Abstract
Purpose
To evaluate local control, survival and toxicity in patients with early-stage endometrioid adenocarcinoma of the uterus treated with adjuvant high-dose-rate (HDR) vaginal brachytherapy (VB) alone using a novel low dose regimen
Methods
We reviewed records of 414 patients with stage IA to stage II endometrial adenocarcinoma treated with VB alone from 2005 to 2011. Of these, 157 patients with endometrioid histology received 24 Gy in 6 fractions of HDR vaginal cylinder brachytherapy and constitute the study population. Dose was prescribed at the cylinder surface and delivered twice weekly in the post-operative setting. Local control and survival rates were calculated by the Kaplan-Meier method.
Results
All 157 patients completed the prescribed course of VB. Median follow-up time was 22.8 months (range, 1.5–76.5). Two patients developed vaginal recurrence, one in the periurethral region below the field and one in the fornix after treatment with a 2.5-cm cylinder. Three patients developed regional recurrence in the para-aortic region. Two patients developed distant metastasis (lung and carcinomatosis). The 2-year rate of vaginal control was 98.6%, locoregional control was 97.9% and disease-free survival was 96.8%. The 2-year overall survival rate was 98.7%. No Grade 2 or higher vaginal, gastrointestinal, genitourinary or skin long-term toxicity was reported for any patient.
Conclusion
Vaginal brachytherapy alone in early-stage endometrial cancer provides excellent results in terms of locoregional control and disease-free survival. The fractionation scheme of 24 Gy in 6 fractions prescribed to the cylinder surface was well-tolerated with minimal late toxicity.
Keywords: Endometrial cancer, vaginal brachytherapy, high-dose-rate
Introduction
Endometrial cancer is the most common gynecologic malignancy in United States[1]. Most patients present with early-stage disease. Seventy percent have International Federation of Gynecologic Oncology (FIGO) Stage I disease at diagnosis and have a favorable prognosis[2]. The primary management of these patients is surgery, including a total hysterectomy and bilateral salpingo-oophorectomy with or without surgical lymph-node staging.
In the adjuvant setting, for early-stage endometrial cancer, prospective randomized trials have shown a benefit in preventing locoregional recurrence with post-operative whole-pelvic radiation therapy that covers the vaginal cuff and pelvic lymph nodes[3–5]. However, late side effects reported in patients who received external-beam radiation (EBRT) include diarrhea, small bowel obstruction, post-lymphadenectomy lower extremity edema, and a potential for avascular necrosis or fracture of the femoral head, among others[4, 6, 7]. Concerns over the 2–3% long-term gastrointestinal(GI) complication rate secondary to EBRT [4] have prompted an increase in the use of vaginal brachytherapy (VB) alone in the adjuvant setting for early-stage endometrial cancer. In a recent report, the Post-Operative Radiation Therapy for Endometrial Carcinoma (PORTEC-2) trial demonstrated the effectiveness of post-operative VB alone compared to EBRT in the prevention of vaginal recurrence[8]. Retrospective studies have also shown the efficacy of VB in preventing vaginal relapse in early-stage endometrial cancer [9–11]. Fewer GI side effects and better quality of life were reported in those receiving post-operative VB alone[8].
In the PORTEC-2 trial, the prescribed dose was 7 Gy for 3 fractions prescribed at the cylinder surface with an equivalent dose in 2 Gy fractions (EQD2) of approximately 50 Gy at the cylinder surface. This is the most common fractionation used worldwide; other common fractionations are listed in Table 1[12, 13]. We hypothesized that by lowering the VB dose to 4 Gy in 6 fractions, with an EQD2 dose of 28 Gy, about half the standard dose, it may be possible to maintain the efficacy of this regimen in term of reducing vaginal recurrences while reducing the likelihood of toxicity. The goal of this retrospective study was to evaluate the efficacy and toxicity of VB alone at this lower dose in patients with early-stage endometrioid endometrial carcinoma treated at our institution.
Table 1.
