Abstract
OBJECTIVES
The Trifecta valve (St. Jude Medical) was introduced into clinical practice as a tri-leaflet stented pericardial valve designed for supra-annular placement in the aortic position. The present study aims to evaluate the preliminary results with this new bioprosthesis.
METHODS
Seventy patients underwent aortic valve replacement (AVR) with the Trifecta valve between August 2010 and December 2011. Thirty-three patients were male and 37 were female (52.9%). Mean age was 74.65 ± 7.63 (range 47–90 years). Prevalent cause of AVR was aortic stenosis in 64 (91.43%) patients. The mean preoperative pressure gradient was 50 ± 17 (range 20–84 mmHg), and the mean aortic valve area was 0.77 ± 0.33. Five (7.14%) patients were operated on due to aortic valve endocarditis. One patient was operated on due to isolated, severe aortic insufficiency. All patients were in New York Heart Association functional class III or IV. Twenty-eight (40%) patients underwent concomitant procedures.
RESULTS
Concomitant procedures were coronary artery bypass grafting (n = 25), mitral valve replacement (n = 1), ablation of atrial fibrillation (n = 1) and septal myomectomy (n = 1). There were no intraoperative deaths. The 30-day in-hospital mortality was 2.85% (2 of 70). One late death occurred during the in-hospital stay due to a multiorgan failure on postoperative day 60. There were 2 (2.85%) perioperative strokes. Mean pressure gradient decreased significantly from a preoperative value of 50 ± 17 mmHg to an intraoperative gradient of 9 ± 4 mmHg (Table 3). The mean gradients were 14, 11, 11, 8 and 6 mmHg for the 19, 21, 23, 25 and 27 mm valve size, respectively. No prosthesis dislocation, endocarditis, valve thrombosis or relevant aortic regurgitation was observed at discharge.
CONCLUSIONS
The initial experience with the Trifecta valve bioprosthesis shows excellent outcomes with favourable early haemodynamics. Further studies with longer follow-up are needed to confirm those preliminary results.
Keywords: Aortic valve replacement, Heart valve bioprosthesis
INTRODUCTION
In 2009, the Trifecta valve (St. Jude Medical Inc., St. Paul, MN, USA) was introduced into clinical practice as a tri-leaflet stented pericardial valve designed for supra-annular placement in the aortic position. Main innovations comprise leaflets from a single pericardial sheet externally mounted on a high-strength titanium stent and a small sewing ring, which is conceived to increase effective orifice area (EOA) and improved haemodynamic performance (Fig. 1). To date, no study has been published on the Trifecta valve because of its recent clinical introduction. Thus, the present study aims to evaluate the preliminary results with this new bioprosthetic aortic valve.
Figure 1:
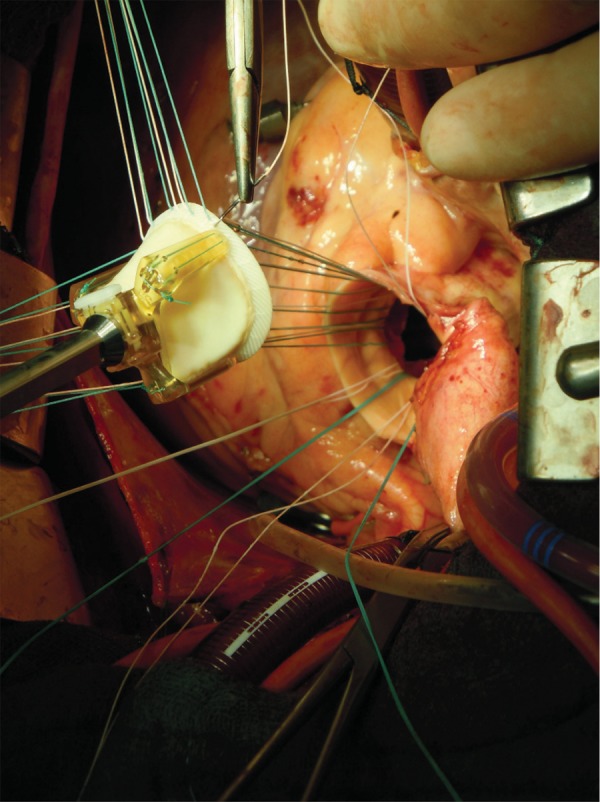
Intraoperative picture of Trifecta valve.
