Abstract
OBJECTIVES
Sternal wound infections occurring after cardiac surgery have a critical impact on morbidity, mortality and hospital costs. This study evaluated the efficacy of a gentamicin–collagen sponge in decreasing deep sternal-wound infections in high-risk cardiac surgery patients.
METHODS
We conducted a quasi-experimental single-centre prospective cohort study in diabetic and/or overweight patients undergoing coronary-artery bypass surgery with bilateral internal mammary artery grafts. The end-point was the rate of reoperation for deep sternal wound infection. The period from January 2006 to October 2008, before the introduction of the gentamicin sponge, was compared with the period from November 2008 to December 2010.
RESULTS
Of 552 patients (median body mass index, 31.5; 37.7% with diabetes requiring insulin), 68 (12.3%) had deep sternal wound infections. Reoperation for deep sternal wound infections occurred in 40/289 (13.8%) preintervention patients and 22/175 (12.6%) patients managed with the sponge. Independent risk factors were female sex and longer time on mechanical ventilation, but not use of the sponge (adjusted odds ratio, 0.95; 95% confidence interval, 0.52–1.73; P = 0.88). The group managed with the sponge had a higher proportion of gentamicin-resistant micro-organisms (21/27, 77.8%) compared with the other patients (23/56, 41.1%; P < 0.01). The median time to reoperation for wound infection was higher with the sponge (21 vs 17 days, P < 0.01).
CONCLUSIONS
A gentamicin–collagen sponge was not effective in preventing deep sternal wound infections in high-risk patients. Our results suggest that a substantial proportion of wound contaminations occur after bypass surgery with bilateral internal mammary artery grafts.
Keywords: Gentamicin, Sponge, Infections, Cardiac surgery
INTRODUCTION
Surgical-site infection (SSI) is a major public health problem. Preventive measures include skin preparation, prophylactic antibiotic therapy, control of the operating room environment, and improvements in surgical techniques. Despite these measures, SSI is the third most common healthcare-associated infection and contributes 13–17% of all such infections [1, 2]. Sternal wound infection (SWI) after cardiothoracic surgery substantially increases illness severity, hospital stay length, mortality, and costs [3, 4]. Sternotomy-healing complications such as instability, non-union, and bone or mediastinal infection occur in 0.5–4.4% of patients after cardiac surgery [5] overall and in up to 12–20% of high-risk patients [6, 7].
Coronary artery bypass grafting (CABG) with bilateral internal thoracic artery (BITA) grafts results in better myocardial revascularization and patency compared with unilateral thoracic mammary artery grafting [8]. However, in diabetic and/or obese patients, BITA grafting increases the risk of SWI compared with unilateral internal thoracic artery or saphenous vein grafting [9]. Removal of the internal thoracic arteries for CABG diminishes the blood supply to the anterior chest wall, thereby increasing the risk of mechanical sternal wound dehiscence and subsequent SWI [10]. A gentamicin-impregnated collagen sponge (GCS) has been developed to prevent SWIs. This resorbable device is designed to be implanted before closure of the incision in orthopaedic, gastrointestinal, or cardiac surgical procedures. The GCS produces high local concentrations of gentamicin and may therefore limit peri- and postoperative bacterial growth, thus preventing deep SSI.
Several studies have evaluated the effectiveness of the GCS in cardiac surgery patients, with conflicting results [11–13] probably due to varying methodologies, study populations, end-points, and follow-up durations. We aimed to assess the effectiveness of the GCS in preventing SSI in high-risk patients undergoing BITA grafting, with special attention to the clinical and microbiological characteristics of deep SWIs (dSWIs).
METHODS
Patients and procedures
We conducted a prospective quasi-experimental before/after interventional study in the cardiac surgery unit of a 1000-bed university hospital, which had approved the use of the GCS. All patients meeting the following criteria were eligible: (i) male or female adult scheduled to undergo BITA grafting, with or without valve repair or replacement surgery, through a full median sternotomy, and (ii) high risk for dSWI, defined as diabetes requiring subcutaneous insulin treatment and/or body mass index (BMI) greater than 30 kg/m². Eligible patients were included in the study before the beginning of the surgery.
The preintervention period extended from January 2006 to October 2008 and the intervention period from November 2008 to December 2010. During the overall period, 5085 patients underwent cardiac surgery with extracorporeal circulation totalizing 5169 procedures.
During the intervention period, the GCS (Syntacoll GmbH, Saal, Germany) was to be used routinely. Each lyophilized sponge (5 by 20 cm) contained 280 mg of bovine collagen and 130 mg of gentamicin (200 mg gentamicin sulphate). The sponge undergoes resorption in 4–14 days. A single sponge was dipped in normal saline solution for a few seconds and then inserted between the two halves of the sternum, along the full length of the cut, immediately before closure of the sternum. Carriers of Staphylococcus aureus received preoperative decontamination with nasal mupirocin. If the preoperative nasal screening revealed the carriage of methicillin-resistant S. aureus, daily skin decontaminations were performed using polyvidone iodine. In all patients, prophylactic cefamandole was started within the hour before the skin incision was made; vancomycin for 36 h plus gentamicin (one perioperative dose) was used instead in individuals carrying MRSA or with an increased risk of MRSA colonization (hospitalization in unit with endemic MRSA) and for patients allergic to cephalosporin or penicillin. According to French law on interventions used as part of the standard of care, without random allocation, informed patient consent was not required for this study.