Common fractionation regimens used for HDR vaginal cylinder brachytherapy, converted into equivalent dose in 2 Gy (EQD2)
| HDR Dose at 0.5 cm | HDR dose (Gy) at surface | Number of HDR fraction | Equivalent dose in 2 Gy(EQD2) at surface |
|---|---|---|---|
| 7 | 9.5 | 3 | 46.3 |
| 5.1 | 7 | 3 | 29.8 |
| 5.6 | 7.6 | 3 | 33.4 |
| 5.5 | 7.5 | 5 | 54.7 |
| 5 | 6.8 | 6 | 57.1 |
| 5 | 6.8 | 5 | 47.6 |
| 5 | 6.8 | 4 | 38.1 |
| 5 | 6.8 | 3 | 28.6 |
| 4.4 | 6 | 5 | 40 |
| 2.9 | 4* | 6 | 28.0 |
| 2.5 | 3.4 | 5 | 19.0 |
Key: HDR, high-dose-rate; Gy, Gray; EBRT, external-beam radiation therapy;
current study
Methods
Patients
The records of 414 patients with endometrial cancer treated with adjuvant high-dose-rate (HDR) VB at Brigham and Women’s/Dana-Farber Cancer Center from November 2005 to May 2011 were reviewed with IRB approval. Eligible patients were those with endometrioid adenocarcinoma who received a vaginal cylinder surface dose of 4 Gy in 6 fractions. Exclusion criteria included other brachytherapy dose regimens (n=218) and non-endometrioid histology (22 papillary serous, 10 clear cell, and 7 carcinosarcoma). A total of 157 patients met eligibility criteria and were included in this analysis. Pathological specimens of all patients were evaluated by a gynecologic pathologist. Surgery consisted of total hysterectomy and bilateral salpingo-oophorectomy with or without lymphadenectomy and pathologic staging according to the International Federation of Gynecologic Oncology (FIGO). As the FIGO staging changed in 2009, the description of tumor extent and depth of myometrial invasion in the original pathology reports were reviewed and re-classified in both the 1988 and 2009 FIGO staging systems.
Vaginal Brachytherapy
Four to 6 weeks after surgery, pelvic examination was performed to evaluate the adequacy of healing of the vaginal cuff and the length of the vagina. The largest possible vaginal cylinder was selected for each patient and inserted comfortably to the vaginal apex; available cylinder sizes wee 2.0, 2.5, 3.0 and 3.5 cm in width. Prior to every brachytherapy fraction, computed tomography (CT) scan was performed in a dedicated brachytherapy suite to assess accuracy of placement to the vaginal apex, to confirm the width of the cylinder and to measure vaginal length. An HDR remote afterloading system with Iridium-192 was used for treatment. The radiation dose was 4 Gy in 6 fractions delivered twice weekly with a prescribed dose at the cylinder surface equal to a total dose of 24 Gy. For this regimen, the biologically effective dose (BED) for tumor (Gy10) recalculated to an equivalent 2-Gy dose was 28 Gy at the cylinder surface. Patients with lymphovascular invasion (LVI) were treated to the full vaginal length corresponding to the bottom of the pubic symphysis as assessed on a sagittal reconstruction of the CT, whereas patients without LVI received treatment to the proximal half of the vaginal length, which most frequently measured 4 cm length, again based on the sagittal CT reconstruction. Treatment planning was based on a straight line, non-CT based reconstruction of a catheter of length equal to the prescribed treatment length. Distance between dwell positions was 5 mm. All plans were normalized to the vaginal surface, that is, to a point halfway along the catheter length at a distance equal to the cylinder radius from the catheter. Dose points, one for each dwell position at a distance equal to the cylinder radius from the catheter, were defined. The dose was optimized so that the average dose to the dose points was equal to the prescription dose.
Outcome Data and Statistics
Patients were followed by the radiation oncologist or referring gynecologist and underwent gynecologic examination including vaginal assessment with each follow-up at 3 month intervals. All available follow-up data from hospital medical records, the referring gynecologist or other physician regarding GI, genitourinary(GU) and vaginal toxicity were reviewed. Relapses as documented on examination, CT, and/or biopsy were recorded as local (vagina), regional (pelvic or paraaortic lymph node) or distant metastasis. Vital status was obtained from the clinical record or the National Death Index. Kaplan-Meier analysis was performed to calculate actuarial rates of local control, disease-free and overall survival using SPSS (IBM Corporation; version 18).
