Abstract
OBJECTIVES
Reports on adverse neurological events following transcatheter aortic valve implantation (TAVI) have focused on strokes, while more subtle postoperative cognitive decline has not yet been systematically investigated. In this study, we prospectively examined neurological and cognitive outcomes in patients undergoing transapical (TA) and surgical aortic valve replacement (AVR).
METHODS
A total of 64 patients with severe symptomatic aortic stenosis were investigated between January 2008 and July 2009. Clinical neurological examination and comprehensive neuropsychological testing were performed before and after the procedure, at discharge and at 3-month follow-up. Diffusion-weighted magnetic resonance imaging (DW-MRI) was applied to detect morphological brain injury.
RESULTS
TA-TAVI patients (n = 27) were older and at higher surgical risk compared with surgical AVR patients (n = 37; mean age 82.2 ± 4.7 vs 67.5 ± 8.9 years; log EuroSCORE 36.4 ± 13.2 vs 2.6 ± 8.5%, both P <0.001). There was one stroke in each group (3.7 vs 2.7%, P = 0.49), both classified as embolic based on imaging characteristics. After TA-TAVI, cognitive tests showed no decline during follow-up, while, after AVR, 7 of 11 tests showed a decline early after surgery. Similarly, with-in patient analysis showed that the rate of individuals with clinically relevant cognitive decline was increased early after AVR (TA-TAVI vs AVR: 18 vs 46% at discharge [P = 0.03]; 28 vs 6% at 3 months [P = 0.04]). New focal ischaemic cerebral lesions were detected on DW-MRI in 58% (7 of 12) of patients after TA-TAVI vs 34% (12 of 35) after AVR (P = 0.13). The number of brain lesions per patient and cumulative embolic load per patient were similar between groups. An association between postoperative cerebral ischaemia and cognitive dysfunction was not found (odds ratio 2.37, 95% confidence interval 0.05–113.75, P = 0.66).
CONCLUSIONS
Cognitive function was only mildly impaired after TA-TAVI when compared with a marked, albeit transient, decline after surgical AVR. Focal embolic brain injury tended to occur more frequently after TA-TAVI, but this was not related to cognitive decline during the 3-month follow-up.
Keywords: Cerebral ischaemia, Cognitive function, Transcatheter aortic valve implantation, Diffusion-weighted magnetic resonance imaging, Aortic stenosis, Bioprosthesis
INTRODUCTION
Surgical aortic valve replacement (AVR) is the current standard therapy for patients with severe symptomatic aortic stenosis [1]. However, transcatheter aortic valve implantation (TAVI) has rapidly gained credibility as a viable alternative to open heart surgery in selected high-risk patients with high-grade aortic stenosis and has emerged as the preferred therapy for inoperable patients [2].
Cardiac surgery implies a substantial risk of neurological complications, with stroke being the most devastating event affecting survival and quality of life. Strokes related to the Edwards SAPIEN transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) using a transapical (TA) approach were noted in as many as 5% at 30 days and 10.3% at 1 year [3]. By far the most frequent neurological complication in modern cardiac surgery, however, is postoperative cognitive decline [4]. The reported incidence of cognitive decrease varies considerably depending on the definition of cognitive decline, time of testing or type of treatment and may occur in as many as 80% of patients early after surgery. Most of the studies, including neuropsychological assessment, have been performed on patients undergoing coronary artery bypass graft surgery (CABG). Differences in operative techniques like open heart surgery with the use of cardiopulmonary bypass (CPB) during conventional AVR or beating heart surgery without CPB and catheter-associated manipulation on the aorta or the aortic valve with the risk of increased microembolization during TAVI may have different effects on postoperative cognitive function, and therefore, data collected on CABG patients may not pertain to other procedures. In addition, neurological outcome research related to TAVI has been focused on strokes and transient ischaemic attacks [2, 3, 5], while studies including a systematic neuropsychological examination and a follow-up beyond the immediate postoperative period (i.e. >1 month) have not yet been published.
Surgical AVR studies, using diffusion-weighted magnetic resonance imaging (DW-MRI), have reported that up to 47% of patients revealed new embolic lesions on postoperative neuroimaging [6–8]. Similar DW-MRI studies after TAVI have disclosed an even greater incidence of new cerebral lesions in up to 84% after the transfemoral (TF) [9] and up to 93% after the TA approach [10].
In the present study, we aimed to assess neurological and cognitive functions after TA-TAVI using a comprehensive battery of tests and with post-discharge examination until 3 months. Additionally, in a subgroup of patients, DW-MRI was performed and the occurrence of new perfusion abnormalities was related to postoperative cognitive decline. Neurological outcome after TA-TAVI was compared with patients who underwent surgical AVR within that time period.