MATERIALS AND METHODS
Patients
This analysis deals with 70 consecutive patients who underwent aortic valve replacement (AVR) with the Trifecta valve at our institution (Department of Cardiac Surgery, Universitätsklinikum Münster, Münster, Germany) between August 2010 (first implantation, 27 August 2010) and December 2011. Patient selection for the implantation of this valve was left to the discretion of the surgeon. Thirty-three patients were male and 37 were female (52.9%). Mean age was 74.65 ± 7.63 (range 47–90 years; Table 1). The mean preoperative pressure gradient was 50 ± 17 (range 20–84 mmHg), and the mean aortic valve area was 0.77 ± 0.33 cm2. All patients were in New York Heart Association functional class III or IV. Informed consent was obtained from each patient, and the study was approved by the institution's human research committee. Transthoracic echocardiography was performed preoperatively and at discharge. Intraoperatively, the correct implantation and function of the valve prosthesis were controlled via transoesophageal echocardiography (TEE). The underlying disease was aortic stenosis in 64 (91.43%) patients. Five (7.14%) patients were operated on due to aortic valve endocarditis. One patient was operated on due to isolated severe aortic insufficiency. The mean additive EuroSCORE was 8.36 ± 2.64. Baseline patient characteristics are listed in Table 1.
Table 1:
Baseline patient characteristics
| Variables | Overall (n = 70) |
|---|---|
| Age, mean ± SD | 74.65 ± 7.63 |
| Sex, female (%) | 37 (52.9) |
| BSA, mean ± SD (m²) | 1.95 ± 0.20 |
| BMI, mean ± SD (kg/m²) | 28.9 ± 4.77 |
| Hypertension (%) | 64 (91.4) |
| Diabetes mellitus (%) | 16 (22.9) |
| COPD (%) | 10 (14.3) |
| Hyperlipidaemia (%) | 45 (64.3) |
| Creatinine, mean ± SD (mg/dl) | 1.27 (0.84) |
| Peripheral vascular disease (%) | 7 (10) |
| Atrial fibrillation (%) | 9 (12.9) |
| EuroSCORE, mean ± SD (%) | 8.36 ± 2.64 |
BSA: body surface area; BMI: body mass index; COPD: chronic obstructive pulmonary disease; SD: standard deviation.
Operative data and statistical analysis
The Trifecta valve was implanted by two expert surgeons. Operations were performed using cardiopulmonary bypass (CPB) with mild hypothermia. Myocardial protection was achieved with cold blood retrograde cardioplegia. Transverse aortotomy was performed approximately 2 cm above the commissures for aortic valve inspection. The aortic annulus was debrided of calcifications. Valve sizing was performed with standard manufacturers' sizers. A non-everted suture technique was used in all patients with an interrupted horizontal mattress suture (2–0 Ethibond, Ethicon, Inc., Somerville, NJ, USA) in a supra-annular position placed around the aortic annulus with pledgets on the ventricular aspect. Good positioning and normal function of the prosthesis were assessed by intraoperative TEE immediately after weaning from CPB. Postoperative anticoagulation consisted of acetylsalicylic acid (100 mg) and low-molecular-weight heparin if there were no contraindications. Oral anticoagulation (vitamin K antagonist) was administered only in the presence of any additional risk factor (atrial fibrillation and ejection fraction <30%).
Baseline data characteristics of patients were prospectively collected. Data are summarized as mean ± standard deviation (SD). Data analysis was performed using commercially available statistical software packages (SPSS for Windows, Version 15.0/SPSS, Inc., Chicago, IL, USA).
RESULTS
There were no emergency cases. Concomitant procedures were coronary artery bypass grafting (n = 25), mitral valve replacement (n = 1), ablation of atrial fibrillation (n = 1) and septal myomectomy (n = 1). Prosthesis sizes implanted were: 19 (n = 8), 21 (n = 21), 23 (n = 18), 25 (n = 19) and 27 mm (n = 4), respectively (Table 2).
Table 2:
Intraoperative data
| Variable | No. of patients | |
|---|---|---|
| Valve size (mm) | 19 | 8 |
| 21 | 21 | |
| 23 | 18 | |
| 25 | 19 | |
| 27 | 4 | |
| Reoperation | 10% | 7 |
| Overall ACC time, mean ± SD (min) | 74.95 ± 22.18 | 70 |
| Overall CPB time, mean ± SD (min) | 113.31 ± 32.45 | 70 |
| Isolated AVR ACC time, mean ± SD (min) | 65.42 ± 13.93 | 42 |
| Isolated AVR CPB time, mean ± SD (min) | 102.04 ± 23.30 | 42 |
ACC: aortic cross clamp; AVR: aortic valve replacement.
