Photolysis of chromium Fischer carbene complexes has become an established synthetic method for the synthesis of a wide range of products including β-lactams, amino acids, peptides, cyclobutanones, and arenes, principally due to the extensive studies by Hegedus and co-workers.1,2 The presumed photogenerated intermediate is a chromium-complexed ketene resulting from insertion of carbon monoxide.3 Acylamino chromium carbene complexes in particular have been utilized as substrates for the photochemical synthesis of products such as β-amino esters, β-lactams, and 2-aminophenols, but can demonstrate markedly different behavior from their nonacylated analogues.4 More broadly, aminocarbene complexes have been the subject of intense study.5 We report herein evidence for the direct, nonphotochemical insertion of carbon monoxide at ambient temperature and subsequent formation of Münchnones as reactive intermediates in reactions of acylamino chromium carbene complexes and demonstrate applications to the synthesis of heterocyclic compounds.
Consideration of the ketene intermediate resulting from photolysis of an acylamino chromium carbene complex immediately suggests the possibility of cyclization to a Münchnone or Münchnone complex (eq 1). Münchnones, and related mesoionic compounds, play an important role in heterocyclic synthesis due to their participation in dipolar cycloaddition reactions.6 Usually generated by the dehydration of acylated amino acids, they have not been formed via an organometallic route to date. Münchnones react with alkynes to form bicyclic intermediates that undergo a cycloreversion reaction losing carbon dioxide and forming pyrroles. Thus, if Münchnones or Münchnone complexes could be formed from acylamino carbene complexes, then a new class of carbene complex plus alkyne annulation reactions leading to pyrroles would be possible.7
 |
(1) |
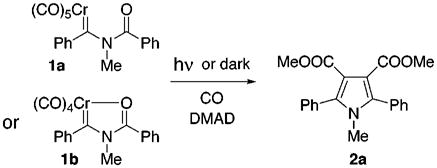
As the initial test of the concept, complex 1a8 was photolyzed under typical conditions in the presence of dimethyl acetylene-dicarboxylate (DMAD).9 Gratifyingly, pyrrole 2a was indeed formed as planned, but in only 41% yield. Use of the more stable chelate complex 1b10 gave an improved 71% yield of the pyrrole. Unfortunately, variations in the photolytic reaction conditions or changes in the alkyne trap resulted in significantly diminished yields. Knowing that carbene complexes react with alkynes,11 a control reaction was performed by mixing carbene 1a and DMAD in THF solvent, pressurizing with 30 psi carbon monoxide, and letting it stand at room temperature in the dark. Remarkably, the pyrrole 2a was formed in 78% yield!
 |
(2) |
Carbene 1b also gave the pyrrole in high yield (90%) under the same conditions of CO pressure in the dark. Reaction of either 1a or 1b using an initial CO purge of the solution, but no applied CO pressure, produced pyrrole, but in lower yields. Confirmation of the Münchnone intermediate, as opposed to a reaction pathway involving initial alkyne insertion,7h,m was accomplished by pressurizing a THF solution of 1b in the absence of alkyne. The dark brown solution changed to light yellow within 24 h and the Münchnone, 3-methyl-2,4-diphenyl-1,3-oxazolium-5-oxide, could be isolated by recrystallization from acetonitrile in 27% yield. The isolated Münchnone spectroscopically matched an authentic sample prepared independently.12 Thus, the presumed initial metal-complexed ketene led to a metal-free Münchnone. In the presence of alkyne trap, chromium hexacarbonyl and carbon dioxide13 were found, in addition to pyrrole, at the end of the reaction which supports the intermediacy of the Münchnone in pyrrole formation. Finally, high yields of pyrrole could be formed from either 1a or 1b by first reacting the carbene complex with CO in the absence of alkyne and then adding alkyne to the yellow Münchnone solution.
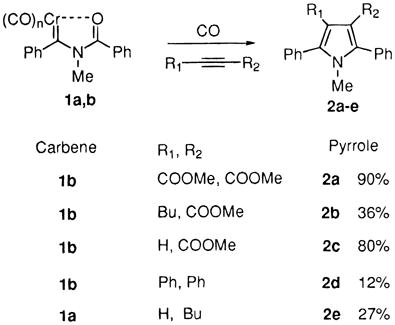
Carbenes 1a and 1b reacted with other alkynes, using either initial addition of alkyne or adding alkyne to the preformed Münchnone, and typical results are shown in eq 3. In concert with literature precedent from the work of Huisgen,14 electron rich or sterically demanding alkynes are not efficient traps of Münchnones. Isolation of sensitive acylaminocarbene complexes can be avoided entirely by using a one-pot procedure of sequential addition of base, acid chloride, and alkyne to transform simple, stable aminocarbene complexes directly to pyrroles (eq 4). Complex 3b leading to pyrrole 2f also demonstrates that the thermal CO insertion is not limited to aryl carbene complexes.