Results
Patient Characteristics
Patient and tumor characteristics of all 157 patients in this cohort are listed in Table 2. The median age at the time of diagnosis was 62 years (range, 35 to 89). Using the FIGO 1988 system, the stage distribution was: 131 patients (83%) with Stage I, 20 (13%) with Stage II and 6 (4%) with Stage IIIA disease. All patients with Stage IIIA had positive peritoneal washings. Using FIGO 2009, 148 patients (94%) had Stage I and 9 (5%) had Stage II disease (Table 2). One hundred twenty-one patients (77%) underwent lymphadenectomy and 21 (13%) had lymph-node sampling; 15 patient (9%) had no pathologic lymph node assessment. Myometrial invasion was less than or equal to 50% in 115 patients (73%) and was more than 50% in 42 (23%). Lymphovascular invasion was found in 28 patients (18%). Five patients received chemotherapy. All patients who received chemotherapy had positive peritoneal cytology.
Table 2.
Patient and tumor characteristics
| Characteristic | # (%) | |
|---|---|---|
| Age, median (range) | 62 (35–89) | |
| Stage (FIGO 1988 system) | IA Grade 3 | 5 (3.2) |
| IB Grade 1 | 9 (5.7) | |
| IB Grade 2 | 48 (30) | |
| IB Grade 3 | 20 (12.7) | |
| IC Grade 1 | 30 (19.1) | |
| IC Grade 2 | 17 (10.8) | |
| IC Grade 3 | 2 (1.2) | |
| IIA Grade 1 | 6 (3.8) | |
| IIA Grade 2 | 2 (1.2) | |
| IIA Grade 3 | 3 (1.9) | |
| IIB Grade 1 | 6 (3.8) | |
| IIB Grade 2 | 3 (1.9) | |
| IIB Grade 1 | 2 (1.2) | |
| IIB Grade 2 | 1 (0.6) | |
| IIB Grade 3 | 3 (1.9) | |
| Stage (FIGO 2009 system) | IA Grade 1 | 15 (9.5) |
| IA Grade 2 | 51 (32.4) | |
| IA Grade 3 | 30 (19.1) | |
| IB Grade 1 | 32 (20.3) | |
| IB Grade 2 | 17 (10.8) | |
| IB Grade 3 | 3 (1.9) | |
| II Grade 1 | 6 (3.8) | |
| II Grade 2 | 3 (1.9) | |
| Myometrial invasion | 0% | 9 (5.7) |
| 1–25% | 59 (37.6) | |
| 26–50% | 47 (28.7) | |
| 51–75% | 32 (20.4) | |
| 76–100% | 10 (6.4) | |
| Lymphatic vessel invasion | Yes | 28 (17.8) |
| Lymphadenectomy | Complete | 121 (77.1) |
| Sampling | 21 (13.4) | |
| No nodes removed | 15 (9.6) | |
| Adjuvant chemotherapy | 5 (3.1) |
The median interval between surgery and the start of brachytherapy was 6.7 weeks (range, 3.2–26 weeks). The overall treatment time was 8 to 42 days (median, 16 days). The most commonly placed vaginal cylinder diameter was 3.5 cm (range, 2–3.5) in 79 patients. The median treated vaginal length was 5 cm (range, 3–9 cm).