There were no intraoperative deaths. The 30-day in-hospital mortality was 2.85% (2 of 70). One patient who had undergone redo AVR died from multiorgan failure on postoperative day 2. Another patient died due to an intraoperative myocardial infarction with consecutive cardiogenic shock on the second postoperative day. One further late death (on the 60th postoperative day) occurred in a patient who developed a late tamponade that required operative re-exploration. The further postoperative course was complicated by pneumonia. The patient finally died from multiorgan failure on postoperative day 60. There were 2 (2.85%) cases of perioperative strokes. One of them fully recovered. There was no endocarditis or valve thrombosis. One patient developed acute renal failure requiring short-term dialysis. Pacemaker implantation was necessary in 3 (4.28%) cases. Three (4.28%) patients required sternal rewiring for deep wound infection and sternal diastasis. None of them needed V.A.C. treatment.
Prosthesis function and haemodynamic performance were assessed intraoperatively and at discharge. Echocardiographic data are shown in Table 3. The mean pressure gradient decreased significantly from a preoperative value of 50 ± 17 to an intraoperative gradient of 9 ± 4 mmHg (Table 3). Details of the haemodynamic performance for each prosthesis size are shown in Table 4. No severe aortic regurgitation caused by a severe leakage was observed at discharge. One patient had a moderate paravalvular leak without haemolysis or necessity of surgical treatment, although he did not suffer from endocarditits preoperatively.
Table 3:
Echocardiographic results
| Variables | Baseline | Intra-operative | Discharge |
|---|---|---|---|
| Aortic valve function | |||
| Maximum aortic gradient (mmHg) | 84 ± 28 | 17 ± 9 | 19 ± 8 |
| Mean aortic gradient (mmHg) | 50 ± 17 | 9 ± 4 | 10 ± 4 |
| Paravalvular leakage | |||
| Moderate | 1 (1.24%) | 1 (1.24%) | |
| Severe | 0 | 0 | |
Table 4:
Echocardiographic haemodynamic data for each valve size
| Variable | Haemodynamic data |
||||
|---|---|---|---|---|---|
| Valve size | 19 (6) | 21 (15) | 23 (14) | 25 (18) | 27 (2) |
| Maximum aortic gradient (mmHg) | 36 ± 7 | 23 ± 9 | 19 ± 4 | 15 ± 4 | 11 ± 5 |
| Mean aortic gradient (mmHg) | 14 ± 4 | 11 ± 5 | 11 ± 3 | 8 ± 2 | 6 ± 2 |
The number of patients is given in parentheses.
COMMENT
The growing elderly population requiring aortic valve surgery has led to the development of new biological valves with better haemodynamic performance. Nowadays, although the effects of mismatch after AVR continue to be a matter of debate [1], there is common consensus that prosthetic valves with low transprosthetic gradients must be preferred.
Although stentless valves have been demonstrated to provide good postoperative gradients [2, 3] with a haemodynamic profile more closely resembling the native aortic valve, it has been shown that they have a substantial learning curve, associated with their technically demanding implantation. Moreover, reoperations are challenging and frequently require aortic root replacement with an increased risk of death [4] in patients with failing stentless valves. The improved design of the recently introduced third-generation stented valves has shown superior performance compared with stentless valves [5]. In this setting, the preference for bovine valves over porcine valves and the supra-annular position in order to place the sewing ring over the annulus, thus minimizing flow-obstruction, seems to be the best approach to optimize haemodynamics [6].
The Trifecta valve is a tri-leaflet stented pericardial valve designed for supra-annular placement in the aortic position. Peculiar characteristics of this valve comprise leaflets from a single pericardial sheet externally mounted on a high-strength titanium stent and a small sewing ring that is conceived to increase EOA and to improve haemodynamic performance. This study, conducted to evaluate the clinical and haemodynamic performance of this new bioprosthetic valve, shows early satisfactory haemodynamic results with a low early-term mortality and morbidity. The 30-day mortality rate was 2.85%. The causes of the two deaths were independent of valve function. This mortality rate is comparable with other studies [7, 8] and seems realistic taking into consideration that in two of three deaths, the presence of advanced age and redo had increased the operative risk.
