Abstract
OBJECTIVES
Recently, the prognosis of patients with non-small-cell lung cancer (NSCLC) has improved, thanks to the standardization of adjuvant chemotherapy and the introduction of molecular-targeted drugs, notably epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and other new anti-cancer agents. However, the survival characteristics and prognosis of patients with recurrent NSCLC after curative resection are not well understood.
METHODS
Of the 430 consecutive patients with NSCLC who underwent complete surgical resection at our institution between January 2004 and July 2011, we included 76 patients with recurrence whose post-recurrence treatment and outcome could be confirmed. We then retrospectively evaluated the effect of prognostic factors on post-recurrence survival.
RESULTS
There were 50 men and 26 women, and the median age at recurrence was 74.5 years. The median time from surgical resection to recurrence was 12.7 months. Thirty-eight of the 76 (50%) patients underwent multimodality treatment with surgery and preoperative and/or postoperative chemotherapy as their initial treatment. For recurrence, systemic chemotherapy was administered to 64 (84%) patients, and the disease control rate for first-line chemotherapy was 55%. The 1- and 2-year post-recurrence survival rates were 68.3 and 45.8%, respectively, and the median post-recurrence survival time was 17.7 months. Six independent prognostic factors were identified: wild-type EGFR, no adjuvant chemotherapy for the primary lung cancer, age ≥80 years at recurrence, a poor Eastern Cooperative Oncology Group performance status at recurrence, symptomatic at recurrence and no systemic chemotherapy for recurrence, which significantly decreased the post-recurrence survival.
CONCLUSIONS
The prognosis of patients with NSCLC recurrence after surgery is currently improving. Our results suggested two new prognostic factors, adjuvant chemotherapy and EGFR mutations, neither of which have been previously reported. Treatment strategies for postoperative recurrence should be established based on a more detailed subdivision of factors, such as histology and molecular markers, in the future.
Keywords: Non-small-cell lung cancer, Post-recurrence survival, Adjuvant chemotherapy
INTRODUCTION
The 5-year survival rates after curative resection for non-small-cell lung cancer (NSCLC) have improved remarkably from 52.6% for patients who underwent resection in 1994 [1] to 61.4% in 1999 [2] and 69.6% in 2004 [3]. This improvement is believed to be a consequence of the increase in the detection of small-sized lung cancers, thanks to improvements in diagnostic imaging, such as computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), as well as other factors, such as the standardization of adjuvant chemotherapy. Several randomized controlled trials (RCTs) were reported in the first half of the 2000s, demonstrating the efficacy of adjuvant chemotherapy followed by complete surgical resection; now, this regimen has been accepted as the standard treatment for pathological Stages II and IIIA NSCLC [4–6]. Preoperative induction chemo- or chemoradiotherapy for superior sulcus tumours [7, 8] and resectable clinical Stage IIIA NSCLC with mediastinal lymph node metastasis [9–12] are also being proactively tested. Thus, attempts are now underway to improve the prognosis of Stage II or more advanced NSCLC with a high risk of recurrence using multimodality treatment, including surgery.
Although the rate of recurrence after the total resection of NSCLC varies according to the pathological stage, it is relatively high at 30–75%, and the prognosis remains poor [13, 14]. Most studies of the treatment for recurrent NSCLC after curative resection and its prognostic factors have investigated patients from before 2000, prior to the establishment of adjuvant chemotherapy as a standard treatment. Thus, few studies have addressed the effect of preoperative induction therapy and adjuvant chemotherapy on the treatment and prognosis after recurrence [15–19].
The objective of this study was to elucidate the effect of preoperative and postoperative chemotherapy, particularly platinum-based chemotherapy (which is the standard regimen for Stage II and IIIA NSCLC), on the post-recurrence prognosis.
MATERIALS AND METHODS
Patients and methods
Of 430 NSCLC patients who underwent curative surgical resection at Kawasaki Medical School Hospital, Kurashiki, Japan, between January 2004 and July 2011, postoperative recurrences had occurred in 109 patients (25%) as of February 2012. Of these 109 patients, complete information on the post-recurrence treatment and prognosis was available for 76 patients (70%), and they were included in this analysis. We retrospectively evaluated the effect of clinical factors, oncological factors, initial treatment (surgical procedures and whether preoperative or postoperative chemotherapy was used and if so, what regimen), treatment for recurrence (regimen and therapeutic efficacy) and other factors of post-recurrence prognosis.
