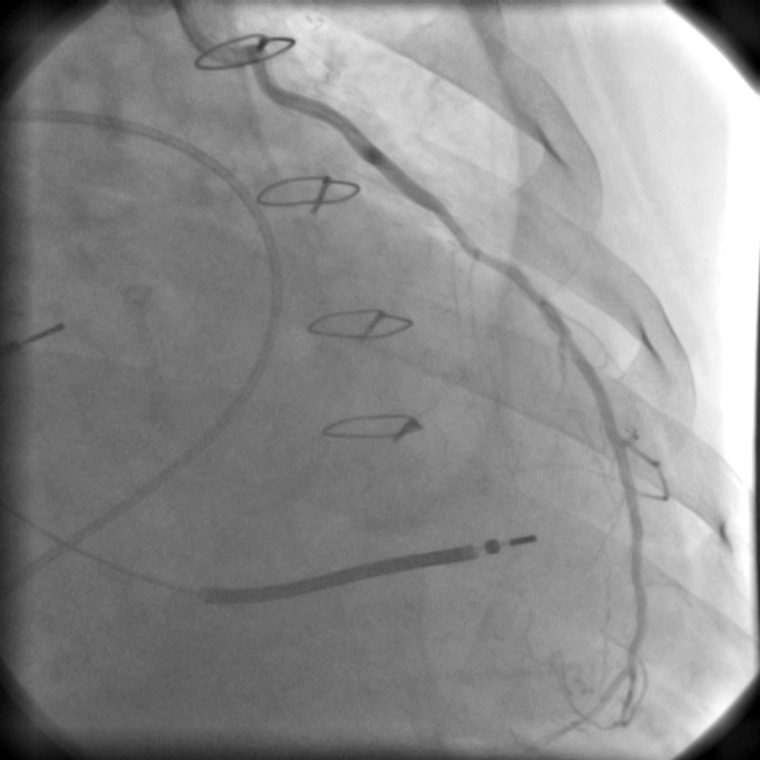
He was transplanted 11 days later with a heart harvested from a 42-year old male donor, with no history of heart disease, a suitable blood type, height and weight, and negative cross-match, who died from traumatic brain injury. The transthoracic echocardiogram showed no evidence of cardiac pathology, regional and global myocardial contractility was unimpaired and the ejection fraction was normal. The donor was not submitted to coronary angiography and the exploration during harvesting confirmed that the heart was good for transplantation. However, during preimplantation preparation, a palpable atherosclerotic lesion was detected in the middle portion of the LAD. Given the risk factors of the recipient, the possibility of progression of the atherosclerotic lesion and the fact that he still had a functional LIMA from the previous CABG, confirmed by a previous angiographic study (Fig. 1) and with good flow observed during the transplantation procedure, this artery was dissected long from the recipient heart and used for revascularization of the distal LAD of the donor heart.
Figure 1:
Angiogram showing LIMA–LDA bypass patency of the recipient before heart transplant.
Total extracorporeal circulation time was 104 min, and the ischaemic period was 124 min. Transplanted heart function by intraoperative transoesophageal echocardiogram was excellent, and no signs of ischaemia were detected in the postoperative period, which was uneventful.
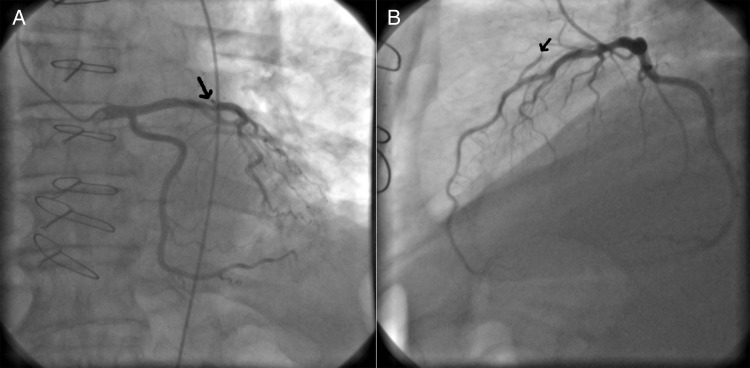
The patient was discharged home on the 12th postoperative day. He is regularly followed in our centre, and a cardiac catheterization and coronary angiogram performed 1 month after transplantation confirmed the presence of the non-occlusive lesion in the LAD, with a functional LIMA (Fig 2). He maintained good left ventricular function, with no areas of hypokinesia in the transthoracic echocardiogram. The patient is well, in class I of the NYHA, 3 years after transplantation. No rejection or ischaemic events were observed, and he is maintained on a three-drug immunosuppressive regime.
Figure 2:
(A) Coronary angiogram performed 32 days after transplantation showing a 50% obstruction in the proximal LAD (arrow). (B) View showing flow competition between the LAD and the ITA (arrow).
DISCUSSION
The increasing number of patients presented for heart transplantation, the low number of hearts that meet the ideal requirements and the growing number of patients dying in the waiting list make it necessary to use a more recipient-oriented assessment, based in less restrictive donor criteria [3]. For the most critic, ‘certainly it makes no sense to replace one diseased heart with another’ as once said DePasquale. That was also the aim of the defined donor criteria—to ensure the use of a non-inferior heart, but a new trend towards donor maximization has been confirmed in the recent literature and several recommendations have been made to expand the donor pool effectively, without raising the risk for complications or decreasing survival [4].
Coronary heart disease in the transplanted heart has been identified as one of the causes of worse long-term outcomes and can be the consequence of two major risk factors—transplant vasculopathy (possibly related to chronic rejection phenomena) and the atherosclerotic disease of the transplanted patient. Hence, heart transplantation using a donor with possible coronary heart disease could be considered a hazardous procedure. However, Abid et al. [5] have reported their experience with the use of donor hearts with coronary heart disease in urgent candidates or in the so-called alternative group (patients with terminal cardiac insufficiency who would otherwise not be transplanted). They found good median-term survival, with a low rate of complications, but an overall graft patency of 82%.
The introduction of these new possible donors must be continuously assessed, as it only makes sense if outcomes are non-inferior to the ones obtained today. In our case, however, we were unexpectedly faced with a palpable coronary lesion with possible obstruction which prompted us to undertake ‘prophylactic’ grafting of the LAD. The presence of a patent LIMA in a previously grafted patient made it the obvious step to take. Here, we believe, lies the uniqueness of our case. The excellent 3-year result, thus far, appears to confirm the appropriateness of the decision.
Conflict of interest: none declared.
REFERENCES
- 1.Jeevanandam V, Furukawa S, Prendergast TW, Todd BA, Eisen HJ, McClurken JB. Standard criteria for an acceptable donor heart are restricting heart transplantation. Ann Thorac Surg. 1996;62:1268–75. doi: 10.1016/0003-4975(96)00626-1. [DOI] [PubMed] [Google Scholar]
- 2.Brock MV, Salazar JD, Cameron DE, Baumgartner WA, Conte JV. The changing profile of the cardiac donor. J Heart Lung Transplant. 2001;20:1005–9. doi: 10.1016/s1053-2498(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 3.Wittwer T, Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Eur Soc Organ Transplant. 2008;21:113–25. doi: 10.1111/j.1432-2277.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 4.Abouna GM. Organ shortage crisis: problems and possible solutions. Transplant Proc. 2008;40:34–8. doi: 10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 5.Abid Q, Parry G, Forty J, Dark JH. Concurrent coronary grafting of the donor heart with left internal mammary: 10-year experience. J Heart Lung Transplant. 2002;21:812–4. doi: 10.1016/s1053-2498(01)00391-6. [DOI] [PubMed] [Google Scholar]