Relapse, survival and toxicity
Median follow-up time was 22.8 months (range, 1.5–76.5 months). Two patients (1.2%) developed vaginal recurrence that was isolated at presentation (Table 3). The first vaginal recurrence occurred in a 53-year-old woman in the periurethral region below the field 15.7 months after surgery and 13 months after VB. This patient initially had a FIGO 1988 Stage IC grade 2 tumor with 95% myometrial invasion without LVI. She received initial VB treatment to a vaginal length of 4 cm and the recurrence was below the field of prior VB. After recurrence, she underwent whole pelvic irradiation with an interstitial brachytherapy boost to the recurrent lesion; she developed lung metastasis after 6 months of salvage treatment without any evidence of disease in the vagina. The other patient with a vaginal recurrence was a 70 year old female with FIGO 1988 Stage IIA grade 3 disease, 80% deep myometrial invasion and no LVI. She was treated with a 2.5-cm cylinder that treated the upper half of the vagina. The time interval between surgery and VB was 2 months. The recurrent tumor presented in the vaginal fornix 21.5 months after VB. After salvage treatment with pelvic radiation and interstitial brachytherapy, there has been no evidence of further relapse, 1.8 years since diagnosis of vaginal recurrence. Three patients (1.9%) developed regional recurrences in the para-aortic region. Two patients developed distant metastasis (lung metastases in one patient and carcinomatosis in another patient). Characteristics of patients with recurrence are shown in Table 3. Two of the 5 patients who received chemotherapy developed recurrence (one in the paraaortic region and one had carcinomatosis). Three patients have died, one from disease after metastasis and two from unknown causes without evidence of disease. The 2-year actuarial rate of vaginal control was 98.6%, locoregional control was 97.9% and disease-free survival was 96.8% (Figure 1). The 2-year actuarial overall survival rate was 98.7% (Figure 2).
Table 3.
Characteristic of patients that recurred
| Stage (FIGO)
|
Grade | Age (years) | MI (%) | LVI | LNE | TTR (months) | Recurrence Site
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 1988 | 2009 | Vagina | PAN | Distant | ||||||
| IB | IA | 3 | 67 | 10 | 0 | 21 | 23.8 | 0 | + | 0 |
| IB | IA | 2 | 70 | 40 | 0 | 12 | 30.6 | 0 | + | 0 |
| IC | IB | 2 | 53 | 95 | 0 | 6 | 15.7 | + | 0 | + |
| IIA | IB | 3 | 70 | 80 | 0 | 21 | 21.5 | + | 0 | 0 |
| IIIA | IA | 2 | 47 | 20 | 0 | 12 | 16.6 | 0 | + | 0 |
| IIIA | IB | 3 | 72 | 50 | + | 32 | 11 | 0 | 0 | + |
Key: MI, depth of myometrial invasion; LVI, lymphovascular space invasion; LNE, number of lymph nodes excised; TTR, time to relapse; PAN, paraaortic lymph node
Figure 1.
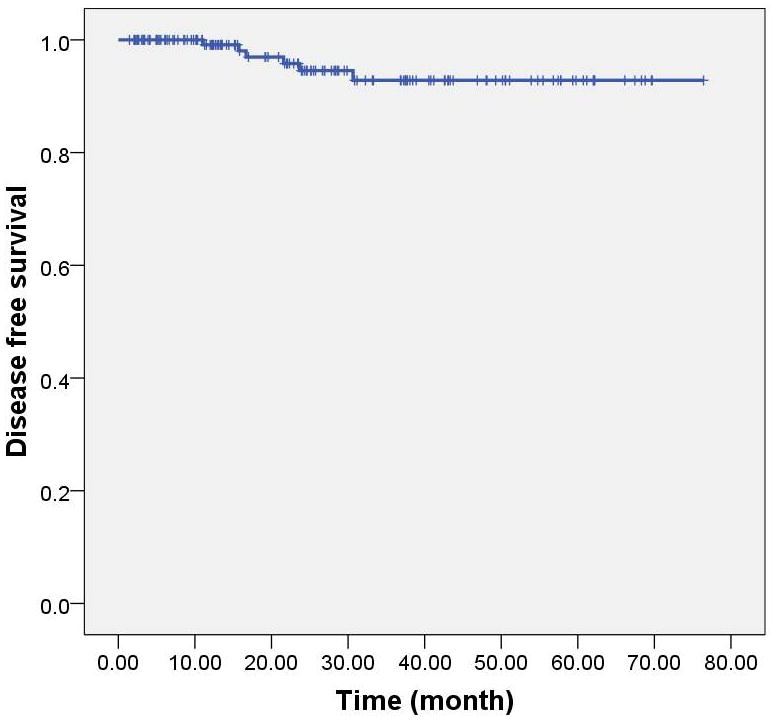
Kaplan-Meier curve depicting disease-free survival for 157 patients with endometrioid adenocarcinoma of the uterus treated with vaginal brachytherapy alone.
Figure 2.