Data including age, sex, smoking status, histopathological diagnosis (histology and pathological stage), surgical procedures, whether preoperative or postoperative chemotherapy was used, and if so, what regimen, epidermal growth factor receptor (EGFR) mutations, Eastern Cooperative Oncology Group performance status (ECOG-PS), symptoms at the time of recurrence, site of recurrence, post-recurrence treatment and response, and survival were gathered from the patients' medical records. Histology was categorized according to the World Health Organization Classification of Tumours, 3rd edition [20], and the TNM classification was assigned according to the International Union Against Cancer staging system [21]. The response to chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) guidelines (version 1.1) [22].
All patients had been preoperatively staged using contrast-enhanced CT, FDG-PET and magnetic resonance imaging (MRI) of brain. Routine mediastinoscopy or endobronchial ultrasound with transbronchial needle aspiration to detect occult mediastinal lymph node metastases was not performed.
As a general rule in surgical procedures, we performed a standard surgical procedure, that is, lobectomy with systematic or selective mediastinal lymph node dissection. However, in high-risk patients who were unable to tolerate a lobectomy as assessed by cardiopulmonary function, sublobar resection was selected. Induction therapy was indicated for clinical Stage IIIA patients with resectable mediastinal lymph node metastasis. Adjuvant platinum-based doublet chemotherapy was indicated for pathological Stage II and IIIA patients. On the other hand, adjuvant therapy with oral uracil-tegafur was indicated for pathological Stage IB patients.
In general, a follow-up examination was done every 3 months for the first 2 years, and thereafter every 6–12 months. The follow-up procedures included a physical examination, the serum level of carcinoembryonic antigen and chest radiography. Screening examinations by CT or FDG-PET were done every 6 or 12 months for 5 years.
Recurrent NSCLC was diagnosed based on a physical examination and diagnostic imaging such as CT, MRI and FDG-PET. Histopathological confirmation of the diagnosis was made only when clinically required; as a result, recurrence was histologically confirmed in only 10 of 76 patients.
Written informed consent was obtained from each patient, and the study was approved by the institutional review board of Kawasaki Medical School (IRB no. 1181).
Statistical analysis
The Kaplan–Meier method was used to analyse survival. The χ2 test was used to compare clinicopathological factors between groups. For univariate analyses of the clinicopathological factors, differences were evaluated using the log-rank test, and a multivariate analysis of independent prognostic factors was conducted using Cox's proportional hazards regression model. Differences were considered significant when the P-value was <0.05.
RESULTS
Patient backgrounds
The patient characteristics are given in Table 1. There were 50 men and 26 women. The median age at the time of recurrence was 74.5 (range, 48–87 years). The median disease-free interval from the initial surgery until recurrence was 12.7 months (range 27 days–66.1 months). Regarding the pathological findings, the most common histology was adenocarcinoma in 53 (70%) patients, and the most common pathological Stage was IIIA in 30 cases (39%). Sixty-seven (88%) patients had been screened for EGFR mutation, and EGFR mutations and wild-type EGFR were found in 28 and 39 patients, respectively.