PATIENTS AND METHODS
Patient selection
Twenty-seven patients with severe symptomatic aortic stenosis who underwent TA-TAVI at our institution were enrolled in this prospective study between January 2008 and July 2009. Seventeen additional patients received TA-TAVI in the above period, but could not be included because of refusal to participate (n = 8), very low general condition (n = 5) or organizational difficulties (n = 4). Patients undergoing TA-TAVI were deemed inoperable because of excessive high risk as estimated by the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE). Patients with aortic stenosis undergoing TA-TAVI were compared with patients undergoing surgical AVR in that period. Patients scheduled for AVR were screened for inclusion in the study. Prior stroke or high-grade carotid stenosis, psychiatric or neurological illness requiring treatment, uncontrolled hypertension, metabolic disease, alcoholism, non-fluency in the German language and acute endocarditis were exclusion criteria. Patients who needed combined heart surgery (multiple valve disease, coronary artery disease, patent foramen ovale etc.) or heart and vascular surgery (ascending aortic aneurysm and carotid revascularization) were also not eligible. Further exclusion criteria were contraindication to MRI scanning (e.g. pacemaker, claustrophobia) or emergencies. To minimize the postoperative dropout rate, patients who were non-compliant were also excluded. Surgical AVR has been extensively detailed in a previous study [7]. All patients agreed to participate in the study and written informed consent was obtained.
Transapical aortic valve implantation technique
The TA procedure was performed as previously described [11]. Briefly, the Edwards SAPIEN™ aortic valve bioprosthesis was used in all patients. A 6-F pigtail catheter was placed in the ascending aorta for aortic root angiography via a TF approach. Access to the left ventricular apex was gained through a 5–7-cm anterolateral minithoracotomy in the fourth to sixth intercostal spaces. Balloon valvuloplasty was routinely performed in all patients during a brief episode of burst rapid ventricular pacing at a rate of 180–220 bpm. The valved stent crimped onto a balloon delivery catheter was inserted through a 26-F Ascendra II TA valve system (Edwards Lifesciences). During a second episode of rapid pacing, rapid deployment of the device was achieved by standard inflation of the balloon. Exact positioning was guided by fluoroscopy and controlled by aortic root angiography. Transoesophageal echocardiography was used to evaluate prosthesis function. The apex was closed by U-shaped sutures. During the procedure, an activated clotting time >250 s was gained by intravenous heparin given when the pigtail catheter was introduced at the beginning of the procedure. Catheters and balloons were carefully deaired and guidewires thoroughly cleaned before use to minimize the risk of embolization. An aortic embolization protection filter was not used. Device-specific medication consisted of acetylsalicylic acid 100 mg once daily lifelong and clopidogrel 75 mg daily for 6 months after a loading dose of 300 mg/d.
Neurological and neuropsychological function assessment
Examinations were performed at baseline, pre-discharge and 3 months thereafter. Neurological status was assessed by a detailed clinical examination. Cognitive function was evaluated using three different standardized neuropsychological tests and the Mini-Mental State Examination (MMSE) in the TA-TAVI group and eight different neuropsychological tests in the AVR group. Stroke and transient ischaemic attack were defined according to the Valve Academic Research Consortium (VARC) recommendation [12]. In case of stroke, the modified Rankin scale (mRS) was assessed to categorize the patient's level of functional independence during daily activities.
The tests applied in the TAVI group were: digit span subtest of the Wechsler Memory Scale-revised (part forward and part backward) [13], wordlist test (subtest of the Nürnberg age inventory; part immediate recall, part delayed recognition) [14] and the Regensburg verbal fluency test [15]. The digit span subtest examines verbal short-term memory and verbal working memory, wordlist test examines verbal learning and delayed recognition. Therefore, five cognitive domains (short-term memory, working memory, verbal learning, delayed recognition and verbal fluency) were explored by the use of the three different tests. Additionally, the MMSE, a 30-point test for the evaluation of cognitive impairment briefly sampling orientation, registration, attention, calculation, recall and language was applied [16]. Thus, a total of six measures (digit span forward, digit span backward, word list immediate recall, word list delayed recognition, verbal fluency and MMSE) was used in the TAVI patients.
Due to the elderly patients' poor general condition, the neuropsychological tests had to be conducted within a short period of time and as bedside testing. Consequently, the battery did not include paper and pencil tests and was less extensive than that used in patients undergoing surgical AVR. The tests administered in AVR patients examined the areas of cognitive flexibility, rate of information processing, attention, verbal memory, visual memory, reasoning and visuo-spatial perception (for details see [7]). Evaluation of cognitive function over time was performed in two ways, one indicating the cognitive measures with decline (group analysis) and the other indicating the rate of patients with decline (with-in patient analysis).
Cerebral magnetic resonance imaging
MRI of the brain was obtained at the same time as the clinical exams. Scans were performed on a 1.5-T MR unit and a circular polarized head coil (Avanto, Siemens Medical Systems AG, Erlangen, Germany). The imaging protocol included a transversal DW, single shot echo-planar sequence of the whole brain. Diffusion images were processed to generate isotopic apparent diffusion coefficient maps using dedicated software allowing for proper classification of the lesions. Transversal fluid-attenuated inversion recovery and transversal T2-weighted turbo spin-echo sequences were also performed. Slice thickness was 5 mm for all sequences. Scans were read by an experienced neuroradiologist blinded to the clinical data. The presence, number, volume and location of all new focal diffusion abnormalities were recorded.