 |
(3) |
 |
(4) |
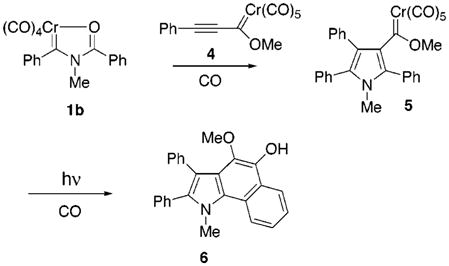
Since unsaturated carbene complexes are quite efficient reactants in Diels Alder cycloaddition reactions15 and there have been a few reports of participation in dipolar cycloaddition reactions,16 we tested the idea of a carbene complex as the dipolarophile to trap the carbene-generated Münchnone.17 Reaction of complex 1b with complex 4 under 30 psi CO in the dark for just 2.25 h resulted in complex 5 in 65% yield (eq 5). An important point is that the direct nonphotochemical carbonyl insertion is critical in this instance as, in general, methoxy-substituted carbene complexes are more photochemically reactive than amino-substituted carbene complexes.1 Using complex 5 as a substrate in our photochemical benzannulation reaction,18 photolysis led regioselectively to indole 6 in 51% yield.
 |
(5) |
The mechanism broadly outlined in eq 1 is our working framework for pyrrole formation. First, chelated complexes such as 1b would be converted to the pentacarbonyl complex which should be more reactive toward CO insertion.4c Then, direct CO insertion, without photolysis, occurs providing a ketene complex. This is an unusual step since while CO insertion readily occurs with chromium carbene complexes lacking a heteroatom substituent directly bonded to the carbene carbon as part of the Dötz reaction,2 CO insertion in heteroatom-substituted chromium carbene complexes has been shown in general to be a photo-chemical, and not thermal, process.1,3 The acyl substituent must be crucial for reducing electron donation from the nitrogen atom to the carbene carbon and thus activating CO insertion. The ketene cyclizes to a metal-free Münchnone as evidenced by our isolation of 3-methyl-2,4-diphenyl-1,3-oxazolium-5-oxide. The demetalation is likely to be facilitated by the presence of CO. The subsequent alkyne cycloaddition and carbon dioxide cycloreversion are well established and supported by our detection of carbon dioxide.6 This proposal is in marked contrast with one put forth by Dötz and co-workers7h for pyrrole formation from an acylamino molybdenum carbene complex wherein initial alkyne insertion was followed by carbonyl insertion and ketene rearrangement to a 2-alkoxy pyrrole. However, in our chromium case, carbonyl insertion clearly precedes alkyne incorporation.
In summary, we have demonstrated the direct, nonphotochemical, insertion of carbon monoxide in acylamino chromium carbene complexes, formation and trapping of Münchnones, and the use of alkynyl carbene complexes as efficient and functional traps of Münchnones for subsequent benzannulation reactions.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health and the National Science Foundation for support of our work. C.A.M. is a recipient of a Camille Dreyfus Teacher-Scholar (1994–1999) award. A.B. is a recipient of a Deutsche Forschungsgemeinschaft postdoctoral fellowship. C.C.A. is a recipient of National Institutes of Health training grant support and support from the Rohm and Haas Company.
Footnotes
Supporting Information Available: Description of syntheses and characterization data for compounds 1a–b, 2a–f, 5, 6, and 3-methyl-2,4-diphenyl-1,3-oxazolium-5-oxide (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews, see: Hegedus LS. Tetrahedron. 1997;53:4105–4128.Hegedus LS. Acc Chem Res. 1995;28:299–305.
- 2.For general reviews, see: Dötz KH, Fischer H, Hofmann P, Kreissl FR, Schubert U, Weiss K. Transition Metal Carbene Complexes. Verlag Chemie; Weinheim, Germany: 1983. Wulff WD. In: Comprehensive Organometallic Chemistry II. Abel EW, Stone FGA, Wilkinson G, editors. Vol. 12. Pergamon Press; New York: 1995. pp. 470–547.Wulff WD. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 5. Pergamon Press; Oxford, England: 1991. pp. 1065–1113.
- 3.Hegedus LS, deWick G, D’Andrea S. J Am Chem Soc. 1988;110:2122–2126. [Google Scholar]
- 4.(a) Hegedus LS, Montgomery J, Narukawa Y, Snustad DC. J Am Chem Soc. 1991;113:5784–5791. [Google Scholar]; (b) Hegedus LS, Lastra E, Narukawa Y, Snustad DC. J Am Chem Soc. 1992;114:2991–2994. [Google Scholar]; (c) Merlic CA, Xu D, Gladstone BG. J Org Chem. 1993;58:538–545. [Google Scholar]
- 5.For reviews, see: de Meijere A. Pure Appl Chem. 1996;68:61–72.Grotjahn DB, Dötz KH. Synlett. 1991:381–390.Schwindt MA, Miller JR, Hegedus LS. J Organomet Chem. 1991;413:143–153.Rudler H, Audouin M, Chelain E, Denise B, Goumont R, Massoud A, Parlier A, Pacreau A, Rudler M, Yefsah R, Alvarez C, Delgado-Reyes F. Chem Soc Rev. 1991;20:503–531.