Table 1:
Patient characteristics
| Clinicopathological background | |
| Age at recurrence (years) | |
| Median | 74.5 |
| Range | 48–87 |
| Sex | |
| Male | 50 |
| Female | 26 |
| Performance status at recurrence | |
| 0 | 23 |
| 1 | 33 |
| 2 | 13 |
| 3 | 6 |
| 4 | 1 |
| Smoking status | |
| Non-smoker | 23 |
| Light smoker | 10 |
| Heavy smoker | 40 |
| Unknown | 3 |
| Smoking index (pack-years) | |
| Median | 31 |
| Range | 6.7–90 |
| Histology | |
| Adenocarcinoma | 53 |
| Squamous cell carcinoma | 15 |
| Large cell carcinoma | 3 |
| Adenosquamous carcinoma | 3 |
| Pleomorphic carcinoma | 2 |
| Pathological stage | |
| IA/IB | 14/14 |
| IIA/IIB | 6/11 |
| IIIA/IIIB | 30/1 |
| Epidermal growth factor receptor mutation status | |
| Mutation | 28 |
| Wild-type | 39 |
| Unknown | 9 |
| Initial treatment | |
| Surgical approach | |
| Thoracotomy | 43 |
| Video-assisted thoracoscopic surgery | 33 |
| Surgical procedures | |
| Pneumonectomy | 0 |
| Lobectomy | 60 |
| Segmentectomy | 6 |
| Wedge resection | 10 |
| Lymph nodes dissection | |
| Systematic lymph node dissection | 56 |
| Mediastinal lymph node sampling | 11 |
| Hilar lymph node sampling | 10 |
| Induction chemotherapy | |
| No | 71 |
| Yes | 5 |
| Adjuvant chemotherapy | |
| No | 41 |
| Yes | 35 |
| Platinum-based | 19 |
| Uracil-tegafur | 16 |
| p-Stage IA | |
| Yes (%) | 4 (28) |
| p-Stage IB | |
| Yes (%) | 10 (71) |
| p-Stage II | |
| Yes (%) | 8 (47) |
| p-Stage III | |
| Yes (%) | 13 (42) |
| Recurrent disease | |
| Symptoms at recurrence | |
| Yes | 25 |
| No | 51 |
| Disease-free survival (months) | |
| Median | 12.8 |
| Range | 0.9–66.1 |
| Recurrent site | |
| Local (intrathoracic) | 42 |
| Distant (extrathoracic) | 16 |
| Both | 18 |
| No. of recurrent foci | |
| Single | 29 |
| Multiple | 47 |
| Therapy for recurrence | |
| Systemic chemotherapy | 64 |
| Palliative care | 12 |
| No. of chemotherapeutic regimen | |
| Median | 2 |
| Range | 1–7 |
| First-line therapeutic response | |
| Complete response | 4 |
| Partial response | 23 |
| Stable disease | 8 |
| Progressive disease | 21 |
| Not evaluable | 8a |
| EGFR-TKIs for recurrence | |
| Yes | 36 |
| No | 40 |
TKIs: tyrosine kinase inhibitors.
an = 6, non-measurable recurrent lesion on the RECIST guideline; n = 2, early discontinuation of chemotherapy due to adverse events.
Initial treatment for NSCLC
The initial treatment was surgery alone in 38 patients; multimodality treatment including surgery and preoperative and/or postoperative chemotherapy was performed in the other 38 (50%) patients. Of these, 5 (7%) patients underwent induction chemotherapy or chemoradiotherapy followed by surgery, and 35 (41%) underwent adjuvant chemotherapy, with 2 patients undergoing both preoperative and postoperative chemotherapy. The induction chemotherapy consisted of platinum-based doublet chemotherapy in all 5 patients, with 2 also undergoing concurrent radiotherapy. The adjuvant chemotherapy consisted of platinum-based doublet chemotherapy in 19 patients and oral uracil-tegafur in 16. Twenty-two (29%) patients had been treated with platinum-based doublet chemotherapy either preoperatively or postoperatively.
Recurrence and post-recurrence therapy
Symptoms were evident in 25 (33%) patients at the time of recurrence. The initial recurrence was intrathoracic in 42 patients, extrathoracic in 16 and a combination of intrathoracic and extrathoracic in 18. Forty-seven (62%) patients developed recurrences in multiple organs.
Treatment for recurrence included systemic chemotherapy in 64 (84%) patients and local therapy only in 2 (stereotactic radiosurgery for brain metastases in 2 patients who had only brain recurrences). The remaining 10 patients received only palliative care because of a poor ECOG-PS, an advanced age and so on. Of the 64 patients who underwent chemotherapy, the response to first-line chemotherapy was a complete response (CR) in 4 patients, a partial response (PR) in 23, stable disease (SD) in 8, progressive disease (PD) in 21 and not evaluable (NE) in 8, with a disease control rate for first-line chemotherapy of 55%. EGFR tyrosine kinase inhibitors (TKIs; gefitinib and erlotinib) were used in 36 (47%) patients; none of the patients were treated with a vascular endothelial growth factor inhibitor (bevacizumab).