Data collection
The study data were extracted from prospectively collected databases. The database in the cardiac surgery unit included demographic data (age, gender), medical background data (BMI, smoking status, chronic obstructive pulmonary disease, dialysis, previous median sternotomy, whether surgery was on an emergency basis, the EuroSCORE [14], the New York Heart Association (NYHA) preoperative functional class, and the left ventricular ejection fraction), and surgical data (CABG only or combined with valve repair, valve repair only, or other procedure, type of graft, operating time, and time on extracorporeal circulation). The following postoperative variables were obtained from the anaesthesiology database: need for early reoperation, use of vasoactive agents, length of stay in the postoperative intensive care unit, and duration of mechanical ventilation. Finally, the infection control unit collected information about dSWIs, including microbiological data. The computerized files were checked for aberrant data and merged for subsequent analysis.
Infection surveillance
The study end-point was the rate of dSWIs occurring within the first 60 postoperative days. We defined dSWI as the need for reoperation accompanied by either of the following findings: discharge of purulent material from the subcutaneous space (sternal bone or mediastinum) or presence of micro-organisms and numerous polymorphonuclear cells in specimens collected during reoperation. This definition included subcutaneous abscess requiring surgical exploration, sternal osteomyelitis without opening of the sternum during revision, and mediastinitis involving the retrosternal space. For simplicity, subcutaneous abscess and sternal osteomyelitis were collapsed into a single entity, designated SCA/OM in this paper. Diagnostic criteria for dSWI were as follows: (i) discharge of purulent material from the subcutaneous space, sternal bone, or mediastinum; or (ii) numerous polymorphonuclear cells with or without micro-organisms in specimens collected during reoperation, drainage material, or wound samples. Patients who underwent reoperation for sternal dehiscence or bleeding were not classified as having dSWI. We routinely reviewed the operating room log to ensure identification of all sternotomy cases and of all cases of dSWI requiring reoperation. Among the 5085 patients who underwent cardiac surgery during the study period, 206 (3.9%) experienced dSWIs, including 132 (2.5%) with SCI/OM and 74 (1.4%) with mediastinitis. The overall yearly dSWI incidence varied from 3.5 to 4.1%, with no significant changes over time (Fig. 1).
Figure 1:
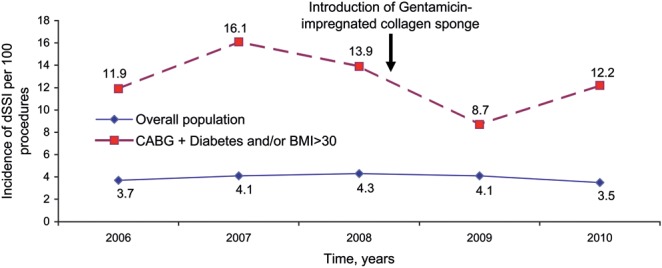
Incidence of deep sternal wound infections (dSWIs) from 2006 to 2010 in the overall population of cardiac surgery patients and in the population of high-risk patients (diabetes and/or obesity with body mass index [BMI] >30 kg/m2) treated with coronary artery bypass grafting (CABG).
Statistical analysis
Contingency tables were used for categorical variables. Unadjusted relative risks (RRs) were determined; chi-square tests or Fisher's exact tests, as appropriate, were performed; and 95% confidence intervals (95%CI) were computed. For continuous variables, the median and inter-quartile range (IQR) were used to describe the data and Student's t-test or Mann–Whitney U-test, as appropriate, to compare values between the preintervention and intervention groups. Next, stepwise multiple logistic regression was performed. Variables associated with P values <0.25 in the bivariate analysis were entered into the model, after categorization of continuous variables, to obtain maximum likelihood estimates. The log-rank test was used to compare the time to dSWI between study groups. A Kaplan–Meier curve of time to dSWI was plotted. Patients with dSWI were censored at the time of diagnosis. These analyses were performed using Stata release 10.0 (Stata Corp LP, College Station, TX). All P values were two sided, and P values <0.05 were considered significant.
RESULTS
Study populations
In total, 552 patients (511 with BITA grafting alone and 41 with or without valve repair or replacement surgery) met our study criteria, 289 (52.4%) during the preintervention period and 263 (47.6%) during the intervention period (Table 1). Among the 263 patients managed during the intervention period, 175 (66.5%) received a GCS and 88 (33.5%) wrongly did not (equally distributed over the 2 years of the intervention period). Both populations differed on BMI (P < 0.01) but not on diabetes characteristics (P = 0.23). The reason for not using GCS in the intervention period was either forgetting or the surgeon's perception that the patient was not overweight, as suggested by a higher BMI in patients with GCS than in patient without GCS during the intervention period (P < 0.01) (Table 1). Several variables differed significantly across groups. GCS-treated patients differed from preintervention patients for left ventricular ejection fraction, operating time, and time on extracorporeal circulation (P < 0.01). Non-GCS-treated patients in the preintervention and intervention periods differed for BMI, EuroSCORE, and time on extracorporeal circulation (P < 0.01). Global mortality rates did not significantly differ between the preintervention and intervention periods (5.2% in 2006–08 vs 4.4% in 2009–10, P = 0.68).
