Abstract
Background
Liposomal drug packaging is well established as an effective means for increasing drug half-life, sustaining drug activity, and increasing drug efficacy, whether administered locally or distally to the site of disease. However, information regarding the relative effectiveness of peripheral (distal) versus local administration of liposomal therapeutics is limited. This issue is of importance with respect to the treatment of central nervous system cancer, for which the blood-brain barrier presents a significant challenge in achieving sufficient drug concentration in tumors to provide treatment benefit for patients.
Methods
We compared the anti-tumor activity and efficacy of a nanoliposomal formulation of irinotecan when delivered peripherally by vascular route with intratumoral administration by convection-enhanced delivery (CED) for treating intracranial glioblastoma xenografts in athymic mice.
Results
Our results show significantly greater anti-tumor activity and survival benefit from CED of nanoliposomal irinotecan. In 2 of 3 efficacy experiments, there were animal subjects that experienced apparent cure of tumor from local administration of therapy, as indicated by a lack of detectable intracranial tumor through bioluminescence imaging and histopathologic analysis. Results from investigating the effectiveness of combination therapy with nanoliposomal irinotecan plus radiation revealed that CED administration of irinotecan plus radiation conferred greater survival benefit than did irinotecan or radiation monotherapy and also when compared with radiation plus vascularly administered irinotecan.
Conclusions
Our results indicate that liposomal formulation plus direct intratumoral administration of therapeutic are important for maximizing the anti-tumor effects of irinotecan and support clinical trial evaluation of this therapeutic plus route of administration combination.
Keywords: convection-enhanced delivery, glioblastoma, irinotecan, liposome, xenograft
The benefits of liposomal drug packaging have been well documented and include improved drug half-life, sustained drug activity, and increased drug efficacy. We and others have shown that liposomal formulation increases the activity of cytotoxic drugs when used in treating intracranial xenografts established from human glioblastoma (GBM), the most common and malignant of primary brain tumors in adults, irrespective of whether the liposomal drug is administered directly into the tumor or peripherally by a vascular route.1–8
Prior studies, however, have not compared the relative efficacy of peripheral and local administration of liposomal therapeutics for treating brain tumors. Information from such comparisons would help increase understanding of the influence of the blood-brain barrier (BBB) in limiting drug access to tumors and the extent to which therapeutic benefit is affected by peripheral administration of liposomal drugs.9,10 This understanding, in turn, could influence clinical trial design for testing novel therapeutics or therapeutic formulations in treating patients with brain tumors.
Although there is widespread understanding and appreciation of BBB-restricted access of therapy to intracranial tumors, this concern tends to be lessened with respect to the conduct of clinical trials by animal model studies, the results of which show activity of peripherally administered therapeutics against brain tumors. Such results, combined with the expectation of repeated administration of therapy through oral or vascular routes, to achieve comparable if not superior anti-tumor activity, relative to a single intratumoral administration of drug, have been instrumental in promoting the preferential use of peripheral administration of therapy for treating patients with brain tumor.
Convection-enhanced delivery (CED) for local administration of therapy is an alternative to vascular administration that bypasses the BBB and delivers therapeutic agents directly into the brain.11 Previously, we have shown that the use of controlled pressure and a specially designed cannula are key to maximizing the distribution of CED-administered therapy in the brains of animal subjects,12,13 which is a critically important issue for effective treatment of GBM, the majority of which grow in an infiltrative, disseminated manner.
Irinotecan has been extensively studied as a therapeutic agent for glioma (reviewed by Vredenburgh et al.14) based on promising in vitro and in vivo preclinical results, its BBB penetrance, and its distinct mechanism of action, as compared with other agents used in the treatment of these tumors. Clinical experience with irinotecan has been at least modestly promising, with this therapeutic showing activity both as a single agent and in combination with other modalities. However, the free drug is associated with complex pharmacologic interactions, less than ideal pharmacokinetic properties, and toxicity. Liposomal formulation improves on irinotecan's pharmacokinetics, reduces toxicity, and, if delivered locally, could be combined with other treatment approaches as an attractive addition to brain tumor therapy.1,4
In the current report, we present the results of a study in which we have conducted experiments for comparing peripheral intravascular and CED administration of nanoliposomal irinotecan for efficacy against 2 distinct orthotopic xenograft models of GBM. Our data reveal that a single administration of nanoliposomal irinotecan by CED is significantly more effective than multiple systemic intravascular administrations of the same therapeutic and is safe for animal subjects. These results provide strong support for CED administration of nanoliposomal irinotecan in the investigational treatment of GBM.
Materials and Methods
Investigational Agent
Nanoliposomal irinotecan (MM-398) is a highly stabilized liposomal formulation containing nano-sized irinotecan crystals complexed with sucrose octasulfate in the liposome interior15 and was generously provided by Merrimack Pharmaceuticals (Cambridge, MA). The preparation of nanoliposomal irinotecan, used in the experiments that follow, had a particle size of 111 nm, as determined by dynamic light scattering, and a drug-to-phospholipid (PL) ratio of 488 g irinotecan/mol PL.
