Abstract
Background and Purpose
This randomized controlled trial tests the efficacy of bilateral arm training with rhythmic auditory cueing (BATRAC) versus dose-matched therapeutic exercises (DMTEs) on upper-extremity (UE) function in stroke survivors and uses functional magnetic resonance imaging (fMRI) to examine effects on cortical reorganization.
Methods
A total of 111 adults with chronic UE paresis were randomized to 6 weeks (3×/week) of BATRAC or DMTE. Primary end points of UE assessments of Fugl-Meyer UE Test (FM) and modified Wolf Motor Function Test Time (WT) were performed 6 weeks prior to and at baseline, after training, and 4 months later. Pretraining and posttraining, fMRI for UE movement was evaluated in 17 BATRAC and 21 DMTE participants.
Results
The improvements in UE function (BATRAC: FM Δ = 1.1 + 0.5, P = .03; WT Δ = −2.6 + 0.8, P < .00; DMTE: FM Δ = 1.9 + 0.4, P < .00; WT Δ = −1.6 + 0.7; P = .04) were comparable between groups and retained after 4 months. Satisfaction was higher after BATRAC than DMTE (P = .003). BATRAC led to significantly higher increase in activation in ipsilesional precentral, anterior cingulate and postcentral gyri, and supplementary motor area and contralesional superior frontal gyrus (P < .05). Activation change in the latter was correlated with improvement in the WMFT (P = .01).
Conclusions
BATRAC is not superior to DMTE, but both rehabilitation programs durably improve motor function for individuals with chronic UE hemiparesis and with varied deficit severity. Adaptations in brain activation are greater after BATRAC than DMTE, suggesting that given similar benefits to motor function, these therapies operate through different mechanisms.
Keywords: rehabilitation, stroke, brain imaging, hemiparesis, neuroplasticity
Introduction
Rehabilitation for stroke survivors with moderate to severe paresis after stroke remains a challenge. Furthermore, there are few randomized controlled trials testing unilateral or bilateral upper-extremity (UE) rehabilitation interventions.1–5 The largest recent trial demonstrated that 2 weeks of constraint-induced movement therapy (CIMT) significantly improves UE function more than usual care, persisting for at least 24 months.6,7 However, CIMT requires the ability to partially extend the wrist and fingers, which limits the success of CIMT in many stroke survivors. In contrast, bilateral arm training with rhythmic auditory cueing (BATRAC) is targeted to rehabilitate stroke survivors with UE impairments that rule out CIMT.
BATRAC, like CIMT, is based on motor learning principles, including repetition, feedback, and goal setting with the aim of overcoming learned nonuse and relative inactivity,6,8–14 but also includes use of the nonparetic arm as a fundamental component of the training based on interlimb coupling theory, where the 2 arms act to form a “neurofunctional” unit.15–17 Evidence from nondisabled people suggest that bilateral arm movements engage additional brain circuits, for example, in the supplementary motor area (SMA) and primary motor cortex18–22 over and above the combination of similar unilateral arm movements. Thus, training these circuits is useful for bilateral movements, and there also appears to be a neurophysiological and functional transfer effect to unilateral movements after short-term training in nondisabled people23 as well as in those with stroke.24 Plausible pathways that are disinhibited or facilitated during bilateral as opposed to unilateral movements include transcallosal,25 ipsilateral uncrossed corticospinal, and bilateral brainstem pathways such as rubrospinal or propriospinal.26 Taken together, the neurophysiological and functional evidence suggests a possible benefit from bilateral arm training to the paretic arm.
Uncontrolled studies with BATRAC27–29 have shown functional benefits, and 1 small controlled study showed increased bihemispheric cortical activation associated with improved UE function after BATRAC, suggesting cortical plasticity.30 The small sample, heterogeneity of the functional and MRI responses, and no assessment of durability led to this randomized controlled trial to test the hypotheses that BATRAC will result in larger and more durable UE functional gains, mediated through remodeling of bihemispheric motor and/or premotor cortical networks, compared with dose-matched unilateral therapeutic exercises (DMTE controls).
Materials and Methods
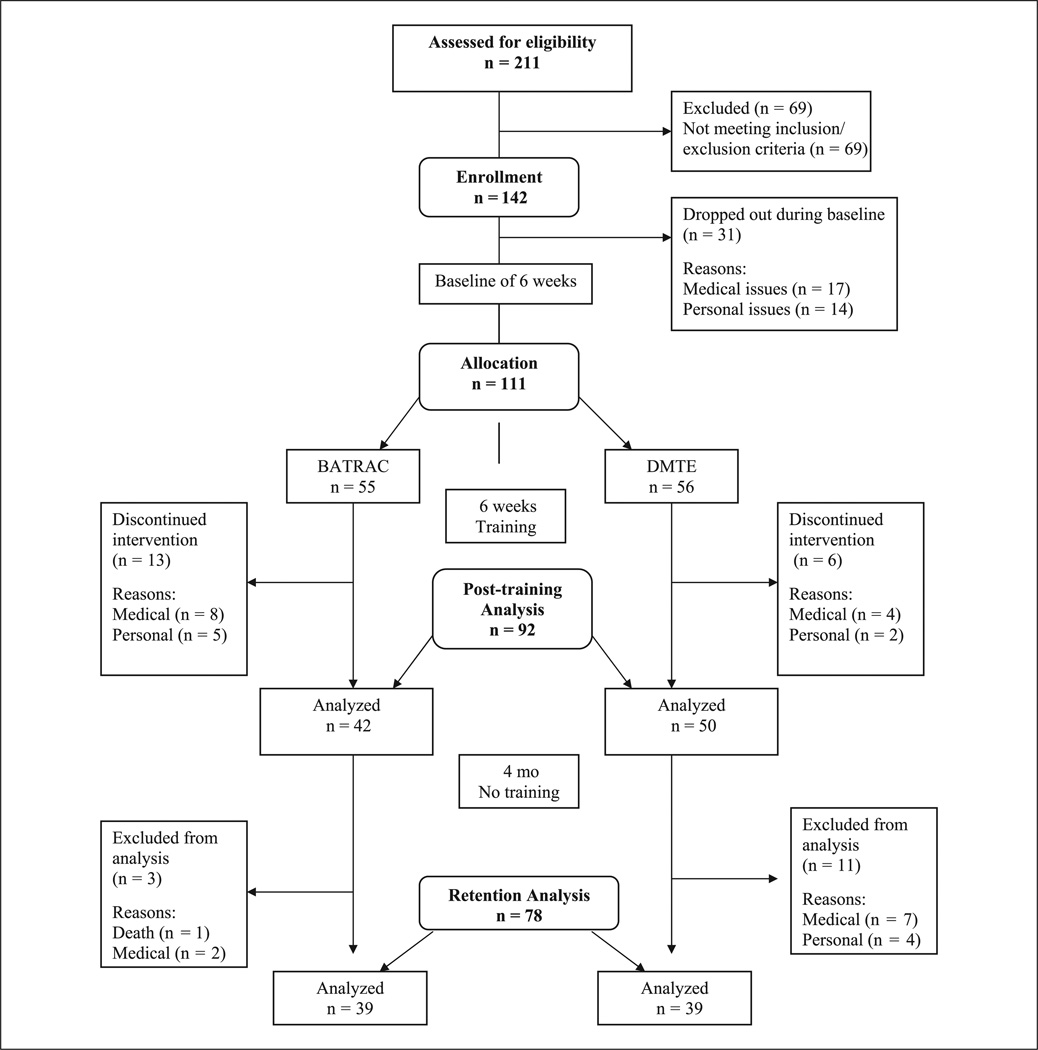
Recruitment, screening, enrollment, and randomization of participants was conducted at the Baltimore Veterans Affairs Medical Center (VAMC) and involved referrals from the University of Maryland (UM) Medical System Hospital and regionwide advertisements (Figure 1). Those included had a unilateral stroke >6 months earlier, could follow simple instructions, had volitional control of the nonparetic arm, and the ability to flex the paretic arm shoulder 3 inches from a neutral position. Exclusion criteria included symptomatic heart disease, and uncontrolled hypertension (>180/100 mm Hg), significant orthopedic or chronic pain conditions, untreated poststroke depression (Center for Epidemiological Studies Depression Scale; cutoff > 16), active cancer, severe obstructive pulmonary disease, and cognitive