Table 1:
Characteristics in the overall population (n = 552), preintervention population (n = 289), intervention-period patients managed with the gentamicin–collagen sponge (n = 175), and intervention-period patients managed without the gentamicin–collagen sponge (n = 88)
| Variables | All (n = 552) | Preintervention period | Intervention period |
|||
|---|---|---|---|---|---|---|
| No GCS (n = 289) | No GCS (n = 88) | P | GCS (n = 175) | P | ||
| Age, median (IQR) | 63.8 (56.5–70.6) | 63.6 (55.8–70.1) | 65.9 (57.3–75.6) | 0.06 | 63.7 (58.3–69.0) | 0.80 |
| Age >70 years, n (%) | 145 (26.3) | 73 (25.3) | 31 (35.2) | 0.07 | 41 (23.4) | 0.66 |
| Female sex, n (%) | 97 (17.6) | 44 (15.2) | 15 (17.0) | 0.08 | 38 (21.7) | 0.08 |
| Body mass index, median (IQR) | 31.5 (33.8–30.1) | 31.4 (30.1–33.6) | 30.8 (28.8–32.0) | <0.01 | 32.1 (30.4–34.7) | 0.11 |
| Body mass index >30 kg/m², n (%) | 425 (77.0) | 226 (78.2) | 63 (71.6%) | 0.19 | 136 (77.7%) | 0.90 |
| Emergency CABG, n (%) | 44 (8.0) | 17 (5.9%) | 8 (9.1%) | 0.33 | 19 (10.8%) | 0.05 |
| Current smoker | 111 (20.1) | 60 (20.8%) | 15 (17.0%) | 0.44 | 36 (20.6%) | 0.96 |
| Arterial hypertension | 423 (76.6) | 226 (78.2%) | 66 (75.0%) | 0.53 | 131 (74.9%) | 0.41 |
| Diabetes requiring insulin | 208 (37.7) | 105 (36.3%) | 30 (34.1%) | 0.70 | 73 (41.7%) | 0.25 |
| Diabetes not requiring insulin | 166 (30.1) | 93 (32.2%) | 19 (21.6%) | 0.06 | 54 (30.9%) | 0.77 |
| Hyperlipidemia | 422 (76.4) | 220 (76.1%) | 72 (81.8%) | 0.26 | 130 (74.3%) | 0.66 |
| Immunosuppression | 1 (0.2) | 0 (0.0%) | 1 (1.1%) | 0.23 | 0 (0.0%) | – |
| COPD | 60 (10.9) | 29 (10.0%) | 12 (13.6%) | 0.34 | 19 (10.9%) | 0.75 |
| Preoperative haemodialysis | 14 (2.5) | 6 (2.1%) | 3 (3.4%) | 0.44 | 5 (2.9%) | 0.75 |
| Repeat CABG | 1 (0.2) | 1 (0.3%) | 0 (0.0%) | 1.00 | 0 (0.0%) | 1.00 |
| NYHA score | ||||||
| 0 | 131 (23.7) | 84 (29.1%) | 15 (17.0%) | 0.16 | 32 (18.3%) | 0.02 |
| I | 108 (19.6) | 56 (19.4%) | 19 (21.6%) | – | 33 (18.8%) | – |
| II | 211 (35.2) | 107 (37.0%) | 37 (42.0%) | – | 67 (38.3%) | – |
| III | 94 (17.0) | 39 (13.5%) | 15 (17.0%) | – | 40 (22.9%) | – |
| IV | 8 (1.4) | 3 (1.0%) | 2 (2.3%) | – | 3 (1.7%) | – |
| LVEF | 57 (48–65) | 60 (50–65) | 60 (50–65) | 0.83 | 55 (45–65) | 0.01 |
| LVEF <50% | 142 (26.2) | 63 (22.1%) | 19 (22.1%) | 0.99 | 60 (35.3%) | <0.01 |
| EuroSCORE, median (IQR) | 3 (2–5) | 4 (2–6) | 4 (2–6) | 0.27 | 4 (2–5) | 0.70 |
| <3 | 207 (37.5) | 111 (38.4%) | 31 (32.6%) | 0.17 | 65 (33.7%) | 0.63 |
| 3–5 | 159 (28.8) | 88 (30.4%) | 25 (28.4%) | – | 46 (26.3%) | – |
| 5–7 | 100 (18.1) | 52 (18.0%) | 12 (13.6%) | – | 36 (20.6%) | – |
| >7 | 86 (15.6) | 38 (13.1%) | 20 (22.7%) | – | 28 (16.0%) | – |
| Preoperative hospital stay (days), median (IQR) | 2 (1–3) | 2 (1–4) | 2 (0–3) | <0.01 | 2 (1–3) | <0.01 |
| Surgical data | ||||||
| Operating time (min), median (IQR) | 200 (180–230) | 205 (180–240) | 200 (180–220) | 0.05 | 200 (180–210) | <0.01 |
| CPB duration (min), median (IQR) | 50 (41–64) | 55 (44.5–69) | 47.5 (38–62) | <0.01 | 45 (38–55) | <0.01 |
| CPB duration, 60 min | 172 (31.2) | 114 (39.4%) | 26 (29.5%) | 0.09 | 32 (18.3%) | <0.01 |
| dSWI | 68 (12.3) | 40 (13.8%) | 6 (6.8%) | 0.09 | 22 (12.6%) | 0.69 |
| Postoperative data | ||||||
| ICU stay >72 h | 185 (66.5) | 92 (31.8%) | 30 (34.1%) | 0.69 | 63 (36.0%) | 0.36 |
| Mechanical ventilation >24 h | 50 (9.1) | 29 (10.0%) | 9 (10.2%) | 1.00 | 12 (6.9%) | 0.26 |
| Use of vasoactive agents | 244 (44.4) | 128 (44.4%) | 41 (44.1%) | 0.66 | 75 (43.1%) | 0.78 |
| Early wound revisionb | 13 (2.4) | 7 (2.4%) | 1 (1.1%) | 0.69 | 5 (2.9%) | 0.77 |
aComparison between the preintervention period and each of the two patient populations during the intervention period (with and without the sponge).
bA wound revision was considered as early if performed during the 96 first hours following CABG.
dSWI: deep sternal wound infection; IQR: inter-quartile range; COPD: chronic obstructive pulmonary disease; CABG: coronary artery bypass grafting; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; CPB: cardiopulmonary bypass; ICU: intensive care unit.