GBM Xenografts
Human GBM primary tissues, GBM43 and SF7796, are maintained as serially passaged subcutaneous xenografts in athymic mice.16,17 Both GBM43 and SF7796 have been modified by lentiviral infection for stable expression of firefly luciferase to enable in vivo bioluminescence imaging, as previously described.18
To prepare tumor cells from subcutaneous xenografts for transfer to the intracranial compartment, excised subcutaneous tumors were placed in culture dishes and minced with a scalpel then mechanically dispersed by repetitive pipetting to create small cellular aggregates that were passed repeatedly through 40-micron nylon mesh filters to produce single-cell suspensions. Cell suspensions were centrifuged at a rate of 1000 rpm for 10min at 4°C and supernatants aspirated before resuspending pellets in 1 mL of sterile DMEM media. Cells were counted and then diluted to 1 × 105 cells/μL for intracranial injection.19
Intracranial Tumor Establishment in Athymic Mice
Five-week-old female athymic mice (nu/nu, homozygous; Simonsen Laboratories, Gilroy, CA), housed under aseptic conditions, received intracranial tumor cell injection as previously described17 and as approved by the University of California San Francisco Institutional Animal Care and Use Committee. In brief, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and then were injected with 3 μL of tumor cell suspension (300 000 cells total) into the right caudate putamen.
Bioluminescence Monitoring of Intracranial Tumor Growth
In preparation for bioluminescence imaging (BLI), mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), then administered 150 mg/kg of luciferin (D-luciferin potassium salt; Gold Biotechnology, St. Louis, MO) via intraperitoneal injection. Ten minutes after luciferin injection, mice were examined for tumor bioluminescence with an IVIS Lumina imaging station (Caliper Life Sciences, Alameda, CA). Regions of interest, defined using Living Image software (Caliper Life Sciences, Alameda, CA), were recorded as photons per second per steradian per square centimeter.19 Beginning at 1 week after intracranial tumor cell injection, mice were imaged twice weekly until deaths were observed in control (untreated) mice, with data from the last imaging session used to evaluate the effect of therapy on tumor growth.
Vascular and Intratumoral Administration of Nanoliposomal Irinotecan
For vascular administration of therapy,20 mice were warmed for 5–10min with either a heating pad or a heat lamp to dilate tail vasculature. Injection sites were then cleaned with an alcohol swab, after which a 28 g a insulin syringe was inserted, with 50 or 100 µL liposomal drug that had been diluted in 5 mM HEPES-buffered saline (pH, 6.5) to a concentration of 0.004 mg irinotecan per microliter, and injected with steady pressure over 5–10 s. For CED administration, our approach was similar to that previously described.21 In brief, infusion cannula were made with silica tubing (Polymicro Technologies, Phoenix, AZ) fused to a 0.1 mL syringe (Plastic One, Roanoke, VA) with a 0.5-mm stepped-tip needle that protruded from the silica guide base. Syringes were loaded with liposomal drug (0.04 mg per microliter) and attached to a microinfusion pump (Bioanalytical Systems, Lafayette, IN). The syringe with silica cannula was mounted onto a stereotactic holder then lowered through a puncture hole made in the skull19,20 and to the same region in the caudate putamen at which tumor cells had been previously injected. Nanoliposomal irinotecan was infused at a rate of 1 μL/min until a volume of 5 or 10 μL had been delivered. Cannulae were removed 2min after completion of infusion.
Mouse Irradiation
Mice were anesthetized via inhalation of 2.5% isoflurane with 1 L of oxygen per minute for 5min prior to being positioned on an irradiation platform located 16.3 cm from a cesium-137 source (J. L. Shepherd & Associates, San Fernando, CA). Their eyes, respiratory tracts, and bodies were protected with lead shielding. Mice received whole brain irradiation at a dose rate of 247 cGy/min22 until 1.5 Gy radiation had been administered. After irradiation, animals were monitored until recovery. For the experiment involving the analysis of combination therapy efficacy, mice were irradiated once daily for 5 consecutive days, Monday through Friday, with the first radiation treatment on day 7 following tumor cell implantation.
Analysis of Irinotecan Content in Intracranial Tumors
For the experiment involving analysis of tumor irinotecan content, athymic mice with intracranial GBM43 were administrated 0.4 mg of irinotecan by tail vein or directly into the tumor mass on day 13 after implantation of tumor cells and 30min after the fifth and final of radiation fractions that had been initiated on day 9. Mice were euthanized 24 h after nanoliposomal irinotecan administration, with brains immediately resected and tumor tissue dissected prior to snap-freezing by immersion in liquid nitrogen. Analysis of irinotecan levels in tumor tissues was as described previously.1 In brief, water was added to tissues at a 20% (w/v) ratio, and tissues were then homogenized with a mechanical homogenizer in an ice bath. Homogenates were extracted for the lactone form of irinotecan with an acidic methanol solution by vortexing and centrifugation at 13 000 rpm for 10min, with the supernatants then transferred to autosampler vials for Dionex high-pressure liquid chromatography (HPLC) analysis.