loss measured using the Folstein Mini Mental State Exam. In all, 54 participants did not undertake the functional magnetic resonance imaging (fMRI): reasons included metallic implants/pacemakers (16); claustrophobia (8); over a certain weight (7); not eligible for transcranial magnetic stimulation, which was initially a cocriterion (5); and low functioning (4). Fourteen participants were in an earlier report,30 so were excluded here. We kept these 14 participants in the functional data set because outcomes were not the emphasis of the previous article. There were no differences in age, time since stroke, or baseline primary outcome measures in those who underwent fMRI versus those who did not. The study was approved by the institutional review boards of the UM Baltimore and Baltimore Veterans Affairs Medical Center, and participants provided informed consent. Participants were recruited for screening between January 2002 and April 2006. After screening, 142 patients were enrolled and 111 randomized after B2 to receive BATRAC or DMTE.
Figure 1.
Study flow
Abbreviations: BATRAC, bilateral arm training with rhythmic auditory cueing; DMTE, dose-matched therapeutic exercise.
Design
Functional measures were collected as follows: (1) at 2 baseline times (B1,2) separated by 6 weeks, (2) after 6 weeks of BATRAC or DMTE intervention, and (3) 4 months after the intervention. Training started after B2. After training, participants were asked to use their paretic arm in daily life but not in new UE training regimens. fMRI data were collected at B2 and after training.
Primary End Points of UE Assessment
Motor impairment was assessed through the Fugl-Meyer (FM) UE test, which is a reliable and valid test of single joint movements, tasks, and reflexes.31–33
Motor function was measured as the time (WT) required to perform 15 tasks of a reliable and valid modified version of the Wolf Motor Function Test (WMFT).34–36
Secondary End Points of UE Assessment
Components of the WMFT, including maximum weight carried (“Wolf weight”), grip strength (“Wolf grip”), and a qualitative assessment of UE performance (“Wolf function”), were determined. The WMFT was administered 3 times at B1 to establish performance stability. The result of the second test was used to measure performance at B1; an analysis showed that performance had stabilized by the second administration.34
The Stroke Impact Scale, a reliable and valid questionnaire for this population, was administered.37,38
Isokinetic strength of elbow flexion/extension movements of both arms were measured on a Kincom Dynamometer (Chattanooga, TN).
Isometric strength of both arms were measured with the Chatillon force dynamometer (The Scale People, Maryland) and a baseline hydraulic hand dynamometer (Kom Kare, New York).
Range of motion measures included shoulder flexion/extension/abduction, elbow flexion/extension, wrist flexion/extension, and thumb opposition, but no mean changes exceeded the recognized 5 measurement error,39 and these data are omitted.
Two verbal assessments of the participant’s perceptions after training were assessed using a 5-point Likert scale where “3” indicates neither satisfied nor dissatisfied with the training and neither improved nor declined after training, respectively. For both questions, a higher score is favorable.
Functional Magnetic Resonance Imaging
fMRI was performed using a 1.5 T scanner (Philips, Eindhoven, The Netherlands) at the Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore.30 Briefly, 60 coronal blood oxygenation-level-dependent weighted scans (echo planar imaging sequence, TE = 40 ms, TR = 3 s, 35–39 slices, slice thickness 5 mm) covering the entire brain were acquired from the nonparetic and then the paretic arm. For each arm, scans were obtained during 3 cycles of rest (10 images) followed by arm movement (10 images) performed in response to an auditory cue given via headsets once every 3 s. During imaging, the arm was strapped to a device that allowed elbow flexion/extension in one plane within a defined range of motion from 45° relative to the standard anatomical position to 60° to 75°, depending on the participant’s paretic arm movement ability. Each participant’s range of motion was also applied to the nonparetic arm and subsequently kept constant. Compliance with the protocol and the presence or absence of mirror movements and head motion was assessed through a video monitor using 2 cameras (head and arms). A T1-weighted image set (3D-MPRAGE, resolution 1 × 1 × 1 mm3) was acquired for anatomical localization. Data were processed using SPM5 software (www.fil.ion.ucl.ac.uk/spm/software/spm5). Standard protocols, including correction for slice timing differences, head motion (<3 mm in any coordinate), and normalization to the MNI coordinate space, were used. Talairach space registration was evaluated individually; the skull was removed and all cortical lesions were masked to avoid image distortion. If not satisfactory, the registration process was repeated without skull removal or with a modified lesion mask, resulting in successful registration for all participants. All image data from participants with left-sided lesions were flipped about the midsagittal plane, so the affected hemisphere was always on the right.