Post-recurrence survival
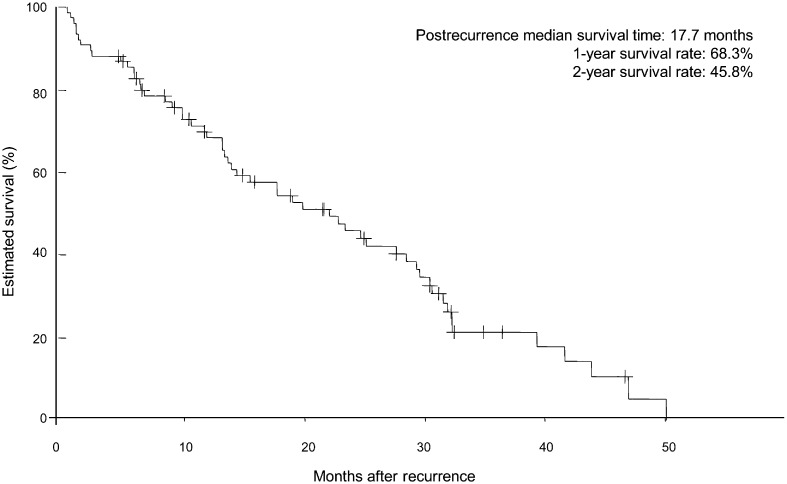
The post-recurrence follow-up period ranged from 10 days to 50.1 months (median, 15.0 months), and the 1- and 2-year post-recurrence survival rates were 68.3 and 45.8%, respectively; the median survival time (MST) after recurrence was 17.7 months (Fig. 1). Post-recurrence survival was analysed with respect to clinical factors (age at recurrence, sex, ECOG-PS at recurrence and smoking status), pathological factors (histology, pathological stage and EGFR mutation status), initial treatment (surgical procedure, whether induction chemo/chemoradiotherapy or adjuvant chemotherapy was used and their regimens) and factors related to recurrence (symptoms at recurrence, postoperative recurrence-free period, site and type of recurrence, use of systemic chemotherapy or EGFR-TKIs, response to first-line chemotherapy). Univariate analyses showed that the patient outcome was significantly poorer for patients with an age of ≥80 years at surgery, non-adenocarcinoma, wild-type EGFR, no adjuvant chemotherapy, no preoperative or postoperative chemotherapy, an age of ≥80 years at recurrence, a poor ECOG-PS at recurrence (PS 2–4), a postoperative recurrence-free period <12 months and no systemic chemotherapy for recurrence. Multivariate analysis of these factors identified six factors as independent prognostic factors: wild-type EGFR, no adjuvant chemotherapy, an age ≥80 years at recurrence, ECOG-PS 2–4, symptomatic and no chemotherapy for recurrence (Tables 2 and 3, Figs 2 and 3). A multivariate analysis of the 64 patients who underwent systemic chemotherapy showed that the response to first-line chemotherapy for recurrence (P <0.001) and the postoperative recurrence-free period (P = 0.026) were independent prognostic factors (Table 4).
Figure 1:
Survival curve illustrating the post-recurrence survival of 76 patients.
Table 2:
Predictors of post-recurrence survival; baseline and primary lung cancer characteristics
| Post-recurrence survival |
P-valuea |
||||
|---|---|---|---|---|---|
| Patients (%) | MST (months) | 2 year (%) | Univariate | Multivariate | |
| Sex | |||||
| Male | 50 (66) | 11.7 | 37.2 | 0.2110 | |
| Female | 26 (34) | 21.6 | 60.5 | ||
| Age at surgery (years) | |||||
| ≤79 | 66 (87) | 17.7 | 49.5 | 0.0128 | 0.460 |
| ≥80 | 10 (13) | 9.2 | 18.0 | ||
| Smoking status | |||||
| Smoker | 50 (66) | 11.7 | 40.2 | 0.4185 | |
| Non-smoker | 23 (30) | 21.6 | 57.5 | ||
| Histology | |||||
| Adenocarcinoma | 53 (70) | 19.8 | 52.9 | 0.0060 | 0.159 |