Immunohistochemistry
Resected mouse brains were fixed in 10% buffered formalin, then paraffin-embedded and sectioned for hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) analysis. To determine cleaved caspase-3 reactivity, unstained sections were processed with a Ventana BenchMark XT automated system and a protocol consisting of pretreatment with 3% ethanolic hydrogen peroxide for 32min at room temperature, epitope retrieval in Tris buffer (pH 8) for 8 min at 90°C, and incubation with primary antibody to cleaved caspase-3 (#9661, Cell Signaling Tech., Danvers, MA) at 0.2 mg/mL for 1 h at 37°C. Total and activated caspase-3–positive cells were counted in 5 high-powered fields within the tumor for each stained tissue section, with percent positive cells averaged for all fields associated with a specific treatment and subjected to statistical analysis as described below.
Statistical Analysis
PRISM 5, version 5.03 (GraphPad Software), was used to conduct all statistical analyses. For survival analysis, significance was determined by the log-rank (Mantel- Cox) test. Animals without tumor burden that died accidentally during anesthesia were excluded from the survival analysis. For all other statistical analyses, either a 2-tailed unpaired t test or Tukey's multiple comparison test was applied.
Results
Comparison of Intravascular Versus CED Administration of Nanoliposomal Irinotecan for Anti-Tumor Activity
Our initial experiment for comparing intracranial GBM xenograft response to peripherally and CED administered nanoliposomal irinotecan used GBM43, which is maintained as a serially propagated subcutaneous xenograft16,17 and has been previously classified as a proneural GBM.23 GBM43 cells, harvested from a disaggregated subcutaneous xenograft, produce rapidly growing intracranial tumors that have been shown to be relatively resistant to radiation therapy.24
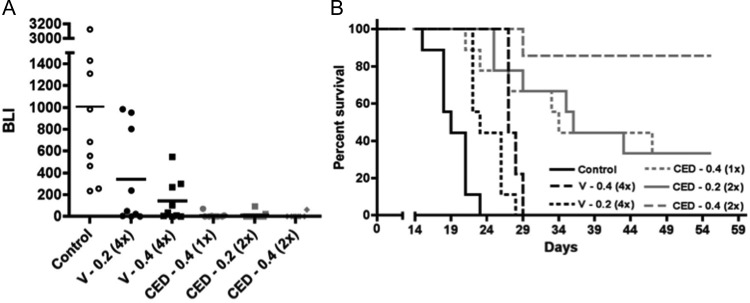
Our experimental design included 3 CED and 2 intravascular administration treatment groups. CED administration was either once at 0.4 mg irinotecan, or twice at either 0.2 or 0.4 mg irinotecan each time. Intravascular administrations were 4 times at either 0.2 or 0.4 mg irinotecan administered each time. Thus, total irinotecan administered by CED was either 0.4 or 0.8 mg irinotecan, whereas total irinotecan administered by intravascular injection was either 0.8 or 1.6 mg. CED administrations were on day 5 only or on days 5 and 8, and intravascular administrations were on days 5, 8, 12, and 15 after tumor cell implantation. BLI of luciferase-modified GBM43 tumors showed an anti-tumor effect from irinotecan administration, regardless of amount or route of administration (Fig. 1A) and significantly (P < .05) or near significantly greater anti-tumor effect of direct over intravascular administration, irrespective of the amounts of administered irinotecan being compared (P values for all 2-way comparisons shown in Table 1).
Fig. 1.
Comparison of intravascular vs CED administration of nanoliposomal irinotecan for anti-tumor activity against intracranial GBM43 and corresponding survival benefit for animal subjects. (A) Treatment group day 15 normalized bioluminescence18 (BLI) distributions (last imaging day in which all control group mice were alive). Route of administration identifiers: V, vascular administration; CED, convection enhanced delivery. Numbers following route of administration identifiers represent mg quantity of irinotecan administered with each dose; numbers in parentheses represent the number of administrations. Direct administrations were on day 5 or on days 5 and 8, and vascular administrations were on days 5, 8, 12, and 15. Student's t-test values for all 2-way comparisons are listed in Table 1s. (B) Corresponding survival plots for each treatment group. Log rank P values for all 2-way comparisons are listed in Table 2 and show that the survival benefit for mice receiving 2 CED administrations of 0.4 mg irinotecan was significantly greater (P < .05) than for any other treatment. Mice surviving at day 60, all of which had no detectable tumor by bioluminescence imaging, were euthanized, with analysis of serial H&E-stained sections of entire brains from 2 of these mice showing no detectable tumor. Control group mice in this experiment were untreated, which were established as valid for comparison by determining, in a separate experiment, that CED of vehicle caused no adverse or beneficial effect on animal survival relative to no treatment (Supplementary Figure 2). Number of mice included in the survival analysis for each treatment group (see Materials and Methods): Control = 9; CED 0.2 (2x) = 9; CED 0.4 (1x) = 9; CED 0.4 (2x) = 7; V 0.2 (4x) = 9; and V 0.4 (4x) = 9.