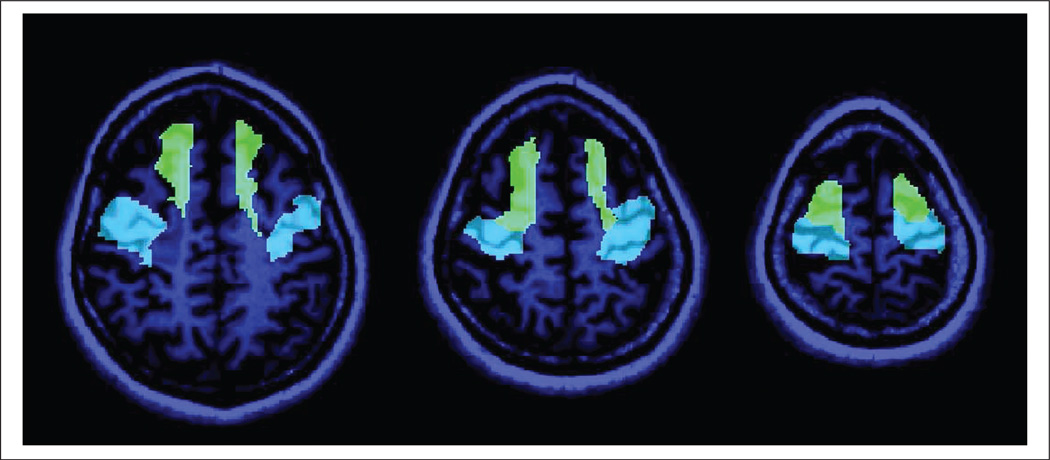
First-level statistical parametric maps were computed, including both the pretraining and the posttraining scans of a given participant. A contrast post–pre was used to identify those voxels whose activation increased between time points. Brain activation was measured by computing the first eigenvariate for each series (ie, the scan for each time point) and for each of 2 prespecified (primary) regions of interest (ROIs) and 6 additional exploratory ROIs and time point. All ROIs were selected from the Automated Anatomical Labeling (AAL) atlas40 (for a detailed visualization refer to http://www.cyceron.fr/web/aal__anatomical_automatic_labeling.html). Primary ROIs were prespecified in the study protocol and were selected based on a priori knowledge of brain activation changes during BATRAC.24 Precentral gyrus and superior frontal gyrus were primary ROIs (Figure 2). Secondary ROIs had not been prespecified and were selected based on a review of single-participant maps. Secondary ROIs included postcentral gyrus, cerebellar hemispheres (anterior and posterior lobes), supramarginal gyrus, anterior cingulate cortex (ACC), and SMA.
Figure 2.
Two primary regions of interest (ROIs), precentral gyrus (blue) and superior frontal gyrus (green), are presented superimposed onto a T1-weighted scan of an exemplary participant. These ROIs were prespecified based on prior studies and defined using the Automated Anatomical Labeling atlas
Randomization and Blinding
Participants were randomized after B2 to receive either BATRAC or DMTE using a stratified block allocation scheme based on initial function (NIH Stroke Scale with 2 as cutoff) and motor dominance of stroke. Because eligibility for fMRI analysis was not a stratification factor, the 2 groups were slightly unbalanced. Testing was conducted in a separate location from the training site by trained testers blinded to group assignment.
Training
Training occurred 3 times per week for 6 weeks, typical of an outpatient clinic, for a total of 18 sessions for each participant. There was a 9-week limit for completing the 18 sessions.30 For BATRAC, participants were seated at the training apparatus that consisted of T-bar handles attached to nearly frictionless linear tracks. They completed 5 minutes of training with the arms moving simultaneously (in phase) away and then toward the body in time to a metronome set at their preferred speed, followed by 10 minutes of rest. Training continued for 5 minutes with the arms moving alternately (antiphase) again with auditory cuing at a preferred speed, followed by 10 minutes of rest. In-phase and antiphase training blocks were repeated once each, achieving a total of 20 minutes of active continuous bilateral arm training in 1 hour for each participant. Frequency was held constant after the third session to allow for initial task adaptation. Participants who were unable to grasp the handles independently had their hands strapped to the T-bar. If necessary, antigravity arm support was provided to avoid an improper arm position during the training; however, the participants were encouraged to produce the forward and backward motions actively and to reach further with their paretic arm throughout the training period by increasing the distance to the target stop. Neither frequency nor resistance was progressed.
DMTE involved a customized set of 4 exercises based on neurodevelopmental principles, including thoracic spine mobilization with weight shifting, scapular mobilization, weight bearing with the paretic arm (elbow fixed), and opening the hand with finger extension. This treatment emphasizes handling techniques that facilitate body and limbs to assume “normal” positions. Participants were encouraged to actively move during the handling. DMTE was performed using the same time schedule as BATRAC (4 cycles of active continuous 5-minute training followed by 10 minutes of rest). Participants of both groups had equal, one-on-one contact with trainers and equal time training, but the number of movements per participant varied according to ability; 5 minutes of active continuous training was sufficient before a rest. A treatment fidelity study, conducted by personnel not affiliated with the study, confirmed study protocols.41
Statistical Analysis
There were separate data analyses for baseline, intervention, and retention phases. An intention-to-treat-analysis included all participants at each time regardless of study completion. Stability of measures from B1 to B2 was modeled in random-effects analysis of variance (ANOVA) (SAS proc mixed, random intercept). The changes in outcome measures during BATRAC versus DMTE were compared using ANOVA adjusted for age, sex, log years since index stroke, the presence or absence of a motor dominant stroke, and the preintervention B2 value of the outcome. A similar model compared changes in outcome measures between groups during retention. A Wilcoxon ranked sums test analyzed the Likert scale data. All analyses were 2 tailed with significance set at P < .05.
Whether BATRAC and DMTE differently affected brain activation during paretic or nonparetic limb movement was analyzed using separate models for each ROI and each brain side (contralesional and ipsilesional). Dependent variables were the difference in the ROIs eigenvariates after therapy minus before. Independent variables included group (BATRAC vs DMTE) and baseline brain activation (eigenvariate) for the respective ROI. Within-group correlations between change in WT and change in ROI activation were assessed using Pearson’s correlation coefficients. Corrections for multiple comparisons were applied for prespecified (4) as well as the secondary (12) ROIs.
Results
A total of 119 subjects were studied during the baseline (8 dropped out between B2 and randomization). Table 1 shows the physical characteristics of the 92 who completed either BATRAC or DMTE. There were no significant differences between study groups with respect to age, gender, time since stroke, side, or dominance of stroke or baseline functional scores.
Table 1.
