Abstract
Recently, some researchers have reported that macrophages and neutrophils were related to severe asthma. Mucus hypersecretion and persistent airway inflammation result from increased expression of mucin gene (MUC5AC). Eosinophilic chronic rhinosinusitis (ECRS) is considered as intractable rhinosinusitis. From the viewpoint of “one way one disease,” we examined whether ECRS is associated with infiltrating macrophages, neutrophils, their promotive factors, and MUC5AC. We examined 21 nasal polyps with CRS. Each specimen was fixed in 10% phosphate-buffered formalin, embedded in paraffin, processed routinely, and then prepared as semithin sections (3.5 μm). We immunohistochemically observed the macrophages by using CD68, neutrophils by using neutrophil elastase and the promotive factors, monocyte chemotactic protein (MCP) 1, IL-17A, and IL-8, in both ECRS and non-ECRS. The number of macrophages (CD68+ cells), IL-17A, and MUC5AC+ cells in ECRS were significantly greater than in non-ECRS. The mean number of MCP-1+ cells in ECRS was greater than that in non-ECRS, but not significantly. There was a significant correlation in all cases between IL-17A and macrophages or MUC5AC+ cells. Neither the numbers of neutrophils (positive cells for neutrophil elastase) nor the IL-8+ cells showed any significant differences between ECRS and non-ECRS. Our study suggested that infiltrating macrophages, IL-17A and MUC5AC, as well as eosinophils could have roles in the development of ECRS.
Keywords: Eosinophilic chronic rhinosinusitis, human, IL-8, IL-17A, macrophage, MCP-1, MUC5AC, nasal polyps, neutrophil
Eosinophils are regarded as key mediators in the pathology and abnormal physiology of asthma.1 Recently, some researchers2,3 reported that macrophages or neutrophils were related to severe asthma. Tagaya and Tamaoki4 indicated that matrix metalloproteinase (MMP) is involved in the airway remodeling of asthma. MMP derived from neutrophils and macrophages is increased in severe asthma.5,6 Bullens7 described that IL-17A could induce IL-8, and IL-8 could recruit neutrophils in human asthmatic airways. Some studies suggested that macrophages are producers of IL-17A.8 Mucus hypersecretion and persistent airway inflammation result from the increased expression of mucin gene (MUC5AC). IL-17A can induce MUC5AC in airways.9 Eosinophilic chronic rhinosinusitis (ECRS) has been considered an intractable rhinosinusitis.10 In ECRS, infiltrating by eosinophils has been thought to take part in severe epithelial damage. The secretion of some proteinases and cytokines of eosinophils can cause remodeling in airway epithelia (e.g., epithelial detachment and basement membrane thickness).4,5 ECRS as well as asthma belong to airway system disease. From the viewpoint of “one way one disease,” we hypothesized that the process of ECRS would be associated with the mobilization of macrophages or neutrophils as well as eosinophils. In the present study, we focused on macrophages and neutrophils as well as their chemoattractants that contribute to the pathological processes of ECRS.
METHODS
Subject
We examined 21 patients with CRS with nasal polyps who were diagnosed based on the criteria of the European Academy of Allergology and Clinical Immunology position paper,11 i.e., they had two or more symptoms including blockage/congestion, discharge, anterior/posterior drip, facial pain/pressure, and reduction or loss of smell for at least 3 months, and either endoscopic signs including polyps, mucopurulent discharge from the middle meatus, or edema/mucosal obstruction primarily in the middle meatus, and/or mucosal changes within ostiomeatal complex and/or sinuses. None of the patients were treated with systemic corticosteroids or other immune-modulating drugs.
The 21 patients were classified into two groups. The ECRS group included 12 patients in whom the eosinophil count was >350/microscopic field (400× magnification in hematoxylin and eosin stain) using the top five fields in terms of rich eosinophilic infiltration. The non-ECRS included nine patients in whom the eosinophil count was <100/microscopic field (400× magnification) in any of the fields. Five cases in which normal mucosal membranes of the sphenoid sinus were removed during surgery for pituitary adenoma were used as controls. All patients gave their written informed consent, and the study was approved by the Ethics Committee of Juntendo University School of Medicine.