Subgroups with and without deep sternal wound infections (dSWIs)
In the overall population of 552 patients, 68 patients had dSWIs and 484 did not. The dSWIs included 48 (8.7%) SCA/OM cases and 20 (3.6%) mediastinitis cases. The yearly dSWI incidence varied between 8.7 and 16.1%, with no significant changes over time.
The dSWI incidence rate was 40/289 (13.8%) in the preintervention population, 22/175 (12.6%) in the GCS population, and 6/88 (6.8%) in the intervention-period patients without GCS. Comparisons of these three groups showed no statistically significant differences.
Median time to reoperation for dSWI was longer in the GCS group than in the preintervention group (21 and 17 days, respectively; P = 0.04). Kaplan–Meier curves for dSWI in GCS and preintervention groups appear in Figure 2.
Figure 2:
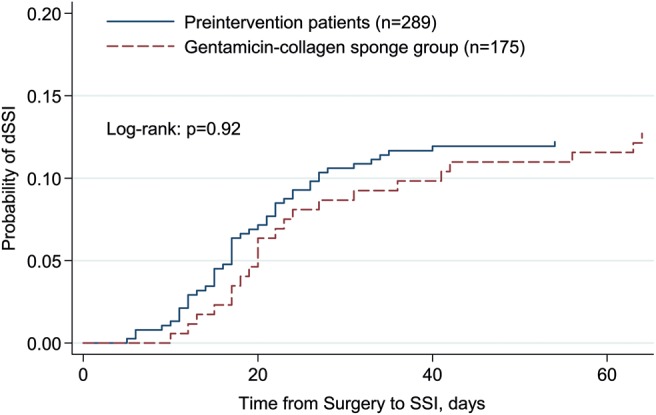
Kaplan–Meier curve of the risk of deep sternal wound infection (dSWI) with and without the gentamicin–collagen sponge.
Risk factors for deep sternal wound infections (dSWIs)
By univariate analysis, the following variables were associated with a higher risk of dSWI: female sex, absence of current smoking, diabetes requiring insulin, chronic haemodialysis, higher NYHA score, higher EuroSCORE, and postoperative complications. GCS use was not associated with the occurrence of dSWI (Table 2).
Table 2:
Characteristics and univariate analysis of variables associated with deep sternal wound infection in 552 cardiac surgery patients at high risk for surgical–site infection
| Variables | dSWI (n = 68) | No dSWI (n = 484) | RR (95% CI) | P |
|---|---|---|---|---|
| Age, median (IQR) | 66.4 (56.2–73.4) | 63.7 (56.6–70.1) | – | 0.24 |
| Age >70 years, n (%) | 22 (32.3) | 123 (25.4) | 1.34 (0.84–2.15) | 0.22 |
| Female sex, n (%) | 33 (48.5) | 64 (13.2) | 4.42 (2.90–6.74) | <0.01 |
| Body mass index, median (IQR) | 31.6 (30.0–34.9) | 31.4 (30.1–33.6) | – | 0.39 |
| Body mass index >30, n (%) | 53 (77.9) | 372 (76.9) | 1.06 (0.62–1.81) | 0.84 |
| Emergency CABG, n (%) | 9 (13.2) | 35 (7.2) | 1.76 (0.94–3.31) | 0.09 |
| Current smoker | 6 (8.8%) | 105 (21.8%) | 0.38 (0.17–0.87) | 0.01 |
| Arterial hypertension | 58 (85.3%) | 365 (75.4%) | 1.86 (0.98–3.55) | 0.07 |
| Diabetes requiring insulin | 36 (52.9%) | 172 (35.5%) | 1.86 (1.19–2.90) | <0.01 |
| Diabetes not requiring insulin | 20 (29.8%) | 146 (30.3%) | 0.97 (0.59–1.58) | 0.94 |
| Hyperlipidemia | 51 (75.0%) | 371 (76.6%) | 0.92 (0.55–1.54) | 0.76 |
| Immunosuppression | 0 (0.0%) | 1 (0.2%) | – | 0.71 |
| COPD | 8 (11.9%) | 52 (10.8%) | 1.09 (0.55–2.17) | 0.77 |
| Preoperative haemodialysis | 5 (7.3%) | 9 (1.9%) | 3.05 (1.45–6.39) | <0.01 |
| Repeat CABG | 0 (0.0%) | 1 (0.2%) | – | 1.00 |
| NYHA score | ||||
| 0 | 23 (33.8%) | 108 (22.3%) | <0.01 | |
| I | 4 (5.9%) | 104 (21.5%) | ||
| II | 23 (33.8%) | 188 (38.8%) | ||
| III | 17 (25.0%) | 77 (15.9%) | ||
| IV | 1 (1.5%) | 7 (1.4%) | ||
| LVEF | 55 (41–65.5) | 58 (48–65) | 0.28 | |
| LVEF <50% | 22 (32.3%) | 120 (25.4%) | 1.38 (0.86–2.21) | 0.22 |
| EuroSCORE, median (IQR) | 3 (2–5) | 5 (3–7) | – | <0.01 |
| <3 | 16 (23.5%) | 191 (39.5%) | <0.01 | |
| 3–5 | 17 (25.0%) | 142 (29.3%) | ||
| 5–7 | 16 (23.5%) | 84 (17.4%) | ||
| >7 | 19 (27.9%) | 67 (13.8%) | ||