Baseline Characteristics for the Treatment Groupsa
| Entire Cohort | fMRI Subcohort | |||||
|---|---|---|---|---|---|---|
| BATRAC (n = 42) |
DMTE (n = 50) |
P Value | BATRAC (n = 17) |
DMTE (n = 21) |
P Value | |
| Age, years | 59.8 (9.9) | 57.7 (12.5) | .37 | 61.2 (13.8) | 54.8 (13.1) | .15 |
| Women, n (%) | 16 (38) | 26 (62) | 10 (59) | 10 (48) | ||
| Men, n (%) | 26 (52) | 24 (48) | .37b | 7 (41) | 11 (52) | .49b |
| Time since stroke*, years | 4.5 (4.1) | 4.1 (5.2) | .68 | 3.9 (2.7) | 3.3 (2.1) | .62 |
| Stroke location, n (%) | ||||||
| Brainstem | 3 (50) | 3 (50) | 1 (6) | 2 (10) | ||
| Cerebellar | 0 (0) | 2 (100) | 0 (0) | 0 (0) | ||
| Cortex | 19 (49) | 20 (51) | 9 (53) | 9 (43) | ||
| Multiple | 3 (100) | 0 (0) | 0 (0) | 0 (0) | ||
| Subcortical | 7 (37) | 12 (62) | 7 (41) | 10 (48) | ||
| Unknown or missing, n (%) | 10 (56) | 14 (44) | .12b | 0 (0) | 0 (0) | .89b |
| Right hand dominant, n (%) | 36 (48) | 39 (52) | 15 (88) | 13 (62) | ||
| Left hand dominant, n (%) | 6 (35) | 11 (65) | .42c | 2 (40) | 8 (38) | .14c |
| Non–motor dominant stroke, n (%) paretic arm is nondominant | 24 (57) | 26 (60) | 10 (59) | 12 (57) | ||
| Motor-dominant stroke, n (%) | 18 (43) | 24 (40) | .81b | 7 (41) | 9 (43) | .92b |
| Right-sided stroke, n (%) | 23 (48) | 25 (52) | 10 (59) | 13 (62) | ||
| Left-sided stroke, n (%) | 18 (42) | 25 (58) | .53b | 7 (41) | 8 (38) | .85b |
| Bilateral stroke, n (%) | 1 (100) | 0 (0) | ||||
| Fugl-Meyer score (maximum 66) | 32.3 (14.1) | 31.0 (14.8) | .67 | 32.0 (12.5) | 27.1 (11.6) | .22 |
| Wolf time score (s) | 54.0 (35.6) | 54.1 (38.5) | .99 | 55.4 (38.5) | 66.0 (35.7) | .39 |
| Wolf function score (maximum 4) | 1.8 (0.6) | 1.9 (0.7) | .41 | 1.74 (0.55) | 1.63 (0.62) | .57 |
| Wolf weight score (in lbs) | 2.6 (2.8) | 2.5 (3.1) | .71 | 2.5 (2.6) | 1.8 (3.3) | .50 |
| Stroke Impact Scale (maximum is 59) | 546 (98) | 559 (104) | .54 | 538 (73) | 556 (81) | .48 |
Abbreviations: fMRI, functional magnetic resonance imaging; BATRAC, bilateral arm training with rhythmic auditory cueing; DMTE, dose-matched therapeutic exercise.
Data are mean (standard deviation) except where otherwise noted. Maximum scores for Wolf time and Wolf weight are unknown but approximately 1 s and 40 lbs, respectively. Wolf time and Stroke Impact Scale have low best scores.
χ2 test.
Fisher’s exact test.
= log-transformed.
End Point Analysis
Preintervention
The 2 primary end points, FM and WT, did not change during the 6-week baseline period. There were no differences in the secondary end point variables except for a decline of 0.18 on the 5-point scale (P < .02) for the Wolf function and 0.12 kg (P < .02) for paretic arm elbow flexion isometric strength.
Intervention
Data (average of B1 and B2 to postintervention) are presented in Table 2 and include primary end points followed by secondary end points. Both interventions improved FM scores, but there was no between-group difference. The FM change ranged from +8 to −5 in BATRAC and +11 to −3 in DMTE. A significant decrease in average WT within the groups also found no significant between-group difference. The WT change ranged from −23.1 to 4.6 in BATRAC and −14.3 to 9.7 in DMTE. There was a significant increase in ability to lift a weight following BATRAC but not DMTE (Wolf weight) and no between-group difference. The model was significant (r2 = 0.22), indicating that being female and having a more recent stroke predicted an improvement in this variable. There was a significant within-group improvement in movement quality (Wolf function) following each intervention but no between-group difference.
Table 2.
Significant Motor Function Changes in Primary and Secondary Measures
| BATRAC | DMTE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure ± SE | n | Baseline | Change | Within- Group P |
n | Baseline | Change | Within- Group P |
Between- Group P |
| Fugl-Meyer (maximum 66) Wolf Motor |
42 | 32.3 ± 2.2 | 1.1 ± 0.5 | .03 | 50 | 31.0 ± 2.1 | 1.9 ± 0.4 | .00 | .22 |
| Time (minimum 120 s) | 42 | 54.0 ± 5.5 | −2.6 ± 0.8 | .00 | 50 | 54.1 ± 5.5 | −1.6 ± 0.7 | .04 | .40 |
| Weight (maximum 10 lbs) | 40 | 2.6 ± 0.4 | 0.4 ± 0.2 | .00 | 47 | 2.5 ± 0.4 | 0.2 ± 0.1 | .44 | .11 |
| Function (maximum 4) | 42 | 1.8 ± 0.1 | 0.0 ± 0.0 | .03 | 50 | 1.9 ± 0.1 | 0.1 ± 0.0 | .00 | .58 |
| Stroke Impact Scale | |||||||||
| Emotion | 37 | 82.1 ± 2.3 | 2.5 ± 1.8 | .24 | 42 | 82.9 ± 2.4 | 3.6 ± 1.7 | .01 | .30 |
| Hand | 37 | 24.6 ± 4.9 | 6.5 ± 3.0 | .03 | 42 | 27.7 ± 5.0 | 6.2 ± 2.5 | .03 | .91 |
| Strength | 37 | 48.3 ± 3.5 | 7.0 ± 3.1 | .01 | 42 | 52.2 ± 3.2 | 3.7 ± 1.9 | .10 | .37 |
| Total score | 37 | 549 ± 17 | 12 ± 6.1 | .14 | 42 | 578 ± 15 | 26 ± 8.9 | .00 | .19 |
| Isokinetic strength (kg/s) | |||||||||
| Nonparetic | |||||||||
| Elbow extension | 41 | 16.6 ± 0.8 | 1.8 ± 0.8 | .03 | 46 | 16.1 ± 0.7 | 0.7 ± 0.8 | .33 | .35 |
| Elbow flexion | 41 | 33.3 ± 2.3 | 1.8 ± 1.1 | .09 | 46 | 36.9 ± 2.7 | −1.5 ± 0.8 | .13 | .03 |
| Paretic | |||||||||
| Elbow extension | 41 | 13.9 ± 1.2 | 1.6 ± 0.8 | .01 | 46 | 13.5 ± 1.4 | 0.8 ± 0.5 | .33 | .24 |
| Isometric strength (kg) | |||||||||
| Nonparetic | |||||||||
| Shoulder extension | 42 | 17.6 ± 0.9 | 1.4 ± 0.4 | .00 | 50 | 18.1 ± 0.8 | 0.6 ± 0.4 | .10 | .29 |
| Wrist extension | 42 | 13.8 ± 0.6 | 0.9 ± 0.3 | .01 | 50 | 12.9 ± 0.4 | 0.5 ± 0.4 | .23 | .25 |
| Wrist flexion | 42 | 11.2 ± 0.6 | 0.6 ± 0.4 | .09 | 50 | 11.4 ± 0.6 | −0.6 ± 0.4 | .10 | .02 |
| Paretic | |||||||||
| Shoulder extension | 42 | 8.1 ± 0.7 | 0.6 ± 0.3 | .05 | 50 | 7.5 ± 0.9 | 0.8 ± 0.3 | .01 | .64 |
| Wrist extension | 42 | 3.5 ± 0.6 | −0.2 ± 0.2 | .25 | 50 | 2.9 ± 0.9 | 0.7 ± 0.2 | .00 | .00 |
| Elbow flexion | 42 | 8.4 ± 0.7 | 0.3 ± 0.3 | .29 | 50 | 7.9 ± 0.7 | 0.9 ± 0.3 | .01 | .30 |
Abbreviations: SE, standard error; BATRAC, bilateral arm training with rhythmic auditory cueing; DMTE, dose-matched therapeutic exercise.