Survival analysis from these treatments showed that all irinotecan treatment groups experienced significant survival benefit relative to control (Fig. 1B). Of importance, comparisons of all CED and intravascular administration groups showed significantly greater benefit from direct administration of therapy, even when the total amount of irinotecan administered by the vascular route was 4 times greater than that delivered directly into the tumor. More striking was the difference in number of mice that experienced apparent cure of tumor from CED of nanoliposomal irinotecan (6 of 7 in the group receiving 0.8 mg irinotecan, and a total of 4 of 18 in the 2 groups receiving 0.4 mg irinotecan), as indicated by a lack of detectable bioluminescence signal in mice at time of euthanasia. Serial sectioning of entire brains from 2 of the mice receiving CED administration of nanoliposomal irinotecan and showing lack of detectable bioluminescence at time of euthanasia revealed no detectable tumor on histopathologic analysis of H&E-stained tissues. None of the mice receiving intravascular administration of nanoliposomal irinotecan experienced cure of tumor.
Body weight monitoring of mice receiving vascular and CED of nanoliposomal irinotecan showed similar patterns of weight loss, irrespective of route of delivery (Fig. 1), with no animal losing >13% initial weight from treatment. All animals receiving liposomal therapy rapidly gained weight on completion of treatment and achieved weights comparable to or exceeding that of untreated control group mice prior to onset of symptoms indicative of tumor burden in control group mice, suggesting that administration of nanoliposomal irinotecan by either route of administration is without extended adverse effect and is well tolerated.
To investigate whether these results might prove to be generalizable to additional subtypes of GBM, we performed a second experiment with SF7796, which was established and has been maintained as a subcutaneous xenograft after initial implantation of a surgical specimen from a patient whose tumor had regrown after standard-of-care therapy (radiation + temozolomide25), which was followed by treatment of the recurrent tumor with bevacizumab26 prior to second surgery. This GBM xenograft has been classified as mesenchymal using the classification scheme described by Verhaak et al.23 For assessing SF7796 response to nanoliposomal therapy, we compared the anti-tumor effect and survival benefit of 0.4 mg irinotecan administered once by CED (day 20 after implantation) with 0.8 mg administered by intravascular route (0.4 mg administered twice: days 20 and 24). As for the previous experiment with GBM43, the results for SF7796 showed irinotecan anti-tumor activity by BLI (Fig. 2A) and significant survival benefit from treatment, irrespective of whether administered directly or intravascularly (Fig. 2B). SF7796 tumor cells produce a diffusely infiltrative intracranial tumor (Fig. 2C), and it is likely that this diffusely infiltrative nature, combined with the later time of treatment initiation for this experiment (day 20 vs day 5 for the initial experiment with GBM43), resulted in none of the mice with intracranial SF7796 experiencing cure of tumor from treatment. However, as before with the GBM43 model (Fig. 1), the survival benefit from one CED treatment was significantly greater than that resulting from multiple (2) intravascular administrations of liposomal irinotecan (P = .048).
Fig. 2.
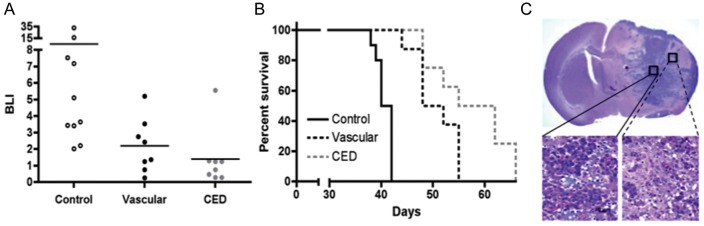
Comparison of intravascular with CED administration of nanoliposomal irinotecan for anti-tumor activity and survival benefit for mice with intracranial SF7796. (A) Treatment group day 35 bioluminescence distributions (last imaging day for which all control group mice were alive). Mice receiving vascular administration received 0.4 mg doses of irinotecan on days 20 and 24 (0.8 mg total dose), whereas mice receiving CED administration were treated just once with 0.4 mg irinotecan on day 20 after tumor cell implantation. Student's t-test values for 2-way comparisons: 0.092 for control vs vascular; 0.061 for control vs CED; and 0.359 for vascular vs CED. (B) Corresponding survival plots for each treatment group. Log rank P values for 2-way comparisons: <0.001 for control vs vascular; <0.001 for control vs CED; and 0.048 for vascular vs CED. Number of mice included in the survival analysis for each treatment group: Control = 10; Vascular = 8; CED = 8. (C) 1.25x (upper) and 40x (lower) magnifications of an H&E-stained coronal section from the brain of a control group mouse that was euthanized on day 39 because of symptoms from increasing tumor burden. Lower left panel: high cellularity at the tumor core. Lower right panel: disseminated tumor cells at the tumor periphery.