Statistical analysis
Continuous variables are presented as mean (±standard deviation [SD]) or median (range) depending on distribution. Groups were compared by Student's t-test or Mann–Whitney U-test as appropiate. Categorical variables are given as frequencies and were compared with the χ2 square or the Fisher exact test as appropriate. To analyse the cognitive data, raw test scores were transformed into z-scores based on the mean (±SD) of the baseline scores of the entire study group. In a primary analysis (group analysis), mean test scores of a variable were compared between two time points. In a secondary analysis (with-in patient analysis), the individual score in a test at baseline was compared with a time after intervention and the number of tests with significant improvement (defined as score difference ≥+1 SD) and significant deterioration (i.e. score difference ≤−1 SD) was determined. A cognitive composite test score (defined as difference between the number of tests with improvement and decline) ≤−2 in TA-TAVI patients and ≤−3 in AVR patients denoted a clinically significant cognitive decline in the individual patient. To detect an association between potential clinical or operative risk factors and new diffusion-weighted imaging (DWI) lesions, patients with and without lesions were compared. The relationship between cerebral ischaemia and postoperative cognitive test performance was determined by linear regression analysis. All analyses were conducted with SPSS version 17 (SPSS, Chicago, IL, USA).
RESULTS
Of 64 patients enrolled in the study, 27 patients received TA-TAVI and 37 surgical AVR. Patients undergoing TA-AVI were significantly older, had more comorbidities and a higher estimated operative risk for mortality than those undergoing surgical AVR (Table 1). Mortality in the TA-TAVI group was 18.5% at 30 days (right ventricular failure n = 1, sepsis n = 2, acute mesenteric ischaemia n = 1, limb ischaemia due to heparin-induced thrombocytopenia n = 1) and 25.9% at 3 months (stroke n = 1 and unknown n = 1). No patient in the AVR group died during follow-up. The stroke rate was 3.7 in the TA-TAVI vs 2.7% in the AVR group (P = 0.49). The stroke event in the TA-TAVI patient was fatal as a result of large multiple bihemispheric embolic infarctions, while that in the AVR patient caused only minor impairment (mRS score = 1).
Table 1:
Baseline characteristics of the study groups
| TA-TAVI (n = 27) | AVR (n = 37) | P-value | |
|---|---|---|---|
| Age (years) | 82.2 ± 4.7 | 67.5 ± 8.9 | <0.001 |
| Female gender | 20 (74.1) | 18 (48.6) | 0.04 |
| Logistic EuroSCORE (%) | 36.4 ± 13.2 | 2.6 ± 8.5 | <0.001 |
| Arterial hypertension | 27 (100) | 28 (75.6) | 0.01 |
| Diabetes mellitus | 8 (29.6) | 3 (8.1) | 0.03 |