- 6.For general reviews, see: Stefaniak L, Jazwinski J. Khim Geterotsikl Soedin. 1995:1180–1199.Osterhout MH, Nadler WR, Padwa A. Synthesis. 1994:123–141.Kobayashi Y, Kumadaki I. Adv Heterocycl Chem. 1982;31:169–206.Ollis WD, Ramsden CA. Adv Heterocycl Chem. 1976;19:1–122.
- 7.Pyrroles have been formed from reactions of group six Fischer carbene complexes, but by entirely different mechanisms: Aumann R, Kuckert E, Krüger C, Angermund K. Angew Chem, Int Ed Engl. 1987;26(6):563–564.Aumann R, Heinen H. J Organomet Chem. 1990;389:C1–C6.Aumann R, Heinen H. J Organomet Chem. 1990;391:C7–C11.Dragisich V, Wulff WD. Organometallics. 1990;9:2867–2870.Dragisich V, Murray CK, Warner BP, Wulff WD, Yang DC. J Am Chem Soc. 1990;112:1251–1253.Denise B, Goumont R, Parlier A, Rudler H, Daran JC, Vaissermann J. J Chem Soc, Chem Commun. 1990:1238–1240.Aumann R, Heinen H, Goddard R, Krüger C. Chem Ber. 1991;124:2587–2593.Grotjahn DB, Kroll FEK, Schäfer T, Harms K, Dötz KH. Organometallics. 1992;11:298–310.Aumann R. Chem Ber. 1993;126:2325–2330.Aumann R, Jasper B, Goddard R, Krüger C. Chem Ber. 1994;127:717–724.Funke K, Duetsch M, Stein F, Noltemeyer M, de Meijere A. Chem Ber. 1994;127:911–920.Danks TN, Velo-Rego D. Tetrahedron Lett. 1994;35:9443–9444.Parlier A, Rudler M, Rudler H, Goumont R, Daran JC, Vaissermann J. Organometallics. 1995;14:2760–2774.Aumann R, Meyer AG, Fröhlich R. Organometallics. 1996;15:5018–5027.Aumann R, Fröhlich R, Zippel F. Organometallics. 1997;16:2571–2580.Aumann R, Yu Z, Fröhlich R. Organometallics. 1998;17:2897–2905.Iwasawa N, Ochiai T, Maeyama K. J Org Chem. 1998;63:3164–3165.
- 8.Hegedus LS, Schultze LM, Montgomery J. Organometallics. 1989;8:2189–2195. [Google Scholar]
- 9.A Pyrex pressure tube containing a solution of carbene complex and 2 equiv of alkyne in THF solvent was pressurized to 40 psi with CO. Photolysis was performed using a 450 W Hanovia medium-pressure mercury lamp and water cooling until the deep orange color of the carbene complex disappeared (2.5 h).
- 10.Complex 1a is an unstable red oil, but complex 1b forms stable olive-green needles. See Supporting Information.
- 11.For a recent review, see: Harvey DF, Sigano DM. Chem Rev. 1996;96:271–288. doi: 10.1021/cr950010w.
- 12.See Supporting Information.
- 13.Hexacarbonylchromium was isolated in 49% yield. Carbon dioxide was trapped with an aqueous barium hydroxide solution as barium carbonate in 88% yield.
- 14.(a) Bayer HO, Huisgen R, Knorr R, Schaefer FC. Chem Ber. 1970;103:2581–2597. doi: 10.1002/cber.19701030831. [DOI] [PubMed] [Google Scholar]; (b) Huisgen R, Gotthardt H, Bayer HO, Schaefer FC. Chem Ber. 1970;103:2611–2624. [Google Scholar]; (c) Gotthardt H, Huisgen R. Chem Ber. 1970;103:2625–2638. doi: 10.1002/cber.19701030831. [DOI] [PubMed] [Google Scholar]
- 15.Powers TS, Jiang W, Su J, Wulff WD. J Am Chem Soc. 1997;119:6438–6439. and references therein. [Google Scholar]
- 16.For a review, see: Alcaide B, Casarrubios L, Domínguez G, Sierra MA. Curr Org Chem. 1998;2:551–574.
- 17.Alkynyl carbene complexes have been reacted with preformed mesoionic compounds: Choi YH, Kang BS, Yoon YJ, Kim J, Shin SC. Synth Commun. 1995;25:2043–2050.
- 18.(a) Merlic CA, Xu D. J Am Chem Soc. 1991;113:7418–7420. [Google Scholar]; (b) Merlic CA, Roberts WM. Tetrahedron Lett. 1993;34:7379–7382. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