Effect of Radiation when Combined with Intravascular or Intratumoral Nanoliposomal Irinotecan Therapy
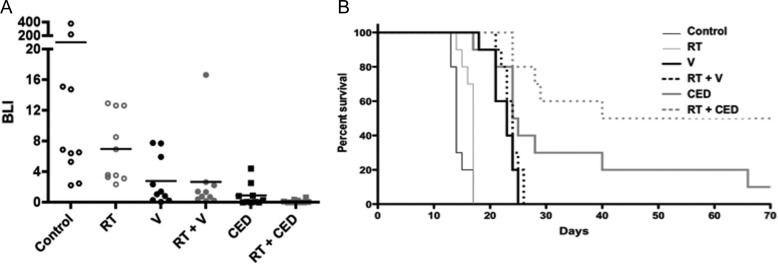
The design for the prior 2 experiments (Figs. 1 and 2) is consistent with that which might be used in a clinical study for treating recurrent GBM and for which investigational therapies are often evaluated in isolation from co-treatment with other therapeutics and/or treatment modalities. To evaluate the activity of nanoliposomal irinotecan in a context consistent with a clinical trial for treating newly diagnosed GBM, we compared the anti-tumor activity of CED with intravascular administration of nanoliposomal irinotecan when used in combination with radiation therapy (RT). For this experiment, radiation was administered at 1.5 Gy/day × 5 (7.5 Gy total) beginning day 7 after implantation of GBM43 cells, with irinotecan administered once by CED (0.4 mg on day 7) or twice by the vascular route (0.4 mg on days 7 and 11). As with the previous GBM43 experiment (Fig. 1), CED of irinotecan outperformed intravascular administration, even though twice as much irinotecan was administered by the vascular route (Figs. 3A and B; P = .035 for survival benefit comparison). RT, which as a monotherapy, provided modest, albeit statistically significant, survival benefit to mice with intracranial GBM43 (Fig. 3B; P = .011 for RT vs control), further increased the anti-tumor effect and survival benefit from nanoliposomal irinotecan, with direct administration of irinotecan + RT providing the most extensive survival benefit of any treatment (Fig. 3B) and resulting in half (5 of 10) of the treatment group mice experiencing apparent cure of tumor. As for previous experiments, therapeutic regimens were well tolerated, with no animal subject experiencing >10% loss of pretreatment body weight at completion of therapy (data not shown).
Fig. 3.
Effect of radiation when combined with intravascular or CED administration of nanoliposomal irinotecan therapy. Radiation treatment for this experiment was 1.5 Gy/day x 5, beginning day 7 and ending day 11, for a total radiation dose of 7.5 Gy. Nanoliposomal irinotecan administration was 0.4 mg on day 7 for CED administration and was 0.4 mg on days 7 and 11 for intravascular administration. (A) Treatment group day 10 bioluminescence distributions (last imaging day at which all control group mice were alive). See Table 3 for all 2-way comparisons using the Student's t-test. (B) Corresponding survival plots for all treatment groups. Log rank P values for all 2-way comparisons are listed in Table 4 and show that CED administration of irinotecan as a monotherapy was significantly better than intravascular and RT monotherapies and that RT + CED of irinotecan was significantly better than all other therapies, including irinotecan monotherapy via CED. The experiment was terminated at 70 days, at which time there was no detectable bioluminescence signal in surviving mice. Ten mice included in the survival analysis for all treatment groups.
For this latter experiment, untreated mice were included for euthanasia at day 7 to obtain samples providing indication of extent of intracranial tumor at time of treatment initiation (Fig. 4A) and at day 12 for all treatment groups (Figs. 4B–F) to allow qualitative comparison of relative tumor size among treatment groups and quantitative IHC analysis of activated caspase 3 staining for assessing the pro-apoptotic effect of different treatments at 1 day after completion of therapy (Fig. 5). Inspection of H&E-stained tissues for sections with the largest tumor areas showed reasonable consistency between treatment effect on amount of H&E-stained tumor at time of completing therapy (Figs. 4B–F) and eventual treatment group survival (Fig. 3B). This was also the case for the activated caspase 3 IHC analysis, which showed the most extensive apoptotic response in mice receiving direct administration of nanoliposomal irinotecan + radiation therapy (Fig. 5; P < .05 for CED + RT vs any other treatment group; Student's t test results for all Fig. 5G; 2-way comparisons are shown in supplementary Table 5).
Fig. 4.
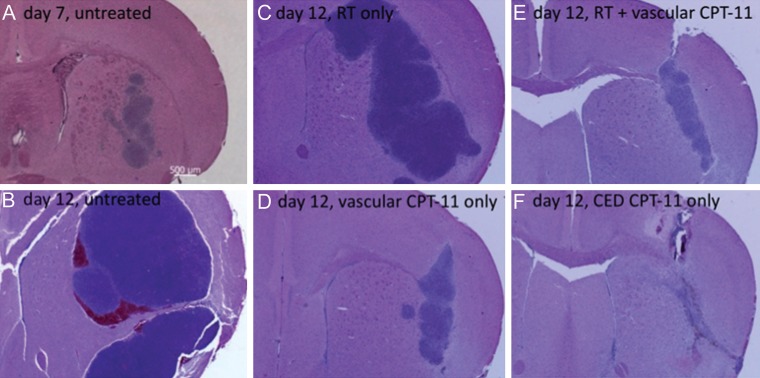
H&E-stained coronal sections (2.5× magnification) from the brain of an untreated mouse that was euthanized on the first day of therapy (A) and from the brains of mice that were euthanized from each treatment group one day after the last day of therapy (day 12: panels B-F). H&E-stained sections from the brain of a mouse receiving RT + CED administration of nanoliposomal irinotecan (not shown) appeared to be similar to those of the mouse receiving local administration of liposomal therapy only (F) and showed a lack of identifiable tumor from combination therapy at day 12 after tumor cell implantation.