| Hyperlipidaemia | 20 (74.1) | 22 (59.4) | 0.17 |
| Smoking | 8 (29.6) | 19 (51.3) | <0.05 |
| Coronary artery disease | 15 (55.5) | 0 | <0.001 |
| Previous cardiac surgery | 4 (14.8) | 0 | 0.03 |
| Ejection fraction (%) | 55.3 ± 8.9 | 60.3 ± 14.4 | 0.12 |
| Peripheral vascular disease | 8 (29.6) | 1 (2.7) | 0.01 |
| Previous cerebral ischaemic event | 6 (22.2) | 0 | 0.01 |
| Porcelain aorta | 1 (3.7) | 0 | 0.42 |
| Educational level (years at school >8) | 4 (14.8) | 12 (32.4) | 0.07 |
Continuous variables are presented as means ± SD and categorical variables as frequencies (percentage).
AVR: aortic valve replacement; TA-TAVI: transapical transcatheter aortic valve implantation.
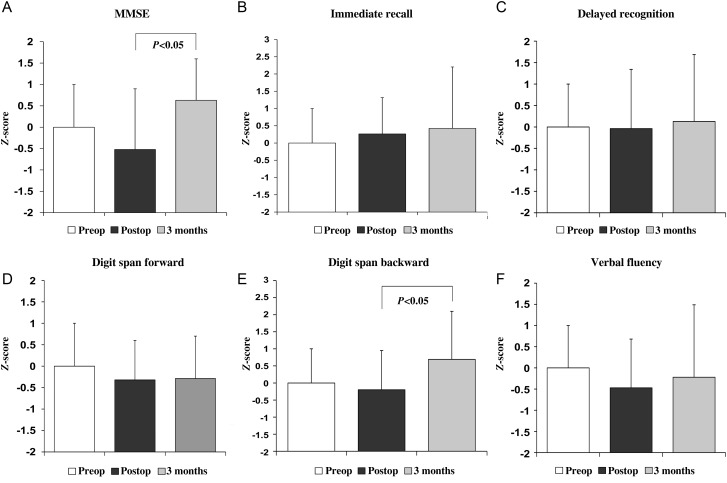
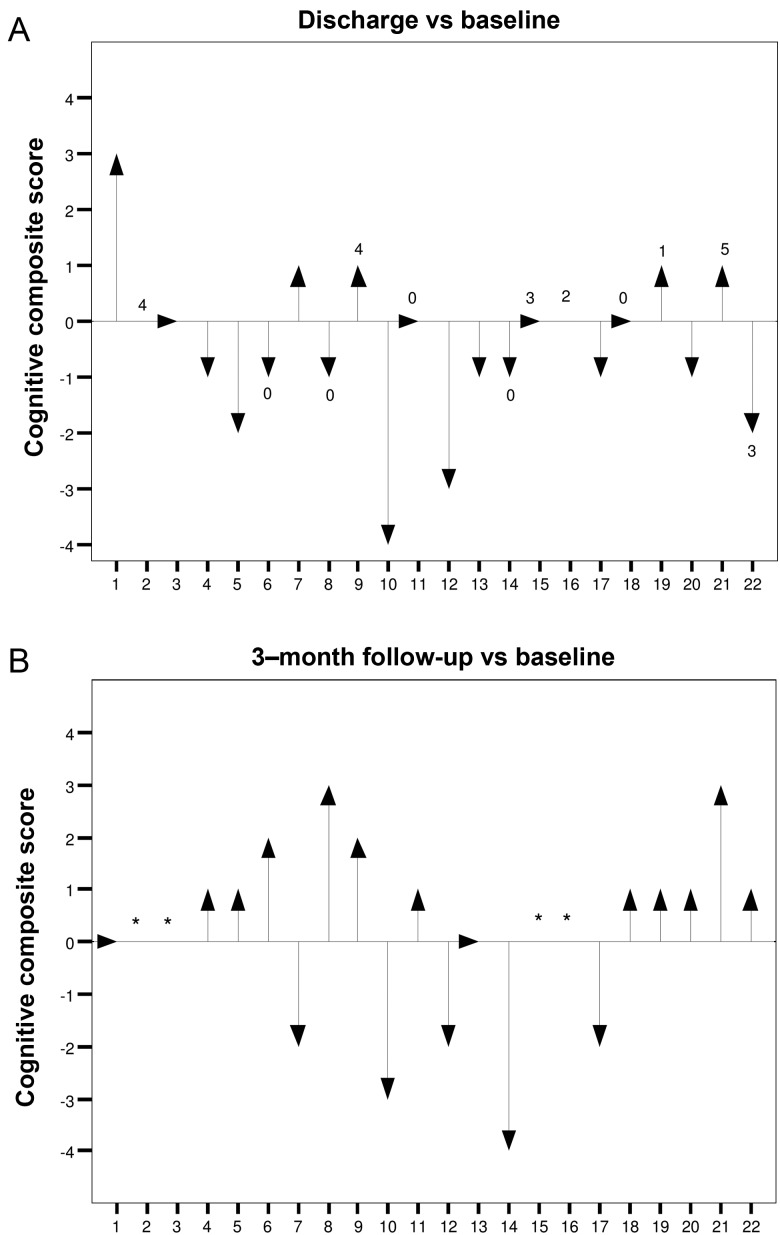
Baseline examination was performed 5.4 ± 8.1 days before TA-TAVI and repeated 10.7 ± 4.9 days (predischarge) and 115.6 ± 49.7 days (follow-up) after intervention. In the TA-TAVI group, 22 patients were available for cognitive testing at discharge (death: n = 4, stroke: n = 1) and 18 at follow-up (death: n = 2, refusal to participate: n = 2). Figure 1 shows cognitive test scores in the TA-TAVI group. In all 6 cognitive domains tested, there was no statistically significant decrease in test performance over time compared with baseline. In the MMSE and digit span backward test, a non-significant decline at discharge (z-score difference, −0.72 ± 1.42 and −0.20 ± 1.15, respectively) was followed by a significant increase by 3 months (z-score difference 0.95 ± 1.20 and 0.98 ± 1.45, respectively, P <0.05). Individual cognitive performance is shown in Fig. 2. The rate of patients with a clinically relevant cognitive deficit (i.e. cognitive composite score ≤−2) was 18 (n = 4 of 22) and 28% (n = 5 of 18) at discharge and follow-up, respectively.
Figure 1:
Change of cognitive function over time in patients undergoing TA-TAVI.
Figure 2:
Individual cognitive function at discharge and 3 months after TA-TAVI. Numbers on top of the arrows indicate the number of new lesions on DW-MRI. *Patient not available for follow-up.
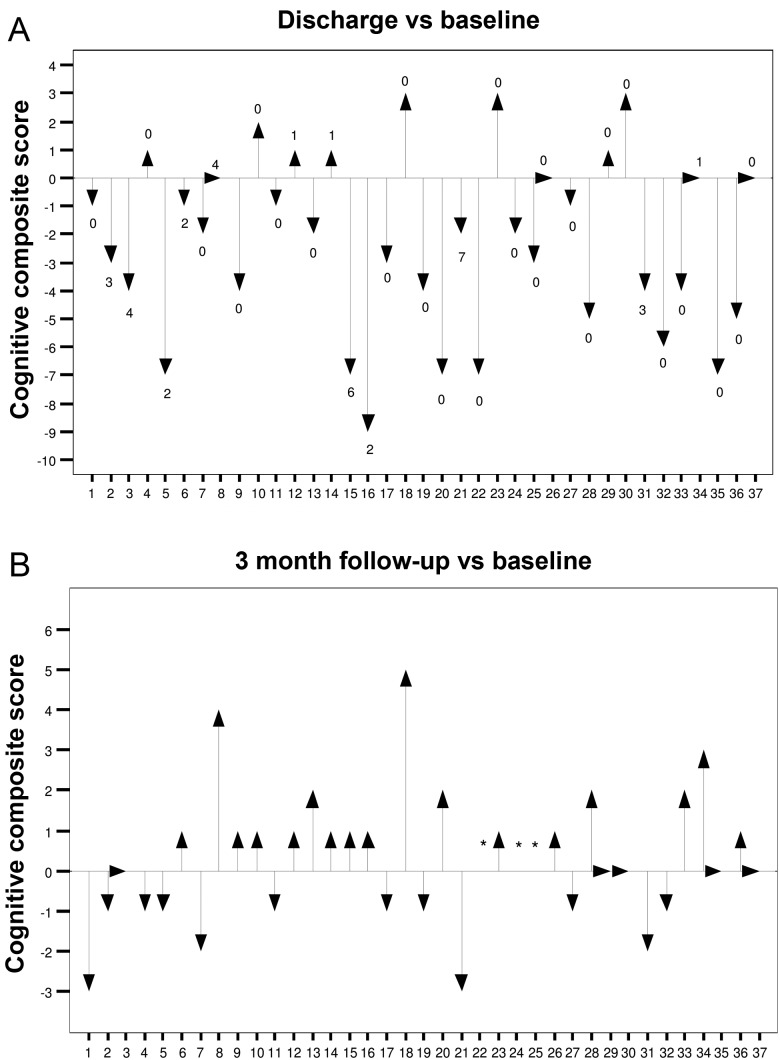
In the AVR group, all 37 patients underwent neuropsychological examination at discharge and 34 at follow-up. At discharge, 7 of the 11 tested cognitive domains declined compared with baseline (Table 2). There was no cognitive test with impairment by 3 months. While 5 of the 7 tests with initial decline returned to baseline, 2 of them showed a gain in test performance (cognitive flexibility and reasoning). The rate of patients with a clinically relevant cognitive deficit (i.e. cognitive composite score ≤−3) was 46 (n = 17 of 37) and 6% (n = 2 of 34) at discharge and follow-up, respectively (Fig. 3).