Kaplan-Meier graph of overall survival for the study population
We evaluated risk for locoregional failure using high-intermediate risk (HIR) criteria as designed by Gynecologic Oncology Group (GOG) 99 and PORTEC-2. The GOG 99 HIR criteria include any age with 3 risk factors (Grade 2–3, lymphovascular invasion, more than 2/3 myometrial invasion) or age less than or equal to 50 years with 2 risk factors or age less than or equal to 70 years with 1 risk factor. The PORTEC-2 HIR criteria are age more than 60 with FIGO 1988 stage IC grade 1–2 or IB grade 3 or any age with FIGO 1988 stage IIA disease. In this study, HIR risk criteria were identified 49 patients by GOG criteria and 56 patients by PORTEC-2 criteria. Two of 49 patients (4%) who met the GOG HIR criteria (one of these also met the PORTEC-2 criteria) had a vaginal recurrence; one patient from each of the HIR GOG and PORTEC criteria had paraaortic lymph node recurrence. There was no difference in relapse rates by risk category. In 28 patients who had lymphovascular invasion, 25 patients had lymph node dissection; after median follow up of 18 months, no vaginal or regional relapse occurred and one patient had distant relapsed (carcinomatosis).
Of 157 patients, 2 patients had grade 1 vaginal stenosis noted. No grade 2 or higher vaginal stenosis or any GI, GU or skin toxicity for either acute or late was reported for any patient as recorded in follow-up examinations.
Discussion
This study demonstrates the feasibility and efficacy of a lower-dose adjuvant brachytherapy regimen prescribed at the cylinder surface. Patients with early-stage endometrial endometrioid adenocarcinoma treated with postoperative HDR brachytherapy alone with a novel schedule of 24 Gy in 6 fractions prescribed at the cylinder surface had excellent locoregional control with low morbidity.
Historically, low dose rate (LDR) brachytherapy was utilized for vaginal cuff treatment; however, this required that the patient be admitted to the hospital. An LDR dose of approximately 60 Gy was prescribed to the vaginal surface[13]. More recently, HDR brachytherapy has increased in popularity. Examples of common fractionation regimens for vaginal cylinder brachytherapy with corresponding EQD2 values are shown in Table 1. The most common HDR fractionation regimen worldwide is 7 Gy for 3 fractions at 5 mm; in the U.S, 42% of physicians reported using[12]. Other regimens in the U.S. include 5 Gy for 3 fractions at 5 mm reported by 8.1%, 5 Gy for 5 fractions by 7%, 7 Gy for 3 fractions at the cylinder surface used by 3.5% and 5 Gy for 4 fractions at 5 mm used by 3.5% [13]. A randomized trial by Sorbe et al. compared a high cumulative dose regimen, 5 Gy for 6 fractions at 5 mm, which resulted in a vaginal shortening in 25% of patients (EQD2 of 55 Gy with an α/β = 10) compared to 2.5 Gy for 6 fractions at 5 mm (EQD2 of 30 Gy at surface) The high-cumulative-dose regimen resulted in vaginal shortening in 25% of patients whereas the other had a significantly lower vaginal-stenosis rate of 3% [14].
In our review, local recurrence occurred in 2 patients of 157 patients, of whom both were successfully salvaged at the site of the recurrent vaginal tumor, though 1 subsequently developed distant metastasis. Three had paraaortic recurrence and 2 had distant recurrence. The 2-year local control rate was 98.6% and the corresponding disease-free survival rate was 97.9%. The rate of vaginal recurrence in the high-intermediate risk group was also 6% (GOG criteria) and 4% (PORTEC-2 criteria). Several retrospective studies of early-stage endometrial cancer patients treated with adjuvant VB alone reported very effective prevention of vaginal recurrence with low rate of GI, GU and vaginal toxicity (Table 4). The doses in those retrospective studies ranged from 15–36 Gy in 3–6 fractions. The rates of vaginal recurrence (0%–9%), pelvic recurrence (0%–3.8%), GI toxicity (0%–3%), GU toxicity (0%–4%) and vaginal toxicity (0%–3%) were low. [9–11, 14–23].
Table 4.