| Non-adenocarcinoma | 23 (30) | 8.4 | 26.5 | ||
| Epidermal growth factor receptor mutation status | |||||
| Mutation | 28 (37) | 22.8 | 64.4 | 0.0378 | 0.012 |
| Wild-type | 39 (51) | 10.5 | 29.8 | ||
| Pathological stage | |||||
| IA–IB | 28 (37) | 13.9 | 43.2 | 0.9820 | |
| IIA–IIIB | 48 (63) | 15.5 | 47.3 | ||
| Initial surgery | |||||
| Lobectomy | 60 (79) | 17.7 | 51.3 | 0.1290 | |
| Limited resection | 16 (21) | 9.8 | 15.5 | ||
| Induction chemotherapy | |||||
| Yes | 5 (7) | 17.7 | 20.0 | 0.6514 | |
| No | 71 (93) | 15.5 | 48.2 | ||
| Adjuvant chemotherapy | |||||
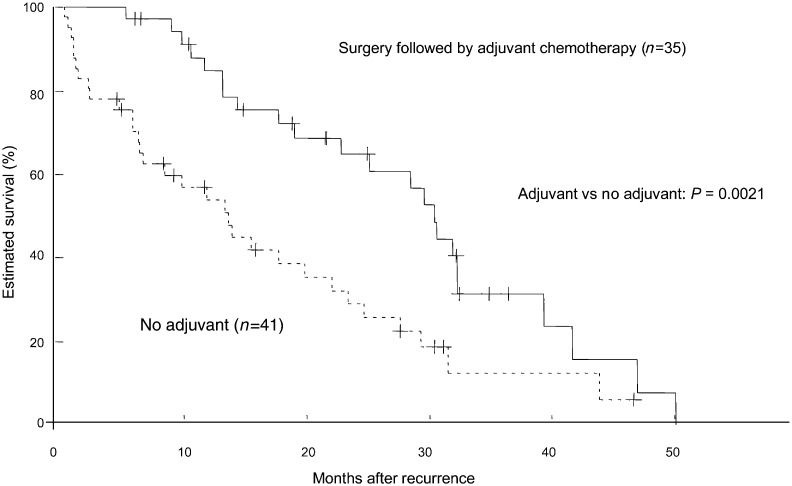
| Yes | 35 (46) | 21.6 | 64.8 | 0.0021 | 0.001 |
| No | 41 (54) | 9.8 | 29.0 | ||
| Chemotherapeutic regimen | |||||
| Paltinum-based | 22 (29) | 19.0 | 49.9 | 0.0082b | 0.291b |
| Uracil-tegafur | 16 (21) | 21.6 | 72.2 | ||
| No | 38 (50) | 9.2 | 32.3 | ||
| Platinum-based chemotherapy | |||||
| Yes | 22 (29) | 13.2 | 47.0 | 0.3921 | |
| No | 54 (71) | 19.0 | 49.9 | ||
MST: median survival time
aLog-rank test for comparison of post-recurrence survival among groups.
bP-value for Paltinum-regimen and uracil-tegafur vs no perioperative chemotherapy.
Table 3:
Predictors of post-recurrence survival: recurrent disease characteristics
| Post-recurrence survival |
P-valuea |
||||
|---|---|---|---|---|---|
| Patients (%) | MST (months) | 2 year (%) | Univariate | Multivariate | |
| Age at recurrence (years) | |||||
| ≤79 | 62 (82) | 17.7 | 49.6 | 0.0182 | 0.001 |
| ≥80 | 14 (18) | 9.2 | 20.4 | ||
| Performance status at recurrence | |||||
| 0–1 | 56 (74) | 18.8 | 58.1 | < 0.0001 | 0.012 |
| 2–4 | 20 (26) | 6.3 | 15.0 | ||
| Symptoms | |||||
| Yes | 25 (33) | 6.5 | 25.1 | 0.0032 | 0.003 |
| No | 51 (67) | 21.5 | 55.4 | ||
| Disease-free interval (months) | |||||
| <12 | 33 (43) | 13.3 | 26.4 | 0.0084 | 0.621 |
| ≥12 | 43 (57) | 19.8 | 65.7 | ||
| Intra- or extrathoracic recurrence | |||||
| Intrathoracic only | 42 (55) | 15.5 | 42.8 | 0.1024 | |
| Extrathoracic only | 16 (21) | 9.0 | 23.8 | ||
| Both | 18 (24) | 19.8 | 56.1 | ||
| Initial recurrence | |||||
| Single site | 29 (38) | 18.8 | 48.0 | 0.167 | |
| Multiple | 47 (62) | 13.6 | 44.7 | ||
| Systemic chemotherapy | |||||
| Yes | 64 (84) | 17.7 | 50.2 | <0.0001 | 0.003 |
| No | 12 (16) | 1.2 | 12.5 | ||
| EGFR-TKIs (gefitinib and erlotinib) | |||||
| Yes | 36 (47) | 15.5 | 42.9 | 0.4205 | |
| No | 40 (53) | 14.4 | 48.2 | ||
EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitors; MST: median survival time.
aLog-rank test for the comparison of post-recurrence survival among groups.