Table 2:
Cognitive test scores in patients undergoing surgical aortic valve replacement (n = 37)
| Cognitive domain | Test | Baseline (E1) | Discharge (E2) | 3 months (E3) | P-value (E1 vs E2) | P-value (E1 vs E3) |
|---|---|---|---|---|---|---|
| Rate of information processing | Trail making A | −0.197 (0.999) | 0.309 (4.128) | −0.292 (1.031) | <0.001 | 0.25 |
| Cognitive flexibility | Trail making B | −0.232 (0.999) | 0.795 (1.577) | −0.342 (0.757) | <0.001 | 0.04 |
| Attention | Divided attention | 0.000 (1.000) | 0.770 (1.750) | 0.106 (1.139) | 0.01 | 0.17 |
| Memory | VLT—immediate recall | −0.004 (0.998) | −0.317 (0.994) | 0.005 (0.839) | 0.08 | 0.62 |
| VLT—delayed recognition | 0.211 (0.998) | −0.820 (1.035) | 0.469 (1.002) | <0.001 | 0.94 | |
| Verbal short-term memory | Digit span forward | −0.003 (1.000) | 0.119 (0.999) | 0.367 (0.932) | 0.30 | 0.01 |
| Verbal working memory | Digit span backward | 0.003 (1.001) | −0.521 (1.017) | 0.015 (0.888) | 0.01 | 0.69 |
| Visual short-term memory | Corsi block tapping forward | 0.003 (0.999) | −0.626 (1.253) | 0.073 (1.122) | 0.01 | 0.92 |
| Visual working memory | Corsi block tapping backward | 0.002 (1.002) | −0.448 (1.418) | 0.164 (1.023) | 0.06 | 0.49 |
| Logical thinking | Horn test no. 3 | −0.001 (1.001) | −0.376 (1.174) | 0.417 (1.075) | 0.02 | 0.01 |
| Visuo-spatial perception | Horn test no. 9 | −0.001 (0.999) | −0.234 (1.240) | 0.324 (1.055) | 0.08 | 0.07 |
Data are z scores (mean ± SD) transformed from raw scores. Note that higher values indicate improved test performance except for timed tests (i.e. Trail making A, Trail making B and divided attention) where higher values denote decline.
E: examination; VLT: verbal learning test.
Figure 3:
Individual cognitive function at discharge and 3 months after surgical AVR. *Patient not available for follow-up.
In addition to neuropsychological testing, MRI studies were performed before and after the procedure. Preprocedural scans were available in 17 (63%) TA-TAVI patients. The reasons for missing baseline scans were pacemaker implants (n = 3), logistic problems (n = 5), MRSA infection (n = 1) and a metal-containing peridural catheter (n = 1). Postprocedural MRI scans could be obtained in 12 patients (Table 3). Six of the 17 patients with preoperative scans were not available after intervention (back pain n = 2, logistic problems n = 1, new pacemaker inserted n = 1, incompliance n = 1, death n = 1), while 1 patient with missing baseline MRI was available postprocedurally. The TA-TAVI patient who suffered a major stroke had no MRI before the procedure due to organizational reasons, and postprocedurally underwent computed tomography for neuroimaging. In the AVR group, all patients could be scanned before surgery, and 95% had MRI early after surgery. Time of postprocedural scan was 11.7 ± 6.2 days after TA-TAVI vs 6.5 ± 3.0 days after AVR (P = 0.02).