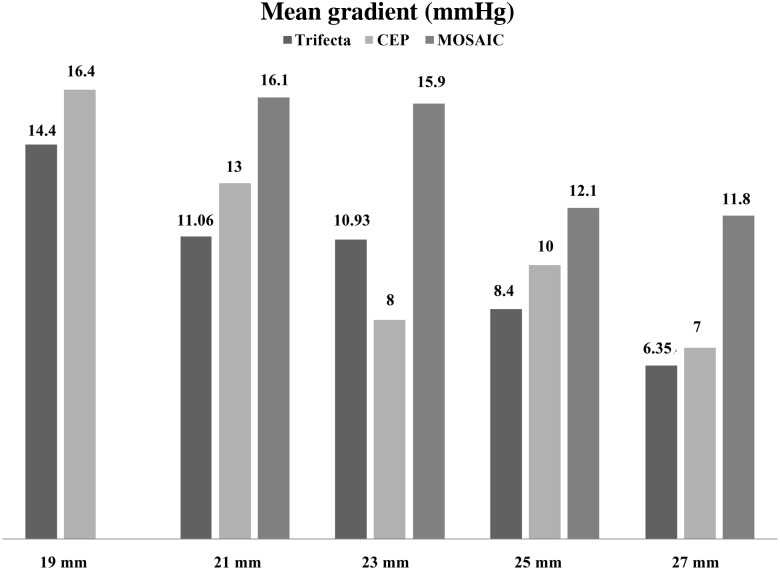
Analyzing the performance of the Trifecta valve, we found lower gradients at discharge compared with other stented biological valves [9, 10] (Fig. 2). For the Mosaic porcine bioprosthesis, Wong et al. have reported mean gradients of 16.1, 15.9 and 12.1 mmHg for the valve sizes 21, 23 and 25 mm, respectively. Similar gradients for Mosaic porcine bioprosthesis were reported by Nozohoor et al. [11]. For the Carpentier-Edwards Perimount (CEP) bovine bioprosthesis, Chambers et al. reported mean gradients of 16, 13, 8 and 10 mmHg for the valve sizes 19, 21, 23 and 25 mm, respectively.
Figure 2:
Mean gradients compared with the Mosaic aortic bioprosthesis [10] and the Carpentier Perimount aortic bioprosthesis [9].
In our study, the mean gradients were 14, 11, 11, 8 and 6 mmHg for the 19, 21, 23, 25 and 27 mm valve sizes, respectively. Comparing those gradients with the above-mentioned studies, we found lower gradients for the Trifecta valve than in all size-matched porcine valves. There were only small differences with lower gradients compared with the size-matched bovine valves, particularly for the 19 and 21 valve sizes. For the 23-mm size valve, Chamber reported lower gradients than for the 25-mm size valve. This discrepancy might be explained by the low number of patients enrolled in his study. The results recently reported by Banbury et al. [12], Dellgren et al. [13] and Pibarot and Dumesnil [14] in a large series of patients with a CEP bioprosthesis showed that the average mean gradient in patients with the 23-mm CEP prosthesis was around 12 mmHg. For the Trifecta valve, we found a minimally better performance than the CEP valve with a mean gradient of 10 mmHg for the 23-mm and minimally better gradients compared with the 25-mm (8 vs 10 mmHg) and the 27-mm (6 vs 7 mmHg) CEP valves. Our data indicate that the Trifecta valve might have a slightly better performance than the matched CEP valve.
A limitation of our study lies in the fact that we focused on early postoperative haemodynamic performance and did not evaluate the impact of late improvement in the haemodynamic performance of Trifecta valves. For this reason, we did not analyse the effect of the improved haemodynamic performance on left ventricular mass regression. Furthermore, temporary effects on echocardiographic measurement of the pressure gradient like pulse rate, fibrillation or not, haemodilution, haematocrite or the degree of preoperative hypertrophy cannot be excluded or quantified. Moreover, due to high variability of aortic valve area at echocardiography during the early postoperative period, we intentionally did not report the aortic valve area. Thus, the frequency of patient–prosthesis mismatch cannot be quantified in the current study. A further possible statistical limitation might lie in the low number of patients.
In conclusion, our data demonstrate good early postoperative results with a low rate of valve-related complications. The Trifecta valve has a better haemodynamic function compared with other bovine aortic valve prosthesis, particularly in small valve sizes. Further studies with longer follow-up focused on valve deterioration and on late valve-related complications are needed to confirm those results.
Conflict of interest: none declared.