Figure 2:
Survival curves illustrating the post-recurrence survival of 35 patients who initially underwent surgery followed by adjuvant chemotherapy and 41 patients who underwent surgery alone. The curves differed significantly for the patients treated with surgery followed by adjuvant chemotherapy vs the patients treated with surgery alone (P = 0.0021).
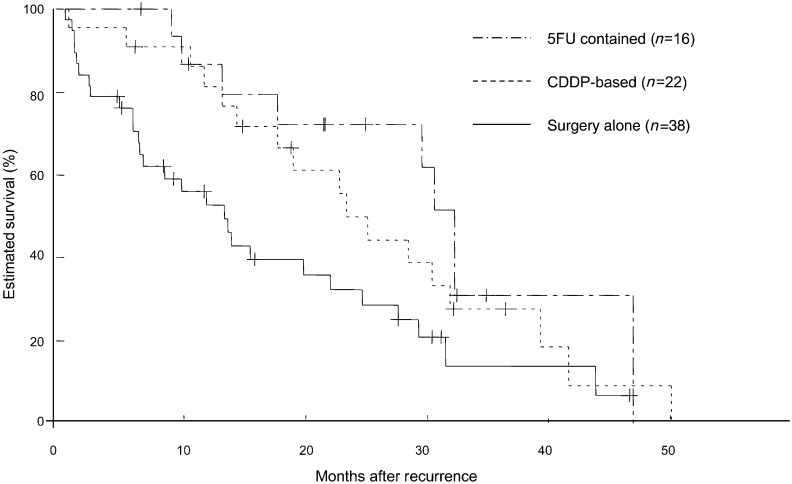
Figure 3:
Survival curves illustrating the post-recurrence survival of 22 patients who received platinum-based induction or adjuvant chemotherapy, 16 who received adjuvant chemotherapy with uracil-tegafur and 38 who did not receive pre- or postoperative chemotherapy. The curves differed significantly for patients who received adjuvant chemotherapy with uracil-tegafur vs those who received no perioperative chemotherapy (P = 0.0089). However, the curves did not differ significantly for patients who received pre- or postoperative platinum-based chemotherapy vs patients who received no perioperative chemotherapy (P = 0.1031) or for patients who received adjuvant chemotherapy with uracil-tegafur compared with those who received pre- or postoperative platinum-based chemotherapy (P = 0.4231).
Table 4:
Predictors of post-recurrence survival for 64 patients who received systemic chemotherapy
| Univariate | Multivariate | |
|---|---|---|
|
P-valuea | ||
| Sex | ||
| Male vs female | 0.6402 | |
| Age at surgery (years) | ||
| ≤79 vs ≥80 | 0.1273 | |
| Age at recurrence (years) | ||
| ≤79 vs ≥80 | 0.0783 | |
| Performance status at recurrence | ||
| 0–1 vs 2–4 | 0.0010 | 0.494 |
| Smoking status | ||
| Smoker vs non-smoker | 0.7829 | |
| Histology | ||
| Ad vs non-ad | 0.2304 | |
| EGFR mutation status | ||
| EGFR mutation vs wild-type | 0.1481 | |
| Pathological stage | ||
| IA–IB vs IIA–IIIB | 0.4776 | |
| Initial surgery | ||
| Lobectomy vs limited resection | 0.4904 | |
| Induction chemotherapy | ||
| Yes vs no | 0.8522 | |
| Adjuvant chemotherapy | ||
| Yes vs no | 0.0507 | 0.207 |
| Perioperative chemotherapy | ||
| Yes vs no | 0.2780 | |
| Platinum-based chemotherapy | ||
| Yes vs no | 0.6443 | |
| Symptoms at recurrence | ||
| Yes vs no | 0.0867 | |
| Disease-free interval (months) | ||
| <12 vs ≥12 | 0.0351 | 0.026 |
| Recurrent site | ||
| Intra vs extra | 0.4756 | |
| Initial recurrence | ||
| Single vs multiple | 0.1266 | |
| EGFR-TKIs (gefitinib and erlotinib) | ||
| Yes vs no | 0.1566 | |
| Response of initial chemotherapy | ||
| CR/PR/SD vs PD | <0.0001 | <0.001 |
Ad: adenocarcinoma; EGFR: epidermal growth factor receptor; TKI: tyrosine kinase inhibitors; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; NE: not evaluable.
aLog-rank test for the comparison of post-recurrence survival among groups.