Retrospective series of post-operative vaginal brachytherapy alone in endometrial cancer patients
| Author | Year | N | Stage | Histology | Median follow-up (months) | HDR Dose (Gy/fx) | Recurrence # (%)
|
DFS | OS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Vaginal | Pelvic | |||||||||
| Current study | 157 | IAG3-IIIA | EAC | 22 | 24/6 | 2 (1.2) | 0 | 96 | 98 (2) | |
| Gaztanaga et al.[15] | 2011 | 122 | IAG3-IIAG2 | AC | 50 | 25/5 | 2 (1.6) | 2 (1.6) | 87.2 | 90.3 (8.5) |
| McCloskey et al.[16] | 2010 | 87 | IB-IIA | AC excluded PS, CC | 52 | 21/3 HDR 30 Gy LDR |
2 (3.8) | 2 (3.8) | ||
| Atahan et al.[9] | 2008 | 128 | IBG2-3, IC | AC | 48 | 27.5/5 | 2 (1.5) | 1 (0.7) | 93 | 96 (5) |
| Obermair et al.[10] | 2008 | 259 | IB-IIA | AC exclude PS, CC | 82 | 36/6 | 10 (3.8) | 8 (3) | 91 | 90 (5) |
| Roper et al.[11] | 2007 | 138 | I-IIIA | All | 107 | 30/3,15/3 | 1(0.7) | 5 (3.6) | 91.7 | 68.5 (10) |
| Martinez-Monge et al.[17] | 2007 | 50 | IA–IIB | EAC | 37 | 25/5 | 0 | 0 | 96 | 96 (5) |
| Alektiar et al.[18] | 2005 | 382 | IB-IIB | AC exclude PS, CC | 48 | 21/3 | 3 (0.8) | 0 | 93 | 93 (5) |
| Sorbe et al.[14] | 2005 | 290 | IA-IB G1-2 | EAC | 64 | 30/6,15/6 | 1 (0.7) | 1 (0.7) | 99 | 95 (5) |
| Jolly et al.[19] | 2005 | 50 | I-II | AC exclude PS, CC | 38 | 30/6 | 2 (4) | 1 (2) | 96 | 97 (4) |
| Solhjem et al.[20] | 2005 | 100 | I | All | 23 | 21/3 | 0 | 0 | 93 | 98 (3) |
| Horowitz et al.[21] | 2002 | 164 | IB-II | AC, SCC | 65 | 21/3 | 2 (1.2) | 2 (2.4) | 87 | 90 (5) |
| Anderson et al.[22] | 2000 | 102 | IB-IC | All exclude CC, CS | 49 | 15/3 | 1(1) | 3 (2.9) | 93 | 84 (5) |
| Ng et al.[23] | 2000 | 77 | IBG3-IC | AC, SCC | 45 | 36 Gy HDR 60 Gy LDR |
7 (9) | 1 (1.2) | 82 | 94 (5) |
Key: HDR, high-dose-rate; LDR, low-dose-rate; AC, Endometrial adenocarcinoma; EAC, Endometrioid endometrial adenocarcinoma; PS, papillary serous carcinoma; CC, clear cell carcinoma; CS, carsinosarcoma; SCC, squamous cell carcinoma; DFS, disease-free survival; OS, overall survival
Randomized trials have reported outcomes using vaginal brachytherapy alone. In the PORTEC-2 trial, VB was 7 Gy for 3 fractions at 5 mm compare to EBRT. The vaginal and locoregional recurrence rates were statistically equivalent. Vaginal recurrence rates were 1.8% in the EBRT arm and 1.6% in the VB arm. Lymph node relapse was significantly increased in the VB arm, 3.8% in VB arm versus 0.5% in EBRT arm. Acute grade 1–2 GI toxicity was significantly lower in VB arm (12% in VB arm versus 54% in EBRT arm). Late grade 3 GI toxicity was 2% with EBRT versus less than 1% with VB. Late grade 3 vaginal atrophy was reported in 2% for the VB arm versus less than 1% in the EBRT arm[8]. Patients in the VB group reported better social functioning scores (p <0.002) and lower bowel symptom and diarrhea scores (p <0.001) than those in the pelvic RT group. Sexual function scores were equivalent (p=0.35) [24]. In the Norwegian Radium Trial, all Stage I endometrial cancer patients received LDR vaginal cuff brachytherapy after surgery with 60 Gy to the vaginal surface and were then randomized to either pelvic radiation or no further therapy. Vaginal and pelvic recurrences were significantly lower in the patients who received pelvic RT (1.9 versus 6.9%, p <0.01) and the largest benefit was seen in patients with high-grade disease. The overall rate of vaginal recurrence in those with high-grade disease was 6%[3]. In a randomized trial of VB versus no additional treatment for patients with low-risk endometrial cancer, the vaginal recurrence rate was 3.1% in the group that did not receive VB versus 1.2% for those that did. GU toxicity were low and more common in VB group, 2.8% in VB group versus 0.6% in no additional treatment group [25].