| Preoperative hospital stay (days), median (IQR) | 2 (1–4) | 2 (1–3) | 0.97 | |
| Surgical data | ||||
| Operating time (min), median (IQR) | 205 (190–240) | 200 (180–230) | – | 0.12 |
| CPB duration (min), median (IQR) | 50 (42–60) | 50 (41–64) | – | 0.81 |
| CPB duration >60 min | 16 (23.5%) | 156 (32.2%) | 0.68 (0.40–1.16) | 0.15 |
| Gentamicin–collagen sponge | 22 (32.3%) | 153 (31.6%) | 1.03 (0.64–1.66) | 0.90 |
| Postoperative data | ||||
| ICU stay >72 h | 33 (48.5%) | 152 (31.4%) | 1.87 (1.20–2.91) | <0.01 |
| Mechanical ventilation >24 h | 18 (26.9%) | 32 (6.6%) | 3.61 (2.30–5.69) | <0.01 |
| Use of vasoactive agents | 41 (61.2%) | 203 (42.1%) | 1.92 (1.22–3.02) | <0.01 |
| Early wound revisiona | 4 (5.9%) | 9 (1.9%) | 2.59 (1.11–6.05) | 0.06 |
| Intervention period 2009–10 | 28 (41.2%) | 235 (48.5%) | 0.77 (0.49–1.24) | 0.25 |
aA wound revision was considered as early if performed during the 96 first hours following CABG.
dSWI: deep sternal wound infection; IQR: inter-quartile range; COPD: chronic obstructive pulmonary disease; CABG: coronary artery bypass grafting; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; CPB: cardiopulmonary bypass; ICU: intensive care unit.
By multivariate analysis, independent predictors of dSWI were female sex (adjusted odds ratio, aOR [95% CI], 6.16 [3.48–10.92], P < 0.01) and mechanical ventilation time longer than 24 h (5.38 [2.66–10.87], P < 0.01). GCS use was not associated with dSWI after adjustment (0.95 [0.52–1.73], P = 0.88) (Table 3).
Table 3:
Multivariate analysis of variables associated with deep sternal wound infection in 552 cardiac surgery patients at high risk for surgical-site infections
| Variables | Full model aOR (95% CI) | P | Final model aOR (95% CI) | P |
|---|---|---|---|---|
| Age >70 years | 1.31 (0.60–2.86) | 0.49 | ||
| Female sex | 5.81 (3.01–11.17) | <0.01 | 6.16 (3.48–10.92) | <0.01 |
| Current smoking | 0.58 (0.23–1.48) | 0.26 | ||
| Arterial hypertension | 1.34 (0.61–2.96) | 0.46 | ||
| Diabetes requiring insulin | 1.54 (0.83–2.84) | 0.16 | ||
| NYHA score | ||||
| I | 0.21 (0.06–0.67) | <0.01 | ||
| II | 0.59 (0.29–1.19) | 0.14 | ||
| III | 0.72 (0.31–1.64) | 0.43 | ||
| IV | 0.41 (0.03–5.50) | 0.50 | ||
| EuroSCORE | ||||
| 3–5 | 0.75 (0.32–1.72) | 0.49 | ||
| 5–7 | 0.72 (0.27–1.92) | 0.51 | ||
| >7 | 0.78 (0.24–2.46) | 0.67 | ||
| Preoperative dialysis | 1.33 (0.31–5.61) | 0.69 | ||
| LVEF <50% | 0.95 (0.48–1.88) | 0.90 | ||
| Emergency operation | 1.89 (0.73–4.89) | 0.19 | ||
| CPB duration >60 min | 0.53 (0.26–1.09) | 0.09 | ||
| Gentamicin–collagen sponge | 0.78 (0.41–1.49) | 0.46 | 0.95 (0.52–1.73) | 0.88 |
| Mechanical ventilation >24 h | 4.66 (2.08–10.43) | <0.01 | 5.38 (2.66–10.87) | <0.01 |
| Early wound revisiona | 3.83 (0.96–15.24) | 0.05 | ||
aA wound revision was considered as early if performed during the 96 first hours following CABG.
NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; CPB: cardiopulmonary bypass.
Microbiological data
Of the 68 patients with dSWIs, 48 (70.6%) had subcutaneous abscesses or sternal osteomyelitis and 20 (29.4%) had mediastinitis (Table 4). The distribution of mediastinitis and other dSWIs was not different in patients with and without GCS.