Following BATRAC, the subsections of Hand and Strength of the Stroke Impact Scale (SIS) improved significantly, but there were no between-group differences. The model for Strength was significant (r2 = 0.27), indicating that a lower initial score for this subsection is a predictor of an improved score after intervention. Following DMTE, the total score and the subsections Hand and Emotion demonstrated significant improvements. The model for Emotion was significant (r2 = 0.30), indicating that a lower initial score for this subsection is a predictor of an improved score after intervention.
There was an increase in isokinetic strength in elbow extension for both arms following BATRAC but not DMTE. BATRAC significantly improved isometric strength in nonparetic arm shoulder extension, wrist extension, and wrist flexion and in paretic arm shoulder extension, whereas DMTE improved strength in paretic arm shoulder and wrist extension and elbow flexion. There was a greater improvement in nonparetic elbow flexion and wrist flexion isometric strength after BATRAC and in paretic wrist extension isometric strength after DMTE.
On the Likert scale questionnaire, satisfaction with BATRAC was significantly higher than with DMTE immediately after training (4.4 vs 3.8; P = .003) and remained slightly higher after the retention period (4.1 vs 3.8; P = NS). Both groups reported comparable perceived improvements immediately after training (BATRAC 4.0 vs DMTE 3.7) and after retention (4.1 vs 3.9).
Retention
During 4-month retention, there were comparable declines in FM scores by 1.1 (P < .04; n = 39) in BATRAC and 1.0 (P < .05; n = 39) in DMTE. The WT and secondary variables that improved after intervention were maintained during retention. However, the SIS total score response during retention differed between groups, improving by 10 after BATRAC and declining by 16 after DMTE (P < .05).
fMRI analysis
In the subset of 17 BATRAC and 21 DMTE patients who underwent fMRI scanning, brain activation during paretic limb movement was differentially affected by the 2 therapies. Among the prespecified ROIs, BATRAC led to a significantly greater increase of activation in the ipsilesional precentral gyrus (contralateral to the moving, paretic limb; between-group P = .011) and contralesional superior frontal gyrus (P = .012; Table 3); see Figure 2. These probabilities remain significant if corrected for 4 comparisons (2 ROIs on each side) using Bonferroni’s correction. A statistical “trend” (.05 < P < .1) was noted for the ipsilesional superior frontal gyrus. Secondary ROIs that increased more after BATRAC than DMTE included ipsilesional SMA, ACC, and postcentral gyrus (Table 3). None of these between-group tests remained significant after correcting for 12 comparisons (6 ROIs on each side). All other regions except the posterior lobe of the cerebellum increased more after BATRAC than DMTE, but no between-group differences were significant.
Table 3.
Relationship Between Changes in UE Function to Brain Activation During Paretic Limb Movement
| BATRAC | DMTE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Side | Mean Increase |
SE | ra | P | Mean Increase |
SE | ra | P | Between- Group P |
| Primary ROI | ||||||||||
| Precentral gyrus | Ipsilesional | 1.17 | 0.20 | −0.45 | .079 | 0.48 | 0.17 | −0.23 | .324 | .011b |
| Contralesional | 1.33 | 0.27 | −0.43 | .093 | 0.83 | 0.23 | −0.45 | .112 | .180 | |
| Superior frontal gyrus | Ipsilesional | 1.54 | 0.17 | −0.48 | .057 | 1.08 | 0.15 | −0.12 | .045 | .053 |
| Contralesional | 1.33 | 0.22 | −0.62 | .010b | 0.58 | 0.19 | −0.25 | .290 | .012b | |
| Secondary ROI | ||||||||||
| SMA | Ipsilesional | 1.34 | 0.17 | −0.12 | .646 | 0.85 | 0.15 | −0.21 | .365 | .039 |
| Contralesional | 1.33 | 0.17 | −0.04 | .855 | 0.89 | 0.15 | −0.13 | .581 | .066 | |
| Anterior cingulate cortex | Ipsilesional | 1.47 | 0.27 | −0.76 | .001b | 0.70 | 0.23 | −0.28 | .229 | .036 |
| Contralesional | 1.24 | 0.24 | −0.68 | .004b | 0.59 | 0.21 | −0.09 | .685 | .052 | |
| Supramarginal gyrus | Ipsilesional | 1.06 | 0.15 | −0.64 | .007 | 0.70 | 0.13 | −0.26 | .260 | .082 |
| Contralesional | 0.96 | 0.12 | −0.69 | .003b | 0.77 | 0.10 | −0.45 | .044 | .239 | |
| Cerebellum, anterior lobe | Ipsilesional | 1.17 | 0.18 | −0.17 | .501 | 1.09 | 0.16 | −0.14 | .531 | .707 |
| Contralesional | 1.03 | 0.17 | −0.17 | .537 | 0.59 | 0.15 | −0.30 | .188 | .060 | |
| Cerebellum, posterior lobe | Ipsilesional | 1.68 | 0.32 | −0.10 | .709 | 2.08 | 0.28 | −0.26 | .273 | .349 |
| Contralesional | 1.55 | 0.22 | −0.39 | .142 | 1.64 | 0.19 | −0.42 | .059 | .767 | |
| Postcentral | Ipsilesional | 0.91 | 0.13 | −0.24 | .376 | 0.50 | 0.11 | −0.55 | .012 | .023 |
| Contralesional | 0.88 | 0.13 | −0.44 | .088 | 0.59 | 0.11 | −0.51 | .022 | .095 | |
Abbreviations: UE, upper extremity; ROI, region of interest; SE, standard error; BATRAC, bilateral arm training with rhythmic auditory cueing; DMTE, dose-matched therapeutic exercise; SMA, supplementary motor area.
Pearson’s r.
Remained significant after Bonferroni corrections.