Immunostaining Analysis for CD68 (Marker of Macrophages), IL-17A, Monocyte Chemotactic Protein 1, Neutrophil Elastase (Marker of Neutrophils), IL-8, and MUC5AC
The nasal polyps were fixed in 10% formalin, embedded in paraffin wax, processed routinely, and then prepared as routine semithin sections (3.5 μm).
Macrophages were observed using mouse anti-human CD68 1:3 (Dako, Tokyo, Japan) and sections treated with immunoblock served as the negative control. The expressions of IL-17A and monocyte chemotactic protein (MCP) 1 were examined by rabbit anti-mouse IL-17A antibody 1:50 (Santa Cruz Biotechnology, Santa Cruz, CA) and anti–MCP-1 antibody 1:200 (R & D, Minneapolis, MN) with rabbit serving IgG1 as the negative control.
Neutrophils were observed using mouse anti-human neutrophil elastase 1:100 (Dako) with mouse IgG1 as the negative control. The expression of IL-8 was examined by goat anti-mouse IL-8 antibody 1:5 (R & D) with immunoblock as the negative control.
MUC5AC was observed using mouse anti-human MUC5AC 1:3 (Bio Science for the World, Santa Barbara, CA); sections treated with mouse IgG1 served as the negative control. The sections were stained with the Ventana /VEW DAB Detection kit using a Ventana automated stainer (Ventana Japan KK, Yokohama, Japan).
The numbers of cells positive for CD68, IL-17A, MUC5AC, MCP-1, and IL-8 and elastase were assessed by the mean of the top three fields in terms of the richness of their infiltration (400× magnification).
Statistical Analyses
The data were expressed as mean ± SD. Statistical analyses were performed using StatMateIII for Windows. Statistical analyses were evaluated using Pearson's correlation coefficient and Student's t-test. A value of p < 0.05 was considered significant.
RESULTS
We identified macrophages using CD68. Most of the CD68+ macrophages (47 ± 23/a field) had infiltrated into the subepithelia of ECRS (Fig. 1), whereas non-ECRS showed few macrophages. A significantly larger mean number of CD68+ macrophages was observed in ECRS (47 ± 23/a field) compared to non-ECRS (20 ± 11/a field) and the control (17 ± 7/a field) group (p < 0.001; Fig. 2). There was no significant difference in the mean number of macrophages between non-ECRS and the control group.
Figure 1.

The number of macrophages with CD68+ reactions of eosinophilic chronic rhinosinusitis (ECRS); arrows, right) was significantly more than in non-ECRS (middle) and control (left).
Figure 2.
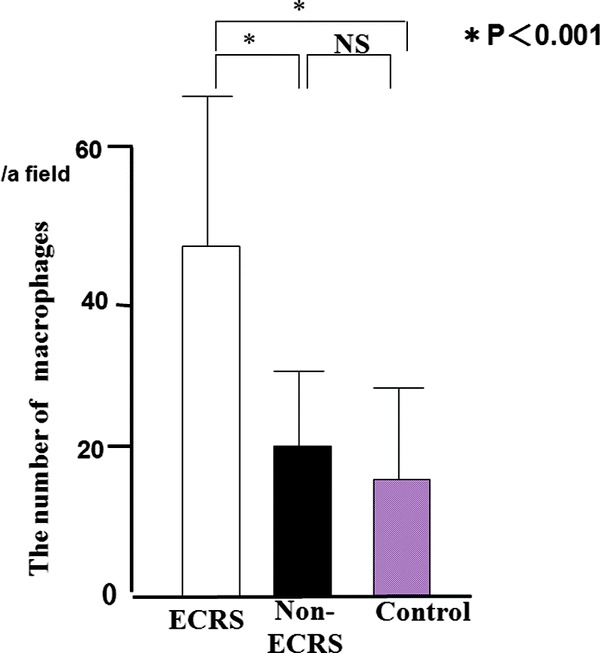
The number of macrophages with CD68 expression in the subepithelia.
IL-17A+ inflammatory cells were observed in CRS (Fig. 3). A significantly larger mean number of IL-17A+ cells was seen in ECRS (48 ± 18/a field) compared with non-ECRS (14 ± 13/a field) and the control group (9 ± 7/a field; Fig. 4). There was no significant difference in the mean number of IL-17A+ cells between non-ECRS and the control group. A significant correlation was recognized in all cases between CD68 and IL-17A+ cells (p < 0.01; Fig. 5).