Fig. 5.
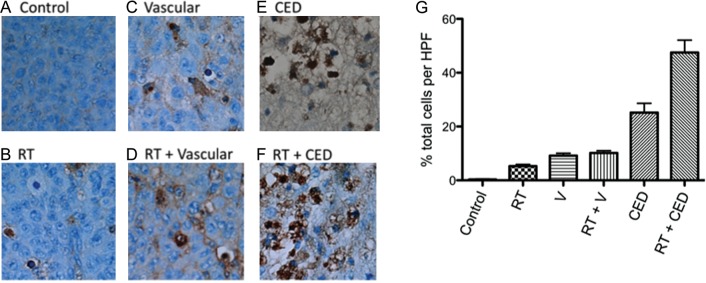
(A–F) Examples of activated caspase 3 staining of tumors in the brains of mice from each of the treatment groups described in Fig. 3. Specimens were obtained from mice that were euthanized on the day after final treatments (day 12). (G) Histogram plot showing average values for percent positive cells from 5 high-powered fields examined in the brains of each of 3 mice from each treatment group: results are therefore based on a total of 15 high-powered fields per treatment group. Student's t test comparison of these values showed that radiation + CED administration of nanoliposomal irinotecan was significantly more effective in inducing apoptotic response, relative to all other treatment groups (P < .05).
Analysis of Irinotecan Content in Intracranial Xenografts
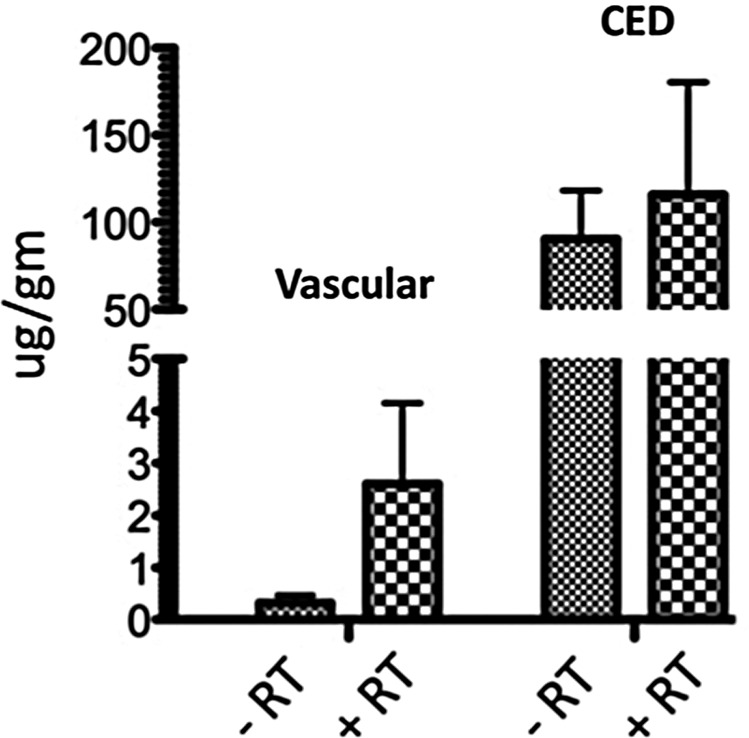
To obtain information addressing whether radiation alters access of peripherally administered nanoliposomal irinotecan to intracranial tumor or, alternatively, alters retention of irinotecan when therapy is administered locally, additional mice received intracranial implantation of GBM43, with tumors allowed to grow until day 9, at which time mice began receiving daily radiation for 5 consecutive days (1.5 Gy/day, 7.5 Gy total), with 0.4 mg nanoliposomal irinotecan administered either by CED or by a vascular route on day 13, the last day of radiation treatment. Twenty-four hours later, the mice were euthanized and tumor was dissected from surrounding normal brain of the euthanized mice, with dissected tumors subsequently examined for irinotecan content. The results of this analysis showed no significant difference in irinotecan content in tumors as a result of mice receiving pretreatment with radiation, although for mice receiving vascular administration of therapy, the mean value for tumor irinotecan was substantially higher in the group receiving radiation (Fig. 6). Not surprisingly, intratumoral irinotecan was substantially and significantly higher in mice receiving CED administration of liposomal therapy, irrespective of radiation pretreatment: 302-fold greater when comparing direct vs vascular administration in nonirradiated mice and 45-fold greater when comparing irinotecan content in mice from irradiated groups.
Fig. 6.