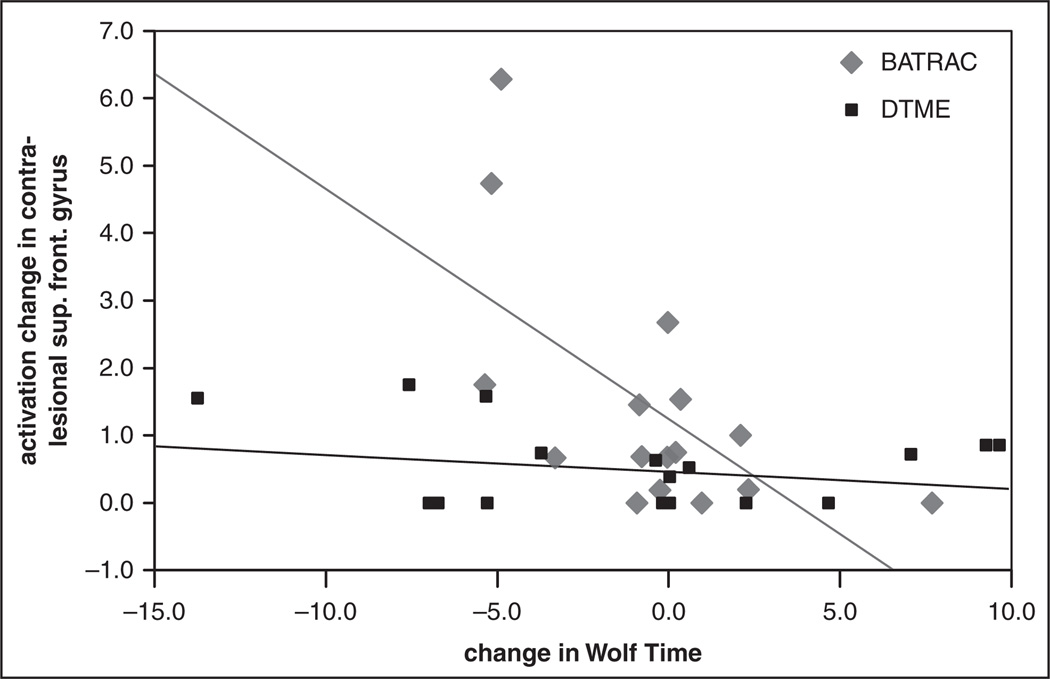
The activation increase in the contralesional superior frontal gyrus predicted 38% of the improvement in WT outcomes after BATRAC (P = .010; if corrected for n = 4 comparisons, 2 primary ROIs on each side, P = .040; Figure 3). Secondary ROIs whose increase in activation correlated with the improvement in WT included the bilateral ACC and supramarginal gyrus. These correlations except one for the ipsilesional supramarginal gyrus remained significant after correcting for 12 comparisons (6 secondary ROIs on each side). The improvement in WT after DMTE was predicted by increased activation in the ipsilesional superior frontal gyrus, contralesional supramarginal gyrus, and bilateral postcentral gyrus; however, none of these correlations remained significant after multiple comparison correction. No area of decreased activation was found after either BATRAC or DMTE. There were no within- or between-group treatment differences in the activation changes for either ROI or side during nonparetic limb movement.
Figure 3.
The increase in activation in the contralesional superior frontal gyrus (ipsilateral to the moving paretic limb) correlated with faster performance in the WMFT (time posttraining − time pretraining) in BATRAC-trained participants (r = −0.62; P = .010). No such correlation was found in the DMTE group
Abbreviations: WMFT, Wolf Motor Function Test; BATRAC, bilateral arm training with rhythmic auditory cueing; DMTE, dose-matched therapeutic exercise.
Discussion
This randomized controlled trial demonstrates that (1) BATRAC is not superior to DMTE, but both improve paretic arm function in stroke survivors, and the improvements are largely maintained for 4 months; (2) BATRAC may operate through activation of primary and secondary motor cortices, whereas DMTE may use additional mechanisms; (3) BATRAC provides higher patient satisfaction than DMTE; and (4) no covariates consistently predicted outcome across variables. Our finding of comparable functional improvements, despite brain activation following BATRAC, disproves our hypothesis.
The improvement found in motor function after 6 weeks of BATRAC is consistent with what was found in previous studies28–30,42; however, DMTE produced better results than expected. Repetition or deliberate practice are major contributors to motor recovery,43,44 and both BATRAC and DMTE involve multiple active repetitions of specific movements. In our effort to control for the repetition built into BATRAC, and matching dose by time, the control group received a viable treatment program. Unlike the EXCITE trial that used a usual care control group,6 other randomized trials of UE rehabilitation that use active dose-matched training as controls also fail to show differences in outcomes between treatments.13,45,46 The within-group gains seen after 6 weeks of either training program were not observed in the 6 weeks between the 2 baseline assessments. Several other trials of UE rehabilitation also demonstrate this point using delayed entry controls6 or attention controls receiving lower-extremity47 or nonmovement exercises.12 Although the latter control nicely for general physiological effects of exercise or confounds of repeated assessments, they do not constitute an active alternative training control targeting the limb of interest comparable to DMTE. Our results indicate, for primary outcomes, that repetition may be more important than features that distinguish the 2 training approaches, such as bilaterality and rhythmic cueing.
There are discrete differences between BATRAC and DMTE in the pattern of improvement across secondary outcome measures. BATRAC, which requires active shoulder and elbow movements of both arms, results in specific active strength gains for these 2 joints across both arms, whereas DMTE, which focuses on static paretic shoulder, elbow, and wrist extension, improves these joint actions. This differential result reflects a prior finding that BATRAC improves temporal and spatial aspects of bilateral reaching, whereas DMTE only improves a temporal aspect of unilateral reaching.48 It may be beneficial to combine selected components of BATRAC and DMTE in sequence or parallel in future studies. BATRAC requires less interaction between trainer and participant, whereas DMTE requires physical support and assistance to facilitate progress. BATRAC might be relatively easier to translate into self-directed training in the clinic or home. The greater sense of satisfaction and positive trend in total SIS scores during retention in the BATRAC group occurred despite the closer trainer–participant relationship with DMTE and could have implications for compliance in community trials.
Although our results suggest that both BATRAC and DMTE are viable as treatment options for stroke survivors with chronic UE deficits, the degree of improvement in the primary end points does not qualify as a clinically significant change according to Van der Lee et al,13 who suggest 10% improvement on an absolute scale. Inspection of the data reveals that both groups had nonresponders, defined by those who maintained or decreased their scores on variables. The treatment effect for our primary variables ranges from 11 to −5 on the FM and an improved timing of −23.1 to 9.7 s for the WT, illustrating a wide range of response. One explanation of the small treatment effects might be low training intensity. Both groups received training for a total of 360 minutes, which is less than other targeted UE interventions in chronic stroke. BATRAC intensity was not progressed in speed or movement resistance. A second explanation might be that the severity deficit range enrolled in this trial was large. Although severity level was not a significant predictor for most variables in this trial, participants at both ends of the spectrum might benefit from a more intensive training regimen to overcome floor or ceiling effects. In currently running studies, we are exploring a more targeted population range and a more intense, progressive form of BATRAC, as well as combining BATRAC with other complementary treatments. Despite the smaller-than-anticipated motor function changes and the lack of superiority of BATRAC, this trial has demonstrated differences between the treatments at the underlying neural mechanism level.