DISCUSSION
In recent years, not only have numerous RCTs established evidence for chemotherapy, but new anti-cancer agents and molecular-targeted drugs have also been introduced, and personalized treatment strategies are now being recommended based on histology and molecular markers [23]. This standardization and personalization of treatment for NSCLC is improving the prognosis of patients with NSCLC. On the other hand, few studies have evaluated treatments for recurrent NSCLC after curative resection. In clinical practice, chemotherapy for recurrence is routinely administered based on the recommended regimen for unresectable advanced NSCLC. However, patients with postoperative recurrent disease can be anticipated to have a poor ECOG-PS and organ function as a result of pulmonary resection or old age. Furthermore, recurrence after pre/postoperative chemotherapy can theoretically be regarded as the proliferation of tumour cells resistant to anti-cancer agents. Therefore, the treatment and prognosis of postoperative recurrence must differ in some aspects from those used for clinical Stage IV NSCLC.
Previous studies of recurrent NSCLC have described a post-recurrence survival rate of 15–20% at 2 years and a post-recurrence MST of 8–13 months [17–19]. In this study, we only investigated patients who had undergone surgery after the mid-2000s, once adjuvant chemotherapy had become established as a standard therapy and found a 2-year post-recurrence survival rate of 45.8% and a post-recurrence MST of 17.7 months, an improvement in outcome compared with previous reports. This improvement likely reflects the effects of new anti-cancer agents, including EGFR-TKIs. This change in lung cancer chemotherapy can also be expected to have exerted an effect on prognostic factors for recurrent NSCLC. Factors previously reported as affecting the prognosis for recurrent NSCLC include ECOG-PS, whether symptoms are present at the time of recurrence, the postoperative recurrence-free period and pre- or postoperative chemotherapy. Among these, adjuvant chemotherapy has been regarded as a predictor of a poor prognosis for patients with recurrent NSCLC, although this topic has only been addressed by a few studies [15–19]. In our study, 38 (50%) patients received pre- and/or postoperative chemotherapy, with 22 undergoing platinum-based doublet chemotherapy pre- or postoperatively and 16 undergoing adjuvant chemotherapy with oral uracil-tegafur. Among these patients, those who underwent adjuvant chemotherapy had a significantly better outcome than those who did not. This finding differs from that of previous studies. Two possible reasons for this discrepancy can be considered: first, the therapeutic efficacy of EGFR-TKIs and other new anti-cancer agents, and secondly, the use of adjuvant chemotherapy with oral fluorouracil. In Japan, oral uracil-tegafur (referred to as UFT) is routinely used for adjuvant chemotherapy for completely resected pathological Stage IB NSCLC [24, 25]. In our study, oral uracil-tegafur was administered to 16 of the 76 (21%) patients, and the outcomes of these 16 patients were comparatively good (Fig. 3).
Because of the retrospective nature of this analysis, our study has some limitations. First, 33 patients (30% of the recurrent patients) had to be excluded because their post-recurrence course of treatment or outcome could not be analysed. The exclusion of 30% of the patients may have affected our results. Secondly, this study analysed only a small number of patients, and this number was lower than those included in previous studies. So, the conclusions drawn by this study limited the significance of the results obtained. Thirdly, screening for EGFR mutations was not performed in 9 of the 76 (12%) patients. Finally, the response to first-line chemotherapy was not evaluable in 8 of the 64 patients (13%) who underwent systemic chemotherapy for recurrence. These patients discontinued chemotherapy at an early stage because of chemotherapy-induced adverse events or an aggravated general condition caused by disease progression. To resolve these problems, analyse the prognosis of patients with recurrent NSCLC, and establish treatment strategies, multicenter, large-scale, prospective studies are required.