Table 4:
Microbiological data among 68 patients with deep sternal wound infection after cardiac surgery
| Variables | Overall (n = 68) | GCS (n = 22) | No GCS (n = 46) | RR (95% CI) | P |
|---|---|---|---|---|---|
| Depth of SWI | |||||
| SCA/OM | 48 (70.6) | 15 (68.2) | 33 (71.7) | 1.00 | – |
| Mediastinitis | 20 (29.4) | 7 (31.8) | 13 (28.3) | 1.18 (0.60–2.35) | 0.63 |
| Causative organisms | |||||
| One organism | 51 (77.3) | 16 (76.3) | 35 (77.8) | – | – |
| More than one organism | 15 (22.7) | 5 (22.7) | 10 (22.2) | – | – |
| Total number of organisms cultured | 83.00 | 27.00 | 56.00 | – | – |
| Enterobacteriaceae | 23 (27.7) | 5 (18.5) | 18 (32.1) | 0.58 (0.24–1.39) | 0.19 |
| Gentamicin-R | 4 (17.4) | 1 (20.0) | 3 (16.7) | 1.20 (0.16–9.18) | 0.62 |
| Staphylococcus aureus | 11 (13.3) | 0 (0) | 11 (19.6) | – | 0.03 |
| Methicillin-S | 11 (100) | 0 (0) | 11 (100) | – | – |
| Gentamicin-R | 0 (0) | 0 (0) | 0 (0) | – | – |
| Coagulase-negative staphylococci | 27 (32.5) | 14 (51.8) | 13 (23.2) | 2.11 (1.16–3.84) | 0.01 |
| Gentamicin-R | 18 (82.6) | 12 (85.7) | 6 (46.1) | 1.86 (0.99–3.47) | 0.07 |
| Enterococcus spp. | 16 (19.3) | 5 (18.6) | 11 (19.7) | 0.94 (0.36–2.44) | 0.90 |
| Gentamicin-R, low-level | 15 (93.7) | 5 (100) | 10 (91.7) | – | – |
| Gentamicin-R, high-level | 1 (6.2) | 0 (0) | 1 (8.3) | – | – |
| Pseudomonas aeruginosa | 3 (3.6) | 1 (3.7) | 2 (3.6) | 1.04 (0.10–10.9) | 0.55 |
| Gentamicin-R | 3 (100) | 1 (100) | 2 (100) | – | – |
| Other organisms | 3 (3.6) | 2 (7.4) | 1 (1.8) | – | – |
| Gentamicin-R | 3 (100) | 2 (100) | 1 (100) | ||
| All gentamicin-R organisms | 44 (53.0) | 21 (77.8) | 23 (41.1) | 1.89 (1.30 –2.75) | <0.01 |
| Wound revision for dSWI | 68 (100) | 22 (100) | 46 (100) | – | – |
| Time to revision, days, median (IQR) | 19.5 (15–26.5) | 21 (17–36) | 17 (13–24) | – | 0.04 |
| Time to revision >19.5 days | 34 (50.0) | 14 (63.2) | 20 (43.5) | 1.46 (0.93–2.31) | 0.19 |
| Bacteremia | 14 (20.9) | 3 (13.6) | 11 (24.4) | 0.57 (0.18–1.84) | 0.51 |
| Hospital mortality | 7 (10.3) | 3 (13.6) | 4 (8.7) | 1.57 (0.38–6.41) | 0.27 |
Data are number (%) unless otherwise indicated.
Gentamicin-R: resistant to gentamicin, SCA/OM: subcutaneous abscess and sternal osteomyelitis.
Overall, 83 micro-organisms were recovered. A single micro-organism was found in 51 patients and two or more micro-organisms in 15. Two patients with dSWIs had negative cultures. The 83 isolates were as follows: Enterobacteriaceae, n = 23 (27.7%); coagulase-negative staphylococci (CoNS), n = 27 (33.5%); Enterococci, n = 16 (19.3%); S. aureus, n = 11 (13.3%); Pseudomonas aeruginosa, n = 3 (3.6%); and other, n = 3 (3.6%). No statistically significant differences were found between the GCS group and both groups without the GCS, regarding Gram-negative species and their resistance to gentamicin. The prevalence of CoNS was significantly higher in GCS patients (14/27, 51.8%) than in those without the GCS (13/56, 23.2%). All 11 S. aureus isolates were susceptible to methicillin and were found in patients managed without the GCS (n = 11, 19.6%).
CoNS recovered from dSWI specimens were more frequently resistant to gentamicin in the GCS-treated patients (12/14, 85.7%) than in the other patients (6/13, 46.1%, P = 0.07). All Enterococci displayed low (n = 15) or high (n = 1) levels of resistance to gentamicin. Overall, 44 of the 83 (53.0%) strains from patients with dSWI were resistant to gentamicin, and the resistance rate was higher with the GCS (n = 21, 77.8%) than without the GCS (n = 23, 41.1%, P < 0.01).
DISCUSSION
In this prospective before/after cohort study, a gentamicin-impregnated collagen sponge did not reduce the rate of dSWI in a high-risk population of patients undergoing CABG with BITA grafts. Our results add to the conflicting data available from several previous studies.
Implants impregnated with antimicrobial agents were developed to limit the growth of organisms in surgical wounds after closure. Gentamicin-impregnated implants have been proposed for various surgical procedures characterized either by a high risk of SSI (e.g. colorectal surgery) or by a low-risk of potentially devastating SSI [15, 16]. Gentamicin has a broad spectrum of bactericidal activity that includes staphylococci and Gram-negative bacteria. Local gentamicin administration is used to avoid diffusion in tissues with disruption of the commensal flora and emergence of bacterial resistance [17].
Several well-performed studies evaluating the effects of a GCS in preventing SWIs in cardiac surgery patients have produced conflicting results [11–13, 18]. In a study done in two centres in all cardiac surgery patients operated through a median sternotomy, the SWI rate was lower in the 967 GCS-treated patients than in the 983 historical patients managed without the GCS (4.3 vs 9.0%) [18]. This study was limited by its quasi-experimental design, with no placebo group. However, a recently published double-blind randomized study in 720 patients concluded that a GCS was superior to a placebo [13]. Finally, in a single-blind randomized study, a GCS was not effective in high-risk patients with diabetes or obesity [12]. These studies differed in terms of methodology, study population, end-point, and follow-up duration.