This series is limited by its retrospective nature. Follow-up and toxicity data were reviewed retrospectively, however all documentation in the medical record was carefully evaluated for any low-grade toxicities, though this may have been underreported by physicians. Patient-reported sexual function was not assessed. In the current study, we have prescribed an EQD2 dose of 28 Gy to the vaginal surface and confirm a low vaginal recurrence rate and low evident vaginal stenosis with this novel low dose regimen.
In conclusion, all patients with endometrioid endometrial carcinoma including those with high intermediate risk may benefit from a vaginal cylinder brachytherapy regimen that reduces the dose from the previous standard of 60 Gy LDR equivalence to approximately 30 Gy. A regimen of 24 Gy in 6 fractions was safe and effective, with equivalent results, meriting further study in a prospective randomized trial.
Acknowledgments
thank you to Barbara Silver for editorial review.
Footnotes
Presented at the World Congress of Brachytherapy meeting, Barcelona, Spain, May 10–12, 2012
Conflict of interest statement: The authors have no financial conflicts of interest to report.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Malkasian GD, Jr, McDonald TW, Pratt JH. Carcinoma of the endometrium: Mayo clinic experience. Mayo Clin Proc. 1977;52:175–80. [PubMed] [Google Scholar]
- 3.Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–27. [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Creutzberg CL, Nout RA, Lybeert ML, Warlam-Rodenhuis CC, Jobsen JJ, Mens JW, Lutgens LC, Pras E, van de Poll-Franse LV, van Putten WL. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e631–8. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Greven KM, Lanciano RM, Herbert SH, Hogan PE. Analysis of complications in patients with endometrial carcinoma receiving adjuvant irradiation. Int J Radiat Oncol Biol Phys. 1991;21:919–23. doi: 10.1016/0360-3016(91)90730-r. [DOI] [PubMed] [Google Scholar]
- 7.Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Stenfert Kroese MC, Nijman HW, van de Poll-Franse LV, Creutzberg CL. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Kroese MC, van Bunningen BN, Ansink AC, van Putten WL, Creutzberg CL. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–23. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 9.Atahan IL, Ozyar E, Yildiz F, Ozyigit G, Genc M, Ulger S, Usubutun A, Kose F, Yuce K, Ayhan A. Vaginal high dose rate brachytherapy alone in patients with intermediate- to high-risk stage I endometrial carcinoma after radical surgery. Int J Gynecol Cancer. 2008;18:1294–9. doi: 10.1111/j.1525-1438.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 10.Obermair A, Cheuk R, Pak SC, Perrin L, Nicklin J, Crandon A, Land R, Chennakes SK, Janda M, Tripcony L. Disease-free survival after vaginal vault brachytherapy versus observation for patients with node-negative intermediate-risk endometrial adenocarcinoma. Gynecol Oncol. 2008;110:280–5. doi: 10.1016/j.ygyno.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Roper B, Astner ST, Heydemann-Obradovic A, Thamm R, Jacob V, Holzel D, Schmalfeldt B, Kiechle-Bahat M, Hoss C, Molls M. Ten-year data on 138 patients with endometrial carcinoma and postoperative vaginal brachytherapy alone: no need for external-beam radiotherapy in low and intermediate risk patients. Gynecol Oncol. 2007;107:541–8. doi: 10.1016/j.ygyno.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 12.Small W, Jr, Erickson B, Kwakwa F. American Brachytherapy Society survey regarding practice patterns of postoperative irradiation for endometrial cancer: current status of vaginal brachytherapy. Int J Radiat Oncol Biol Phys. 2005;63:1502–7. doi: 10.1016/j.ijrobp.