The different brain activation responses suggest that BATRAC and DMTE may produce their improvements through different mechanisms. We previously showed that participants who improve arm function after BATRAC show bihemispheric, mainly contralesional, activation of the premotor cortex by fMRI, whereas those who do not improve lack this activation.30 The present data confirm this finding, showing that activation increases in the contralesional superior frontal gyrus after BATRAC but not DMTE and that this increase is associated with improved arm function. The superior frontal gyrus ROI is the same region identified in our previous analysis as well as by other investigators49–54 as a region modified during recovery of UE function. In contrast to BATRAC, DMTE is associated with smaller increments in brain activation; distributed among different brain regions (except in the ipsilesional premotor cortex, where activation increase correlates with DMTE-related WT improvement). Also, the DMTE-related changes in brain activation did not meet strict statistical criteria applying to multiple comparisons. Therefore, despite similar improvements in arm function, BATRAC and DMTE appear to operate through different brain mechanisms, or DMTE makes more use of adaptations that are outside the brain or not measured by fMRI. These differences may be a result of the different circuitry used in bilateral and unilateral arm movements as described earlier.
Supramarginal gyrus and ACC are other brain regions where changes in brain activation correlated with improved arm function after BATRAC. The supramarginal gyrus may be involved in attention,55 handwriting movements (left hemisphere region in right-handed participants),56 and spatial perception57 and is shown to change activation during recovery of function.51,58 Activation changes in this region during BATRAC may be related to the participant paying more attention while moving the paretic arm or reflect improvements in spatial perception of the paretic arm after BATRAC therapy. Both mechanisms could improve arm function on WMFT and FM tests. The ACC is involved, among other tasks, in error-based movement learning.59 Activation in this region after BATRAC may reflect motor learning mechanisms that are recruited by the therapy.
Other neurorehabilitative therapies based on motor learning strategies are associated with brain activation. Juenger et al60 showed that CIMT leads to increases and shifts of activation in frontal and motor cortices, mainly in the lesioned and, to a lesser extent, in the nonaffected hemisphere in chronic stroke survivors,61 which parallels alterations in brain structure.54 A recent review concluded that no single pattern of CNS change is observed during recovery; rather, the pattern of neuroplasticity seems to depend on the training intervention and the patient’s deficits caused by the initial lesion.62 Our findings support a differential activation change resulting from the training program.
Conclusion
We found that 6 weeks of BATRAC or DMTE improves global arm impairment and function comparably in chronic stroke survivors. Each treatment produced common and different improvements in UE function that were sustained for at least 4 months. The improvements after BATRAC appear to be mediated, at least in part, by cortical remodeling centered in the ipsilesional precentral gyrus and the contralesional superior frontal gyrus (premotor cortex), whereas DMTE seems to affect other neuroplastic processes. A BATRAC intervention of increased intensity and duration, coupled with DMTE and other UE interventions, may be necessary to capitalize on this neuroplasticity to maximize improvements in UE function.
Acknowledgments
The authors thank the participants and the Baltimore research team of Kathleen Michael, PhD; Susan Kopunek, RN; Jill Ohloff, BS, PTA; Tzu Yun Chang, MS, PT; Federico Villagra, PhD; and recruiters, trainers, testers, and nurses in the Pepper Center Cores and GRECC. A patent was awarded to the University of Maryland for BATRAC (#7121981, inventors JW, SMW).
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: P60AG12583; PI AG, NIDDR H H133G010111, the Baltimore Veterans Administration Geriatrics Research, Education and Clinical Center (GRECC). AL was supported by DFG SFB 550, C 12.
Footnotes
Declaration of Conflicting Interests
The author(s) declared a potential conflict of interest (e.g. a financial relationship with the commercial organizations or products discussed in this article) as follows: As inventors of the subject technology, Jill Whitall and Sandy McCombe Waller anticipate receiving licensing income from their institution (UMB), under its Intellectual Property Policy.
References
- 1.French B, Thomas LH, Leathley MJ, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD006073.pub2. CD006073. [DOI] [PubMed] [Google Scholar]
- 2.Masiero S, Carraro E. Upper limb movements and cerebral plasticity in post-stroke rehabilitation. Aging Clin Exp Res. 2008;20:103–108. doi: 10.1007/BF03324755. [DOI] [PubMed] [Google Scholar]
- 3.McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits? NeuroRehabilitation. 2008;23:29–41. [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart KC, Cauraugh JH, Summers JJ. Bilateral movement training and stroke rehabilitation: a systematic review and meta-analysis. J Neurol Sci. 2006;244:89–95. doi: 10.1016/j.jns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18:833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- 6.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 7.Wolf SL, Winstein CJ, Miller JP, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taub E, Miller N, Novack T, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 9.Lin K-C, Wu C-Y, Liu J-S, Chen Y-T, Hsu C-J. Constraint-induced therapy versus dose-matched control intervention to improve motor ability, basic/extended daily functions, and quality of life in stroke. Neurorehabil Neural Repair. 2009;23:160–165. doi: 10.1177/1545968308320642. [DOI] [PubMed] [Google Scholar]
- 10.Stoykov ME, Lewis G, Corcos DM. Comparison of bilateral and unilateral training for upper extremity hemiparesis in stroke. Neurorehabil Neural Repair. 2009;23:945–953. doi: 10.1177/1545968309338190. [DOI] [PubMed] [Google Scholar]
- 11.Boake C, Noser EA, Ro T, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:4–21. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- 12.Rijntjes M, Hobbeling V, Hamzei F, et al. Individual factors in constraint-induced movement therapy after stroke. Neurorehabil Neural Repair. 2005;19:238–249. doi: 10.1177/1545968305279205. [DOI] [PubMed] [Google Scholar]
- 13.Van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar T, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 14.Volpe BT, Lynch D, Rykman-Berland A, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:305–310. doi: 10.1177/1545968307311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- 16.Chan M, Tong R, Chung K. Bilateral upper limb training with functional electric stimulation in patyients with chronic stroke. Neurorehabil Neural Repair. 2009;23:357–365. doi: 10.1177/1545968308326428. [DOI] [PubMed] [Google Scholar]
- 17.Mudie M, Matyas T. Responses of the densely hemiplegic upper extremity to bilateral training. Neurorehabil Neural Repair. 2001;15:129–140. doi: 10.1177/154596830101500206. [DOI] [PubMed] [Google Scholar]
- 18.Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature. 1998;395:274–278. doi: 10.1038/26220. [DOI] [PubMed] [Google Scholar]
- 19.Donchin O, Gribova A, Steinberg O, Bergman H, Cardoso de Oliveira S, Vaadia E. Local field potentials related to bimanual movements in the primary and supplementary motor cortices. Exp Brain Res. 2001;140:46–55. doi: 10.1007/s002210100784. [DOI] [PubMed] [Google Scholar]