In this study, we investigated the post-recurrence outcome and prognostic factors for patients who had undergone surgery for NSCLC. The prognostic factors included EGFR mutation, adjuvant chemotherapy, ECOG-PS, age, symptoms at the time of recurrence and the use of systemic chemotherapy for recurrence. Although the post-recurrence outcome was better than in previous studies, the outcome for recurrent NSCLC remains poor. The use of adjuvant chemotherapy for initial treatment in accordance with the treatment guidelines is important. Further investigation and standardization of post-recurrence treatment are required.
Conflict of interest: All authors declare that they have no competing interests.
REFERENCES
- 1.Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: A Japanese lung cancer registry study. Lung Cancer. 2005;50:227–34. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, Mori M, et al. A Japanese lung cancer registry study—prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 3.Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004—demographic and prognosis change over decade. J Thorac Oncol. 2011;6:1229–35. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al. National Cancer Institute of Canada Clinical Trials Group; National Cancer Institute of the United States Intergroup JBR.10 Trial Investigators. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Rosell R, Lena MD, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 7.Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Clin Oncol. 2007;25:313–8. doi: 10.1200/JCO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 8.Kunitoh H, Kato H, Tsuboi M, Shibata T, Asamura H, Ichonose Y, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group Trial 9806. J Clin Oncol. 2008;26:644–9. doi: 10.1200/JCO.2007.14.1911. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, et al. Randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–8. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 10.Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JB, Jr, Lee JS, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86:673–80. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 11.van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst. 2007;99:442–50. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M, Rube C, Hoffknecht P, Macha HN, Freitag L, Linder A, et al. German Lung Cancer Cooperative Group. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9:636–48. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 13.Al-Kattan K, Sepsas E, Fountain SW, Townsend ER. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg. 1997;12:380–4. doi: 10.1016/s1010-7940(97)00198-x. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Ginsberg RJ, Venkatraman ES, Bains MS, Downey RJ, Korst RJ, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol. 2002;20:1989–95. doi: 10.1200/JCO.2002.08.092. [DOI] [PubMed] [Google Scholar]
- 15.Ichinose Y, Hara N, Ohta M, Motohiro A, Kuda T, Aso H. Postoperative adjuvant chemotherapy in non-small cell lung cancer: prognostic value of DNA ploidy and post-recurrent survival. J Surg Oncol. 1991;46:15–20. doi: 10.1002/jso.2930460105. [DOI] [PubMed] [Google Scholar]
- 16.Ichinose Y, Yano T, Yokoyama H, Inoue T, Asoh H, Tayama K, et al. Postrecurrent survival of patients with non-small-cell lung cancer undergoing a complete resection. J Thorac Cardiovasc Surg. 1994;108:158–61. [PubMed] [Google Scholar]
- 17.Yoshino I, Yohena T, Kitajima M, Ushijima C, Nishioka K, Ichinose Y, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg. 2001;7:204–9. [PubMed] [Google Scholar]
- 18.Williams BA, Sugimura H, Endo C, Nichols FC, Cassivi SD, Allen MS, et al. Predicting postrecurrence survival among completely resected non-small-cell lung cancer patients. Ann Thorac Surg. 2006;81:1021–7. doi: 10.1016/j.athoracsur.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, et al. Survival after recurrent non-small-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–18. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 20.Travis WD, Colby TB, Corrin B, Shimosato Y, Brambilla E. Histological typing of tumors of lung and pleura. In: Sobin LH, editor. World Health Organization International Classification of Tumours. 3rd edn. New York: Springer-Verlag; 1999. pp. 21–47. [Google Scholar]
- 21.Sobin L, Gospodarowicz M, Witterkind C. International Union Against Cancer: TNM Classification of Malignant Tumors. 7th edn. New York: John Wiley & Sons, Inc.; 2009. [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger DS, Bepler G, Bueno R, Chang A, Chang JY, Chirieac LR, et al. National Comprehensive Cancer Network (NCCN) Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:548–82. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 24.Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. Japan Lung Cancer Research Group on Postsurgical Adjuvant Chemotherapy. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–21. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 25.Hamada C, Tanaka F, Ohta M, Fujimura S, Kodama K, Imaizumi M, et al. Meta-analysis of postoperative adjuvant chemotherapy with tegafur-uracil in non-small-cell lung cancer. J Clin Oncol. 2005;23:4999–5001. doi: 10.1200/JCO.2005.09.017. [DOI] [PubMed] [Google Scholar]