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Small W, Jr, Beriwal S, Demanes DJ, Dusenbery KE, Eifel P, Erickson B, Jones E, Rownd JJ, De Los Santos JF, Viswanathan AN, Gaffney D. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy. 2012;11:58–67. doi: 10.1016/j.brachy.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Sorbe B, Straumits A, Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: a randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys. 2005;62:1385–9. doi: 10.1016/j.ijrobp.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 15.Gaztanaga M, Cambeiro M, Villafranca E, Vila M, Jurado M, Moreno M, Martinez-Monge R. Long-term results of 1-week intravaginal high-dose-rate brachytherapy alone for endometrial cancer. Brachytherapy. 2011 doi: 10.1016/j.brachy.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 16.McCloskey SA, Tchabo NE, Malhotra HK, Odunsi K, Rodabaugh K, Singhal P, Lele S, Jaggernauth W. Adjuvant vaginal brachytherapy alone for high risk localized endometrial cancer as defined by the three major randomized trials of adjuvant pelvic radiation. Gynecol Oncol. 2010;116:404–7. doi: 10.1016/j.ygyno.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Monge R, Nagore G, Cambeiro M, Garran C, Villafranca E, Jurado M. Intravaginal 1-week high-dose-rate brachytherapy alone for Stages I–II endometrial cancer. Brachytherapy. 2007;6:195–200. doi: 10.1016/j.brachy.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Alektiar KM, Venkatraman E, Chi DS, Barakat RR. Intravaginal brachytherapy alone for intermediate-risk endometrial cancer. Int J Radiat Oncol Biol Phys. 2005;62:111–7. doi: 10.1016/j.ijrobp.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Jolly S, Vargas C, Kumar T, Weiner S, Brabbins D, Chen P, Floyd W, Martinez AA. Vaginal brachytherapy alone: an alternative to adjuvant whole pelvis radiation for early stage endometrial cancer. Gynecol Oncol. 2005;97:887–92. doi: 10.1016/j.ygyno.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Solhjem MC, Petersen IA, Haddock MG. Vaginal brachytherapy alone is sufficient adjuvant treatment of surgical stage I endometrial cancer. Int J Radiat Oncol Biol Phys. 2005;62:1379–84. doi: 10.1016/j.ijrobp.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz NS, Peters WA, 3rd, Smith MR, Drescher CW, Atwood M, Mate TP. Adjuvant high dose rate vaginal brachytherapy as treatment of stage I and II endometrial carcinoma. Obstet Gynecol. 2002;99:235–40. doi: 10.1016/s0029-7844(01)01672-6. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JM, Stea B, Hallum AV, Rogoff E, Childers J. High-dose-rate postoperative vaginal cuff irradiation alone for stage IB and IC endometrial cancer. Int J Radiat Oncol Biol Phys. 2000;46:417–25. doi: 10.1016/s0360-3016(99)00427-7. [DOI] [PubMed] [Google Scholar]
- 23.Ng TY, Perrin LC, Nicklin JL, Cheuk R, Crandon AJ. Local recurrence in high-risk node-negative stage I endometrial carcinoma treated with postoperative vaginal vault brachytherapy. Gynecol Oncol. 2000;79:490–4. doi: 10.1006/gyno.2000.6005. [DOI] [PubMed] [Google Scholar]
- 24.Nout RA, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Stenfert Kroese MC, van Bunningen BN, Smit VT, Nijman HW, van den Tol PP, Creutzberg CL. Quality of life after pelvic radiotherapy or vaginal brachytherapy for endometrial cancer: first results of the randomized PORTEC-2 trial. J Clin Oncol. 2009;27:3547–56. doi: 10.1200/JCO.2008.20.2424. [DOI] [PubMed] [Google Scholar]
- 25.Sorbe B, Nordstrom B, Maenpaa J, Kuhelj J, Kuhelj D, Okkan S, Delaloye JF, Frankendal B. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: a controlled randomized study. Int J Gynecol Cancer. 2009;19:873–8. doi: 10.1111/IGC.0b013e3181a6c9df. [DOI] [PubMed] [Google Scholar]