Analysis of irinotecan content in intracranial GBM43 xenograft tumors 24 h after either vascular or CED administration of 0.4 mg nanoliposomal irinotecan and either in the presence or absence of radiation, with the last of 5 radiation treatments administered 30min prior to the single irinotecan administration at day 13 subsequent to tumor cell implantation. The results show that RT does not cause a significant difference in tumor irinotecan, whether administered directly or by vascular route, but do show a significantly higher amount of irinotecan content in tumors receiving direct administration of therapy (P < .05). Analyzable samples were 2–4 for each treatment group.
Discussion
In the current report, we have presented results addressing the relative activity and efficacy of intravascular and CED administration of nanoliposomal irinotecan in treating mice with orthotopic GBM xenografts. To the best of our knowledge, there are no previously published reports involving a route of administration comparison for liposomal therapy in treating an experimental animal model of GBM. Although our study is not exhaustive with respect to potential experimental variations, we feel that there is sufficient consistency of results for the variables tested in 2 distinct intracranial xenograft models to support the interpretation of CED administration of irinotecan nanoliposomes as being the more effective administration for maximizing anti-tumor effect of this therapy. Our cumulative experience in this area of research indicates that it is the combined effects of nanoliposomal packaging for extending the biologic half-life of active drug1–5 and bypassing the limiting influence of the BBB through direct intratumoral administration of therapy1–4 that are important for maximizing the anti-tumor effect of cytotoxic chemotherapy.
Our interpretation of these results is not at odds with clinical trial designs that use intravascular administration of liposomal therapy to treat GBM, which is an approach that, according to our results, could well provide benefit to patients with brain tumors. Our results do, however, support a clinical trial design in which nanoliposomal irinotecan is administered locally.
The advantage of CED is multifactorial. The combination of cannulae that minimize reflux21 and the liposomal formulation of irinotecan allows for more robust and uniform distribution of the therapeutic. Catheter placement into an intact tumor, confirmed by neuro-navigational methods with direct imaging assessment of catheter position and subsequent convective infusion of the liposome using real-time imaging,13 would be the optimal strategy for initial clinical studies of this agent. This clinical setting would eliminate the risk of drug reflux back into a surgical cavity seen when using CED strategies at the time of surgical resection and minimize the risk of improper placement of catheters, often seen after expected changes in the geometry of the cavity hours to days after resection.
The increased efficacy of CED administration of therapy is consistent with the substantial disparity in irinotecan content of xenograft tumors removed 24h after treatment of animal subjects with equivalent vascular and intratumoral amounts of liposomal drug (Fig. 6). In a previous study, we showed that CED of nanoliposomal irinotecan sustains higher intracranial levels of irinotecan, relative to intracranial administration of free irinotecan, and that CED administration of nanoliposomal irinotecan outperforms direct intratumoral administration of equivalent-free irinotecan.1 Thus, liposomal formulation is important to maximizing anti-tumor activity and, potentially, clinical benefit from CED administration of irinotecan.
For the present study, it is noteworthy that the nanoliposomal irinotecan preparation used for direct intracranial administration was the same as that used for intravascular administration, and this formulation was developed for intravascular administration of therapy. Thus, it is conceivable that alternative nanoliposomal irinotecan formulations may further improve on the intracranial distribution and efficacy of CED administration of nanoliposomal irinotecan. Despite the use of vascular-optimized nanoliposomal irinotecan, our results suggest the diffusion of locally administered liposomal therapy to an extent that is effective in eradicating, at least in some instances, tumor that occupies a substantial portion of total brain (Figs. 4A and F). Because mice experiencing apparent cure of intracranial tumor (Figs. 1 and 3) were treated with 5–10 μL of liposomal therapy, our results support the concept that this therapeutic approach involving local administration of therapy is sufficiently scalable to anticipate efficacy against brain tumor in patients with GBM.
In addition to the superior efficacy of local administration of nanoliposomal irinotecan, it is important to emphasize the apparent safety of direct intracranial administration of therapy, as indicated by body weight monitoring of treated mice (Fig. 1), and lack of an indication of neurologic deficit (e.g., ambulation, activity, and seizure) from CED of liposomal therapy. Future preclinical studies will focus on dose escalation experiments to identify maximum tolerable amounts of nanoliposomal irinotecan that can be administered directly into brain and the optimization of convection-enhanced delivery approaches for sustained CED administration of nanoliposomal irinotecan.
Finally, our results show that CED administration of nanoliposomal irinotecan can be used with radiation therapy for further improvement of anti-tumor effect and survival benefit, relative to either monotherapy, and thereby support the potential use of direct administration of liposomal therapy with subsequent standard-of-care therapy for treating GBM: RT + temozolomide.25 With irinotecan being administered locally, one would anticipate a lack of adverse effects associated with the peripheral administration of 2 cytotoxic therapies and that local administration of nanoliposomal irinotecan could be used safely with conventional treatment for newly diagnosed GBM. Thus, our results support the clinical trial evaluation of direct intra-tumoral administration of nanoliposomal irinotecan, both as a single agent in the treatment of recurrent GBM and as part of a combination therapy for patients with newly diagnosed GBM.