Table 3:
Findings on MRI after transapical and surgical aortic valve replacement
| TA-TAVI (n = 12) | AVR (n = 35) | P-value | |
|---|---|---|---|
| Patients with new lesions | 7 (58) | 12 (34) | 0.131 |
| Total number of lesions | 22 | 37 | 0.224 |
| Localization | |||
| Frontal | 14 (64) | 9 (24) | 0.003 |
| Parietal | 1 (5) | 2 (5) | 0.451 |
| Occipital | 4 (18) | 10 (27) | 0.192 |
| Temporal | 1 (5) | 1 (3) | 0.476 |
| Cerebellum | 2 (9) | 10 (27) | 0.072 |
| Others | 0 | 5 (14) | 0.087 |
| Patients with single lesion | 1 (8) | 3 (9) | 0.397 |
| Patients with multiple lesions | 6 (50) | 9 (26) | 0.525 |
| Patients with bihemispheric lesions | 5 (42) | 6 (17) | 0.465 |
| Embolic lesions per patient | 1.8 ± 1.9 (0–5) | 1.1 ± 1.9 (0–7) | 0.149 |
| Single lesion volume (mm3) | 171.7 ± 282.9 (15–1205) | 218.6 ± 437.3 (50–1900) | 0.713 |
| Time of postprocedural scan (days) | 11.7 ± 6.2 (6–26) | 6.5 ± 3.0 (2–32) | 0.016 |
Categorical data are given as frequency (percentage) and continuous data as mean ± SD (range).
On postoperative MRI scans of the brain, 7 (58%) TA-TAVI patients had areas of restricted diffusion suggestive of new focal ischaemia (Table 3). Total number of lesions was 22, with a median of 1.5 lesions per patient (range 0–5). All new DWI lesions were clinically silent. Lesions typically occurred in >1 vascular territory in all but 1 patient with multiple lesions suggesting an embolic pathogenesis. More than half of the lesions were detected in the frontal lobe, and 64% were on the right side. The cumulative embolic load per patient was smaller than 1 cm2 in 6 of the 7 patients. After AVR, 12 of the 35 (34%) patients with postoperative MRI were found to have a total of 37 new lesions. The number of patients with new lesions, total number of lesions, lesions per patient and cumulative lesion volume per patient were similar between both groups (Table 3).
To assess the effect of potential risk factors on structural brain injury detected on DW-MRI, multiple variables (e.g. age, pre-existing brain abnormalities, hypertension, procedure time, time of postoperative scanning) were analysed. A difference between TA-TAVI patients with or without new DWI lesions was not found. The presence of postoperative new cerebral ischaemia was not related to cognitive dysfunction (odds ratio 2.37, 95% confidence interval 0.05–113.75, P = 0.66). Early cognitive impairment was also not predictive for late cognitive performance (P = 0.46).
DISCUSSION
A considerable number of elderly patients with severe aortic stenosis are considered not to be candidates for conventional replacement of the aortic valve due to comorbidity and age [2]. TAVI has emerged as a less invasive treatment for high-risk patients with aortic stenosis, and meanwhile, a considerable amount of literature is available on TAVI addressing procedural success, general clinical outcome and echocardiographic follow-up. Neurological assessment after TAVI has focused on documenting clinically apparent strokes and transient ischaemic attacks [3]. However, the extent of brain injury due to ‘silent ischaemia’ may be conceivably higher and responsible for more subtle cognitive or intellectual decline. In fact, postoperative cognitive decline is the most frequent complication in modern cardiac surgery [4], and microembolization has been suggested to play a crucial role in the development of postoperative cognitive deterioration [4, 17, 18]. Studies using postinterventional cerebral DW-MRI after TF- and TA-TAVI have recently reported a high number of new, albeit mostly clinically silent focal hyperintense signals, suggestive of small embolic lesions [9, 10, 19–21], and neuromonitoring with intraoperative transcranial Doppler ultrasound has documented considerable procedural microembolization [22, 23].
Hence, TAVI is expected to be associated with increased downstream (micro-)embolization, but the effect on cognitive function is not known. Using various neuropsychological tests, the present study is the first to systematically investigate cognitive function following TA-TAVI, which is supposed to be less invasive than surgical valve replacement with the use of extracorporeal circulation. One of the main results was that we found no evidence of a relevant cognitive impairment in any domain immediately and 3 months after TA-TAVI. The rate of patients with an individual clinically relevant cognitive deficit was also moderate at discharge and during follow-up. All 4 TA-TAVI patients with a cognitive deficit at discharge subsequently improved in function. In comparison, surgical AVR patients revealed a marked cognitive impairment at discharge as reflected by both the number of cognitive domains with decline (7 of 11) and the number of patients with an individual cognitive deficit (48%). The reasons for the comparably mild cognitive changes in the TA-TAVI patients remain unknown. Of note, TAVI patients were much older and had more comorbidity predisposing to more extensive atherosclerotic disease of the cerebral vasculature, both factors predisposing to even more severe neurological morbidity after intervention. On the other hand, it might be argued, that the younger, less comorbid patients undergoing surgical AVR a priori have a higher level of cognitive function than the TA-TAVI patients and, therefore, show a more pronounced decline. As long as a direct comparison between low-risk AVR and equally low-risk TA-TAVI patients is considered somewhat unethical due to the lack of randomized, controlled clinical trials, such a question has to remain open at present.