Here, we focussed on a population at high risk for infection after CABG with BITA grafts because of the presence of obesity and/or diabetes [9, 10, 19]. In this high-risk population, the pathophysiology of SWI may involve delayed wound healing due to sternal ischaemia leading to mechanical wound dehiscence, with possible postoperative contamination [10]. The effects of any preventive measure should be greater in high-risk patients than in the general cardiac surgery population. However, the GCS releases gentamicin only during the first few postoperative days and may, therefore, fail to decrease the risk of SWI related to postoperative contamination during delayed wound healing. Friberg et al. reported a significant decrease in wound-fluid gentamicin concentrations during the first 24 postoperative hours [20], suggesting a decline in the antimicrobial activity of gentamicin during the first few days after GCS implantation. That the time to reoperation for dSWI was significantly longer in GCS-treated patients than in the other patients in our study supports this hypothesis.
The precise description of the microbiology of dSWIs showed that GCS-treated patients had a higher prevalence of CoNS and a lower prevalence of S. aureus than did patients without GCS. That CoNS were the most common micro-organisms is consistent with earlier data [21, 22]. Moreover, resistance to gentamicin probably contributed to the selection of some species. Thus, GCS-treated patients had the same risk of dSWI but were more likely to be infected with gentamicin-resistant organisms than were non-GCS-treated patients. A landmark study has established that antibiotic-resistant CoNS become predominant when subjected to selective pressure of antibiotic surgical prophylaxis [23].
Our patients were followed for 60 days after surgery. Among the 68 patients with dSWI, 13 (19.1%) underwent reoperation more than 30 days after surgery. The duration of postoperative follow-up is a crucial factor in studies of dSWI, given the possibility of postoperative contamination due to delayed wound healing. Restricting surveillance to 30 days, although often done, may underestimate the true SWI rate.
Our study has several limitations. First, its quasi-experimental design makes it sensitive to confounding factors, such as changes in other preventive measures or differences in patient comorbidity profiles. However, the dSWI rate in the overall population of cardiac surgery patients remained stable over the 5-year study period, varying from 3.5 to 4.1 dSWI per 100 procedures, with no significant differences between the preintervention and intervention periods. This evolution of the SSI rate was also observed in the high-risk patient population. The equal distribution over time of patients who should have, but failed to, receive GCS should not have contributed to these variations. In addition, dSWI surveillance was performed for several years using the same methodology (same 2 infection control personnel and senior surgeon) and included a strong end-point, i.e. the need for reoperation for dSWI [24]. The same technique of skeletonized BITA graft harvesting was used throughout the 5-year study period by the same surgical team without modifications in surgical, antibiotic prophylaxis and hygienic techniques. Despite a few differences in baseline characteristics, no major variations were found regarding dSWI incidence or patient characteristics between patients managed without GCS (constituting a useful control group) and the GCS group. Second, our end-point was dSWI requiring reoperation, which encompassed subcutaneous abscess without evidence of bone involvement, osteomyelitis, and mediastinitis. Although this definition does not match the CDC definition, we were able to differentiate true mediastinitis, defined as a need for sternal re-opening, from other dSWIs. Our definition of dSWI is reproducible, provided that all surgeons make the same decision about reoperation when faced by the same clinical and laboratory abnormalities. Third, it was suggested recently that gentamicin might be lost in the dipping solution [25]. However, the increased time to dSWI in GCS-treated patients and the microbiology of dSWI cases do not support a major impact of gentamicin elution into the dipping solution on the in situ concentration or antimicrobial activity of the antibiotic. Finally, this study was performed in a single cardiac surgery centre. SWIs are due to multifactorial causes linked to patient risk factors, surgical technique, operating room environment, and preventive measures, and our results may not be generalizable to other cardiac surgery units.
In conclusion, in our study, a gentamicin-impregnated collagen sponge was not effective in preventing dSWIs in a high-risk population of cardiac surgery patients. Further studies are needed to establish firm conclusions, given the conflicting nature of the results available in the literature, and to identify which patients might be most likely to benefit from GCS implantation. Our results suggest wound contamination in a substantial proportion of patients after CABG with BITA grafts.
ACKNOWLEDGEMENTS
We acknowledge Xavier Arrault, who participated in the data collection of patients receiving GCS and Laurence Armand-Lefevre, who participated in the routine activities in the Bacteriology laboratory of the Bichat-Claude Bernard Hospital.
Conflict of interest: none declared.