Supplementary Material
Funding
This study was supported by National Cancer Institute (grant CA097257 to T. O., M. D. P., M. S. B., K. B., C. D. J.).
Supplementary Material
Acknowledgments
We thank Raquel Santos, Edgar Lopez-Lepe, Jacqueline De La Torre, and Christina Ng for their expert technical assistance.
Conflict of interest statement. D. C. D., A. K., J. B. F., and D. B. K. are employees of Merrimack Pharmaceuticals, the supplier of the therapeutic investigated in the study reported herein. All other authors declare no conflict of interest.
References
- 1.Noble CO, Krauze MT, Drummond DC, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 2.Saito R, Krauze MT, Noble CO, et al. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neuro Oncol. 2006;8:205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamashita Y, Krauze MT, Kawaguchi T, et al. Convection-enhanced delivery of a topoisomerase I inhibitor (nanoliposomal topotecan) and a topoisomerase II inhibitor (pegylated liposomal doxorubicin) in intracranial brain tumor xenografts. Neuro Oncol. 2007;9:20–28. doi: 10.1215/15228517-2006-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krauze MT, Noble CO, Kawaguchi T, et al. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and Pegylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007;9:393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serwer LP, Noble CO, Michaud K, et al. Investigation of intravenous delivery of nanoliposomal topotecan for activity against orthotopic glioblastoma xenografts. Neuro Oncol. 2011;13:1288–1295. doi: 10.1093/neuonc/nor139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riondel J, Jacrot M, Fessi H, et al. Effects of free and liposome-encapsulated taxol on two brain tumors xenografted into nude mice. In Vivo. 1992;6:23–27. [PubMed] [Google Scholar]
- 7.Sharma US, Sharma A, Chau RI, Straubinger RM. Liposome-mediated therapy of intracranial brain tumors in a rat model. Pharm Res. 1997;14:992–998. doi: 10.1023/a:1012136925030. [DOI] [PubMed] [Google Scholar]
- 8.Grahn AY, Bankiewicz KS, Dugich-Djordjevic M, et al. Non-PEGylated liposomes for convection-enhanced delivery of topotecan and gadodiamide in malignant glioma: initial experience. J Neurooncol. 2009;95:185–197. doi: 10.1007/s11060-009-9917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serwer LP, James CD. Challenges in drug delivery to tumors of the central nervous system: an overview of pharmacological and surgical considerations. Adv Drug Deliv Rev. 2012;64:590–597. doi: 10.1016/j.addr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 10.de Vries NA, Beijnen JH, Boogerd W, van Tellingen O. Blood-brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother. 2006;6:1199–1209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 11.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamot C, Nguyen JB, Pourdehnad M, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg. 2008;108:989–998. doi: 10.3171/JNS/2008/108/5/0989. [DOI] [PubMed] [Google Scholar]
- 14.Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS. Experience with irinotecan for the treatment of malignant glioma. Neuro-Oncol. 2009;12:80–91. doi: 10.1215/15228517-2008-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond DC, Noble CO, Guo Z, et al. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 16.Pandita A, Aldape KD, Zadeh G, Guha A, James CD. Contrasting in Vivo and in Vitro Fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosomes Cancer. 2004;39:29–36. doi: 10.1002/gcc.10300. [DOI] [PubMed] [Google Scholar]
- 17.Giannini C, Sarkaria JN, Saito A, et al. Patient Tumor EGFR and PDGFRA Gene Amplifications Retained in an Invasive Intracranial Xenograft Model of GBM. Neuro-Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinca EB, Sarkaria JN, Schroeder MA, et al. Bioluminescence Monitoring of Intracranial Glioblastoma Xenograft Response to Primary and Salvage Temozolomide Therapy. J Neurosurg. 2007;107:610–616. doi: 10.3171/JNS-07/09/0610. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa T, James CD. Establishing Intracranial Brain Tumor Xenografts With Subsequent Analysis of Tumor Growth and Response to Therapy using Bioluminescence Imaging. J Vis Exp. 2010;41 doi: 10.3791/1986. doi:pii:1986.10.3791/1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serwer L, Hashizume R, Ozawa T, James CD. Systemic and Local Drug Delivery for Treating Diseases of the Central Nervous System in Rodent Models. J Vis Exp. 2010;42 doi: 10.3791/1992. doi:pii:1992.10.3791/1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187:46–51. doi: 10.1016/j.jneumeth.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozawa T, Faddegon BA, Hu LJ, et al. Response of intracerebral human glioblastoma xenografts to multi-fraction radiation exposures. Int J Radiat Oncol Biol Phys. 2006;66:263–270. doi: 10.1016/j.ijrobp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Verhaak RGW, Hoadley KA, Purdom E, et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarkaria JN, Carlson BL, Schroeder MA, et al. Use of an Orthotopic Xenograft Model for Assessing the Effect of EGFR Amplification on Glioblastoma Radiation Response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 25.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain M. Bevacizumab for the treatment of recurrent glioblastoma. Clin Med Insights Oncol. 2011;5:117–129. doi: 10.4137/CMO.S7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.