While cognitive decline is frequently seen after conventional cardiac surgery, particularly when CPB is used, no previous study has systematically investigated cognitive functioning by the use of tests onto a spectrum of various cognitive areas after TAVI that is performed, while the heart is beating and the circulation not interrupted. Recently, Kahlert et al. [9] reported on 32 TF-TAVI patients in whom cognitive function was screened by the MMSE test. Compared with baseline, the authors did not find an impairment of cognitive function during follow-up as indicated by stable peri-interventional MMSE scores. In a prospective multicenter study by Rodes-Cabau et al. [19] that included 29 TF- and 31 TA-TAVI patients treated with the Edwards SAPIEN device, there was also no significant difference in the MMSE score between the two groups. When interpreting these data, one has to take into consideration that the MMSE is a crude, global measure for cognitive impairment. Developed as a quick screening tool to provide quantitative information on cognitive impairment and to record cognitive changes over time [16], it does not allow a detailed assessment of cognitive function. Hence, it cannot be precluded that more comprehensive cognitive testing in the two recent studies might have been able to detect subtle changes in some areas of cognition.
DW-MRI, a sensitive surrogate marker of ischaemic brain injury, has shown new perfusion abnormalities in as many as 47% of patients after AVR [6–8]. After TAVI, DW-MRI studies revealed an even greater incidence of new cerebral lesions. In TF studies, new ischaemic lesions were reported to range from 66 to 84% [9, 19], and in TA studies from 66 to 93% [10, 21]. With an incidence of 58% of new DWI lesions in our patients, our data parallel those reported by other studies with 25 and 31 patients undergoing MRI [19, 21]. In one smaller series including 14 patients using the Edwards SAPIEN valve, 13 (93%) patients were found to have new embolic lesions [10]. One of the reasons for the variability in the reported incidence of new lesions after the procedures may be that the time when the scan is obtained varies between studies. With an acquisition time of 48 h after TAVI, the high number of newly detected lesions in the above study is probably due to this protocol. Although all lesions were neurologically silent, their impact on cognitive function remained open since the study did not include cognitive testing [10].
Conflicting results are reported regarding the clinical significance of cerebral embolic lesions detected on postoperative examination after cardiac surgery. In a review of 22 cardiac surgery studies using transcranial Doppler monitoring or DW-MRI and cognitive testing [24], no association was found in 15 studies, and in only one of the seven studies using DW-MRI such an association was assumed [25]. After TAVI, the vast majority of new focal embolic lesions were silent [9, 10, 20, 21], and in the two studies that included the assessment of cognitive function by use of MMSE, a correlation between the occurrence of new lesions and cognitive function was not found [9, 19].
There are some limitations to the study. The numbers are small, affecting the statistical power of the analysis. However, this is a single-centre study, and the TA procedure was introduced into clinical practice with the availability of the CE mark for the Edwards SAPIEN prosthesis in January 2008. Many patients could not have MRI scanning because of claustrophobia, pacemaker or logistic difficulties. The study was meant as an observational study without prespecified power or sample size for any of the outcomes. At present, AVR is the treatment of choice in patients with symptomatic severe degenerative aortic stenosis, and the indication for TAVI is restricted to patients with aortic stenosis whom surgeons considered not to be suitable candidates for conventional AVR due to coexisting conditions. In fact, in this study, all patients undergoing TA-TAVI were declined for traditional surgery because of comorbidities associated with excessive operative risks. TA-TAVI patients were significantly older (82.2 ± 4.7 vs 67.5 ± 8.9 years) and the predicted operative mortality was much greater (EuroSCORE 36.4 ± 13.2 vs 2.6 + 8.5% years) compared with AVR patients (Table 1). Because the indication for TAVI was different from AVR, however, it was impossible to obtain a control group of comparable profile. These differences have to be kept in mind when comparing the two groups in terms of clinical outcomes. Also, this is important when considering baseline cognitive functioning and the changes in performance over time. As long as the results of ongoing trials in which patients are randomly assigned to standard surgical AVR or TAVI are being awaited, the two treatment groups will continue to differ markedly and, hence, comparisons naturally remain a matter of controversy due to different baseline performances. Until exactly matched groups of TA-TAVI and surgical AVR patients are available, the effect of both treatment options on neurocognitive outcome may only be analysed within these limitations.
In conclusion, TA-TAVI in elderly high-risk patients with severe aortic stenosis can be performed without clinically significant cognitive decline during a 3-month follow-up. Although cerebral embolism was frequently seen following TA-TAVI, an association between new ischaemic lesions and cognitive function was not found. After surgical AVR, cognitive function was severely impaired, but resolved completely in 3 months.
ACKNOWLEDGEMENTS
We are indebted to Christian Lösch for statistical support and Jan Felix Fritsch for his excellent and continuous support in data analysis. We thank Sabrina Lück for data acquisition.
Conflict of interest: none declared.