REFERENCES
- 1.Barennes H, Andriatahina T, Latthaphasavang V, Anderson M, Srour LM. Misperceptions and misuse of Bear Brand coffee creamer as infant food: national cross sectional survey of consumers and paediatricians in Laos. BMJ. 2008;337:a1379. doi: 10.1136/bmj.a1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Center for Disease Prevention and Control. ECDC europa; Surveillance of healthcare-associated infections in Europe 2007. [published 15 February 2012,]. Available from: http://www.ecdc.europa.eu/en/publications/Publications/120215_SUR_HAI_2007.pdf. 19 September 2012, date last accessed. [Google Scholar]
- 3.Finkelstein R, Rabino G, Mashiah T, Bar-El Y, Adler Z, Kertzman V, et al. Surgical site infection rates following cardiac surgery: the impact of a 6-year infection control program. Am J Infect Control. 2005;33:450–4. doi: 10.1016/j.ajic.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Anderson DJ, Kaye KS, Classen D, Arias KM, Podgorny K, Burstin H, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S51–61. doi: 10.1086/591064. [DOI] [PubMed] [Google Scholar]
- 5.Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. Ann Thorac Surg. 2004;77:676–83. doi: 10.1016/S0003-4975(03)01523-6. [DOI] [PubMed] [Google Scholar]
- 6.Ridderstolpe L, Gill H, Granfeldt H, Ahlfeldt H, Rutberg H. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg. 2001;20:1168–75. doi: 10.1016/s1010-7940(01)00991-5. [DOI] [PubMed] [Google Scholar]
- 7.Harrington G, Russo P, Spelman D, Borrell S, Watson K, Barr W, et al. Surgical-site infection rates and risk factor analysis in coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2004;25:472–6. doi: 10.1086/502424. [DOI] [PubMed] [Google Scholar]
- 8.Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–5. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 9.Toumpoulis IK, Theakos N, Dunning J. Does bilateral internal thoracic artery harvest increase the risk of mediastinitis? Interact CardioVasc Thorac Surg. 2007;6:787–91. doi: 10.1510/icvts.2007.164343. [DOI] [PubMed] [Google Scholar]
- 10.Grover FL, Johnson RR, Marshall G, Hammermeister KE. Impact of mammary grafts on coronary bypass operative mortality and morbidity. Department of Veterans Affairs Cardiac Surgeons. Ann Thorac Surg. 1994;57:559–68. doi: 10.1016/0003-4975(94)90546-0. discussion 568–559. [DOI] [PubMed] [Google Scholar]
- 11.Eklund AM, Valtonen M, Werkkala KA. Prophylaxis of sternal wound infections with gentamicin-collagen implant: randomized controlled study in cardiac surgery. J Hosp Infect. 2005;59:108–12. doi: 10.1016/j.jhin.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Ferguson TB, Jr, Lin M, Garg J, Mark DB, Scavo VA, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: a randomized trial. JAMA. 2010;304:755–62. doi: 10.1001/jama.2010.1152. [DOI] [PubMed] [Google Scholar]
- 13.Schimmer C, Ozkur M, Sinha B, Hain J, Gorski A, Hager B, et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: a controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg. 2011;143(1):194–200. doi: 10.1016/j.jtcvs.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 15.Bennett-Guerrero E, Pappas TN, Koltun WA, Fleshman JW, Lin M, Garg J, et al. Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med. 2010;363:1038–49. doi: 10.1056/NEJMoa1000837. [DOI] [PubMed] [Google Scholar]
- 16.Andersson RE, Lukas G, Skullman S, Hugander A. Local administration of antibiotics by gentamicin-collagen sponge does not improve wound healing or reduce recurrence rate after pilonidal excision with primary suture: a prospective randomized controlled trial. World J Surg. 2010;34:3042–8. doi: 10.1007/s00268-010-0763-2. [DOI] [PubMed] [Google Scholar]
- 17.Leyh RG, Bartels C, Sievers HH. Adjuvant treatment of deep sternal wound infection with collagenous gentamycin. Ann Thorac Surg. 1999;68:1648–51. doi: 10.1016/s0003-4975(99)00836-x. [DOI] [PubMed] [Google Scholar]
- 18.Friberg O, Svedjeholm R, Soderquist B, Granfeldt H, Vikerfors T, Kallman J. Local gentamicin reduces sternal wound infections after cardiac surgery: a randomized controlled trial. Ann Thorac Surg. 2005;79:153–61. doi: 10.1016/j.athoracsur.2004.06.043. discussion 161–152. [DOI] [PubMed] [Google Scholar]
- 19.Momin AU, Deshpande R, Potts J, El-Gamel A, Marrinan MT, Omigie J, et al. Incidence of sternal infection in diabetic patients undergoing bilateral internal thoracic artery grafting. Ann Thorac Surg. 2005;80:1765–72. doi: 10.1016/j.athoracsur.2005.04.061. discussion 1772. [DOI] [PubMed] [Google Scholar]
- 20.Friberg O, Jones I, Sjoberg L, Soderquist B, Vikerfors T, Kallman J. Antibiotic concentrations in serum and wound fluid after local gentamicin or intravenous dicloxacillin prophylaxis in cardiac surgery. Scand J Infect Dis. 2003;35:251–4. doi: 10.1080/003655400310000184. [DOI] [PubMed] [Google Scholar]
- 21.Tammelin A, Hambraeus A, Stahle E. Mediastinitis after cardiac surgery: improvement of bacteriological diagnosis by use of multiple tissue samples and strain typing. J Clin Microbiol. 2002;40:2936–41. doi: 10.1128/JCM.40.8.2936-2941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery—microbiology and pathogenesis. Eur J Cardiothorac Surg. 2002;21:825–30. doi: 10.1016/s1010-7940(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 23.Archer GL, Armstrong BC. Alteration of staphylococcal flora in cardiac surgery patients receiving antibiotic prophylaxis. J Infect Dis. 1983;147:642–9. doi: 10.1093/infdis/147.4.642. [DOI] [PubMed] [Google Scholar]
- 24.Lucet JC. Surgical site infection after cardiac surgery: a simplified surveillance method. Infect Control Hosp Epidemiol. 2006;27:1393–6. doi: 10.1086/509853. [DOI] [PubMed] [Google Scholar]
- 25.Peirano G, van Greune CH, Pitout JD. Characteristics of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli from community hospitals in South Africa. Diagn Microbiol Infect Dis. 2011;69:449–53. doi: 10.1016/j.diagmicrobio.2010.11.011. [DOI] [PubMed] [Google Scholar]