REFERENCES
- 1.Cotoni DA, Palac RT, Dacey LJ, O'Rourke DJ. Defining patient-prosthesis mismatch and its effect on survival in patients with impaired ejection fraction. Ann Thorac Surg. 2011;91:692–9. doi: 10.1016/j.athoracsur.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 2.D'Onofrio A, Auriemma S, Magagna P, Favaro A, Cannarella A, Piccin C, et al. Aortic valve replacement with the Sorin Pericarbon Freedom stentless prosthesis: 7 years’ experience in 130 patients. J Thorac Cardiovasc Surg. 2007;134:491–5. doi: 10.1016/j.jtcvs.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Borger MA, Carson SM, Ivanov J, Rao V, Scully HE, Feindel CM, et al. Stentless aortic valves are hemodynamically superior to stented valves during mid-term follow-up: a large retrospective study. Ann Thorac Surg. 2005;80:2180–5. doi: 10.1016/j.athoracsur.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 4.Borger MA, Prasongsukarn K, Armstrong S, Feindel CM, David TE. Stentless aortic valve reoperations: a surgical challenge. Ann Thorac Surg. 2007;84:737–43. doi: 10.1016/j.athoracsur.2007.04.061. discussion 743–4. [DOI] [PubMed] [Google Scholar]
- 5.Totaro P, Degno N, Zaidi A, Youhana A, Argano V. Carpentier-edwards PERIMOUNT Magna bioprosthesis: a stented valve with stentless performance? J Thorac Cardiovasc Surg. 2005;130:1668–74. doi: 10.1016/j.jtcvs.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Ruzicka DJ, Hettich I, Hutter A, Bleiziffer S, Badiu CC, Bauernschmitt R, et al. The complete supraannular concept. Circulation. 2009;120:S139–45. doi: 10.1161/CIRCULATIONAHA.109.844332. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli G, Mirone S, Ius P, Polesel E, Bottio T, Salvador L, et al. Fifteen-year results with the Hancock II valve: a multicenter experience. J Thorac Cardiovasc Surg. 2006;132:602–9. doi: 10.1016/j.jtcvs.2006.05.031. 609. e1–4. [DOI] [PubMed] [Google Scholar]
- 8.Toole JM, Stroud MR, Kratz JM, Crumbley AJ, 3rd, Bradley SM, Crawford FA, Jr, et al. Twenty-five year experience with the St. Jude Medical mechanical valve prosthesis. Ann Thorac Surg. 2010;89:1402–9. doi: 10.1016/j.athoracsur.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 9.Chambers JB, Rajani R, Parkin D, Rimington HM, Blauth CI, Venn GE, et al. Bovine pericardial versus porcine stented replacement aortic valves: early results of a randomized comparison of the Perimount and the Mosaic valves. J Thorac Cardiovasc Surg. 2008;136:1142–8. doi: 10.1016/j.jtcvs.2007.12.086. [DOI] [PubMed] [Google Scholar]
- 10.Wong SP, Legget ME, Greaves SC, Barratt-Boyes BG, Milsom FP, Raudkivi PJ. Early experience with the Mosaic bioprosthesis: a new generation porcine valve. Ann Thorac Surg. 2000;69:1846–50. doi: 10.1016/s0003-4975(00)01167-x. [DOI] [PubMed] [Google Scholar]
- 11.Nozohoor S, Nilsson J, Luhrs C, Roijer A, Sjogren J. Influence of prosthesis-patient mismatch on diastolic heart failure after aortic valve replacement. Ann Thorac Surg. 2008;85:1310–7. doi: 10.1016/j.athoracsur.2007.12.069. [DOI] [PubMed] [Google Scholar]
- 12.Banbury MK, Cosgrove DM, Thomas JD, Blackstone EH, Rajeswaran J, Okies JE, et al. Hemodynamic stability during 17 years of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg. 2002;73:1460–5. doi: 10.1016/s0003-4975(02)03445-8. [DOI] [PubMed] [Google Scholar]
- 13.Dellgren G, David TE, Raanani E, Armstrong S, Ivanov J, Rakowski H. Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124:146–54. doi: 10.1067/mtc.2002.121672. [DOI] [PubMed] [Google Scholar]
- 14.Pibarot P, Dumesnil JG. Is the hemodynamic performance of the Carpentier-Edwards Perimount valve really equivalent to that of stentless valves? Ann Thorac Surg. 2003;76:656–7. doi: 10.1016/s0003-4975(03)00269-8. [DOI] [PubMed] [Google Scholar]