REFERENCES
- 1.Bonow RA, Carabello BA, Chatterjee K. ACC/AHA Guidelines for the management of patients with valvular heart disease: executive summary. Circulation. 2006;114:450–527. doi:10.1161/CIRCULATIONAHA.106.177303. [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. doi:10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Daneault B, Kirtane AJ, Kodali SK, Williams MR, Genereux P, Reiss GR, et al. Stroke associated with surgical and transcatheter treatment of aortic stenosis. J Am Coll Cardiol. 2011;58:2143–50. doi: 10.1016/j.jacc.2011.08.024. doi:10.1016/j.jacc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Newman MF, Mathew JP, Grocott HP, Mackensen GB, Monk T, Welsj-Bohmer KA, et al. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368:694–703. doi: 10.1016/S0140-6736(06)69254-4. doi:10.1016/S0140-6736(06)69254-4. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, et al. One-year outcomes of cohort 1 in the Edwards SAPIEN aortic bioprosthesis European outcome (SOURCE) registry. The European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2011;124:425–33. doi: 10.1161/CIRCULATIONAHA.110.001545. doi:10.1161/CIRCULATIONAHA.110.001545. [DOI] [PubMed] [Google Scholar]
- 6.Stolz E, Gerriets T, Kluge A, Kloevekorn WP, Kaps M, Bachmann G. Diffusion-weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: implications for future neuroprotective trials. Stroke. 2004;35:888–92. doi: 10.1161/01.STR.0000120306.82787.5A. doi:10.1161/01.STR.0000120306.82787.5A. [DOI] [PubMed] [Google Scholar]
- 7.Knipp SC, Matatko N, Schlamann M, Wilhelm H, Thielmann M, Forsting M, et al. Small ischemic brain lesions after cardiac valve replacement detected by diffusion-weighted magnetic resonance imaging: relation to neurocognitive function. Eur J Cardiothorac Surg. 2005;28:88–96. doi: 10.1016/j.ejcts.2005.02.043. doi:10.1016/j.ejcts.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 8.Floyd TF, Shah PN, Price CC, Harris F, Radcliffe SJ, Acker MA, et al. Clinically silent cerebral ischemic events after cardiac surgery: their incidence, regional vascular occurrence, and procedural dependence. Ann Thorac Surg. 2006;81:2160–6. doi: 10.1016/j.athoracsur.2006.01.080. doi:10.1016/j.athoracsur.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 9.Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation. A diffusion-weighted magnetic resonance imaging study. Circulation. 2010;121:870–8. doi: 10.1161/CIRCULATIONAHA.109.855866. doi:10.1161/CIRCULATIONAHA.109.855866. [DOI] [PubMed] [Google Scholar]
- 10.Astarci P, Glineur D, Kefer J, Hoore WD, Renkin J, Vanoverschelde JL, et al. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and transapical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg. 2011;40:475–9. doi: 10.1016/j.ejcts.2010.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Walther T, Simon P, Dewey T, Wimmer-Greinecker G, Falk V, Kasimir MT, et al. Transapical minimally invasive aortic valve implantation. Multicenter experience. Circulation. 2007;116(Suppl I):I-240–45. doi: 10.1161/CIRCULATIONAHA.106.677237. [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials. A consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. doi:10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Härting C, Markowitz HJ, Neufeld H, Calabrese P, Deisinger K, Keßler J. Die Wechsler Memory Scale-Revised. Deutschsprachige Adaptation. Bern: Huber; 2000. [Google Scholar]
- 14.Oswald WD, Fleischmann UM. Nürnberger-Alters-Inventar. Göttingen: Hogrefe; 1995. [Google Scholar]
- 15.Aschenbrenner S, Tucha O, Lange KW. Regensburger Wortflüssigkeitstest. Göttingen: Hogrefe; 2000. [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. Mini-mental-state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–9. doi: 10.1161/01.str.25.7.1393. doi:10.1161/01.STR.25.7.1393. [DOI] [PubMed] [Google Scholar]
- 18.Kruis RWJ, Vlasveld FAE, Van Dijk D. The (Un)Importance of cerebral microemboli. Semin Cardiothorac Vasc Anesth. 2010;14:111–8. doi: 10.1177/1089253210370903. doi:10.1177/1089253210370903. [DOI] [PubMed] [Google Scholar]
- 19.Rodes-Cabau J, Dumont E, Boone RH, Larose E, Bagur R, Gurtvich R, et al. Cerebral embolism following transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;57:18–28. doi: 10.1016/j.jacc.2010.07.036. doi:10.1016/j.jacc.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 20.Ghanem A, Müller A, Nähle CP, Kocurek J, Werner N, Hammerstingl C, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation. J Am Coll Cardiol. 2010;55:1427–32. doi: 10.1016/j.jacc.2009.12.026. doi:10.1016/j.jacc.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Arnold M, Schulz-Heise S, Achenbach S, Ott S, Dörfler A, Ropers D, et al. Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. J Am Coll Cardiol Interv. 2010;3:1126–32. doi: 10.1016/j.jcin.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Drews T, Pasic M, Buz S, Unbehaun A, Dreysse S, Kukucka M, et al. Transcranial Doppler sound detection of cerebral microembolism during transapical aortic valve implantation. Thorac Cardiovasc Surg. 2011;59:237–42. doi: 10.1055/s-0030-1250495. doi:10.1055/s-0030-1250495. [DOI] [PubMed] [Google Scholar]
- 23.Kahlert P, Al-Rashid F, Doettger P, Mori K, Plicht B, Wendt D, et al. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation. 2012;126:1245–55. doi: 10.1161/CIRCULATIONAHA.112.092544. [DOI] [PubMed] [Google Scholar]
- 24.Vermeer SE, longstreth WT, Koudstaal P. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–9. doi: 10.1016/S1474-4422(07)70170-9. doi:10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 25.Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39:1427–33. doi: 10.1161/STROKEAHA.107.502989. doi:10.1161/STROKEAHA.107.502989. [DOI] [PubMed] [Google Scholar]