- 20.Donchin O, Gribova A, Steinberg O, Mitz AR, Bergman H, Vaadia E. Single-unit activity related to bimanual arm movements in the primary and supplementary motor cortices. J Neurophysiol. 2002;88:3498–3517. doi: 10.1152/jn.00335.2001. [DOI] [PubMed] [Google Scholar]
- 21.Toyokura M, Muro I, Komiya T, Obara M. Relation of bimanual coordination to activation in the sensorimotor cortex and supplementary motor area: analysis using functional magnetic resonance imaging. Brain Res Bull. 1999;48:211–217. doi: 10.1016/s0361-9230(98)00165-8. [DOI] [PubMed] [Google Scholar]
- 22.Toyokura M, Muro I, Komiya T, Obara M. Activation of pre-supplementary motor area (SMA) and SMA proper during unimanual and bimanual complex sequences: An analysis using functional magnetic resonance imaging. J Neuroimaging. 2002;12:172–178. doi: 10.1111/j.1552-6569.2002.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith AL, Staines WR. Cortical adaptations and motor performance improvements associated with short-term bimanual training. Brain Res. 2006;1071:165–174. doi: 10.1016/j.brainres.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 24.Renner CI, Woldag H, Atanasova R, Hummelsheim H. Change of facilitation during voluntary bilateral hand activation after stroke. J Neurol Sci. 2005;239:25–30. doi: 10.1016/j.jns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Stinear JW, Byblow WD. Disinhibition in the human motor cortex is enhanced by synchronous upper limb movements. J Physiol. 2002;543:307–316. doi: 10.1113/jphysiol.2002.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinear JW, Byblow WD. The contribution of cervical propriospinal premotoneurons in recovering hemiparetic stroke patients. J Clin Neurophysiol. 2004;21:426–434. doi: 10.1097/00004691-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Whitall J, McCombe Waller S, Silver KHC, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 28.McCombe Waller S, Whitall J. Fine motor control in adults with and without chronic hemiparesis: Baseline comparison to nondisabled adults and effects of bilateral arm training. Arch Phys Med Rehabil. 2004;85:1076–1083. doi: 10.1016/j.apmr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 29.McCombe Waller S, Whitall J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clin Rehabil. 2005;19:544–551. doi: 10.1191/0269215505cr829oa. [DOI] [PubMed] [Google Scholar]
- 30.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berglund K, Fugl-Meyer A. Upper extremity function in hemiplegia. Scand J Rehabil Med. 1986;18:155–157. [PubMed] [Google Scholar]
- 32.Filiatraut J, Arsenault A, Dutil E, Bourbonnais D. Motor function and activities of daily living assessments: a study of three tests for persons with hemiplegia. Am J Occup Ther. 1991;45:806–810. doi: 10.5014/ajot.45.9.806. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh IP, Hsu M, Sheu C, Lee S, Hsieh CL, Lin JH. Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale. Neurorehabil Neural Repair. 2008;22:737–744. doi: 10.1177/1545968308315999. [DOI] [PubMed] [Google Scholar]
- 34.Whitall J, Savin DN, Jr, Harris-Love M, Waller SM. Psychometric properties of a modified Wolf Motor Function Test for people with mild and moderate upper-extremity hemiparesis. Arch Phys Med Rehabil. 2006;87:656–660. doi: 10.1016/j.apmr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL. Minimal detectable change scores for the Wolf Motor Function Test. Neurorehabil Neural Repair. 2009;23:662–667. doi: 10.1177/1545968309335975. [DOI] [PubMed] [Google Scholar]
- 36.Wolf S, Catlin P, Ellis M, Archer A, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 37.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The Stroke Impact Scale version 2.0: evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 38.Duncan PW, Wallace D, Studenski S, Lai SM, Johnson D. Conceptualization of a new stroke-specific outcome measure: the Stroke Impact Scale. Top Stroke Rehabil. 2001;8:19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT. [DOI] [PubMed] [Google Scholar]
- 39.Norkin C, White D. Measurement of joint motion: A guide to goniometry. 2nd ed. Philadelphia, PA: F.A. Davis Company; 1995. [Google Scholar]
- 40.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in spm using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 41.Shaughnessy M, Michael KM, Resnick B, Nahm E, Kopunek S, Orwig D. Clinical trials and tribulations: challenges to realizing intervention research. J Gerontol Soc Am. 2005;45:670. [Google Scholar]
- 42.Richards LG, Senesac CR, Davis SB, Woodbury ML, Nadeau SE. Bilateral arm training with rhythmic auditory cueing in chronic stroke: not always efficacious. Neurorehabil Neural Repair. 2008;22:180–184. doi: 10.1177/1545968307305355. [DOI] [PubMed] [Google Scholar]
- 43.Sawaki L, Butler AJ, Leng X, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3–9 months after stroke. Neurorehabil Neural Repair. 2008;22:505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003;41(suppl):7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 45.Desrosiers J, Bourbonnais D, Noreau L, Rochette A, Bravo G, Bourget A. Participation after stroke compared to normal aging. J Rehabil Med. 2005;37:353–357. doi: 10.1080/16501970510037096. [DOI] [PubMed] [Google Scholar]
- 46.Platz T, Eickhof C, van Kaick S, et al. Impairment-oriented training or Bobath therapy for severe arm paresis after stroke: a single-blind, multicentre randomized controlled trial. Clin Rehabil. 2005;19:714–724. doi: 10.1191/0269215505cr904oa. [DOI] [PubMed] [Google Scholar]
- 47.Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2006;87:1–9. doi: 10.1016/j.apmr.2005.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCombe Waller S, Liu W, Whitall J. Temporal and spatial control following bilateral versus unilateral training. Hum Mov Sci. 2008;27:749–758. doi: 10.1016/j.humov.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Schaechter JD. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol. 2004;73:61–72. doi: 10.1016/j.pneurobio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 51.Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page SJ, Szaflarski J, Eliassen J, Pan H, Cramer SC. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23:382–388. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (atom) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 56.McKeever WF. An x-linked three allele model of hand preference and hand posture for writing. Laterality. 2004;9:149–173. doi: 10.1080/13576500244000292. [DOI] [PubMed] [Google Scholar]
- 57.Golay L, Schnider A, Ptak R. Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behav Brain Funct. 2008;4:43. doi: 10.1186/1744-9081-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ertelt D, Small S, Solodkin A, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36(suppl 2):T164–T173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 59.Grafton ST, Schmitt P, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage. 2008;39:1383–1395. doi: 10.1016/j.neuroimage.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juenger H, Linder-Lucht M, Walther M, Berweck S, Mall V, Staudt M. Cortical neuromodulation by constraint-induced movement therapy in congenital hemiparesis: an fMRI study. Neuropediatrics. 2007;38:130–136. doi: 10.1055/s-2007-985904. [DOI] [PubMed] [Google Scholar]
- 61.Dong Y, Winstein C, Albistegui-DuBois R, Dobkin BH. Evolution of fMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after chronic stroke. Neurorehabil Neural Repair. 2007;21:412–428. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kreisel SH, Hennerici MG, Bazner H. Pathophysiology of stroke rehabilitation: the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis. 2007;23:243–255. doi: 10.1159/000098323. [DOI] [PubMed] [Google Scholar]