Figure 3.
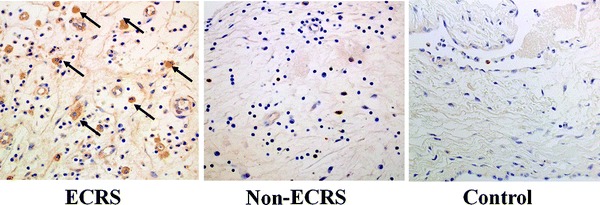
Immunohistochemical demonstration of IL-17A in chronic rhinosinusitis (CRS). The number of cells with IL-17A+ reactions of eosinophilic CRS (ECRS; arrows, right) was significantly more than in non-ECRS (middle) and controls (left).
Figure 4.
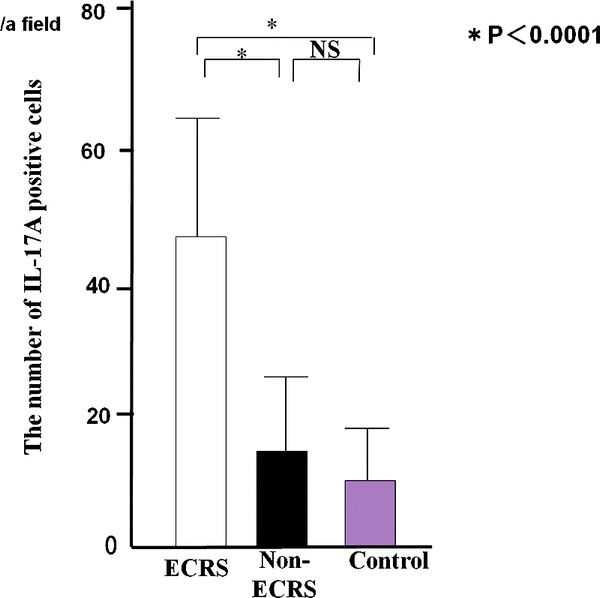
The number of IL-17A+ cells in chronic rhinosinusitis (CRS).
Figure 5.
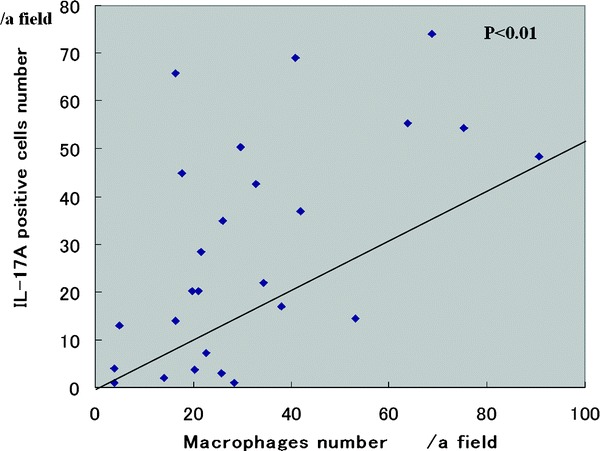
Relationship between the number of IL17A and CD68+ cells.
The mean percentages of MUC5AC+ epithelial cells reaction in ECRS (26.5 ± 21.1%/a field) were significantly greater than in non-ECRS (9.2 ± 7.5/a field) and the control group (7.0 ± 5.1/a field; Fig. 6; p < 0.05). There was a significant correlation between MUC5AC and IL-17A+ cells in all cases (Fig. 7; p < 0.05)).
Figure 6.
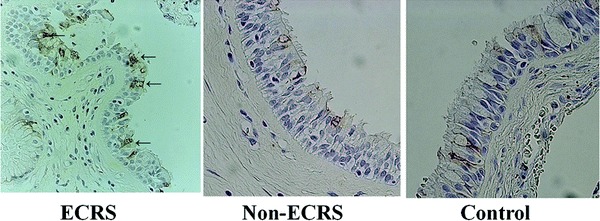
The number of MUC5AC positive cells in ECRS (arrows, left) was significantly greater than in non-ECRS(middle) and control (right).
Figure 7.
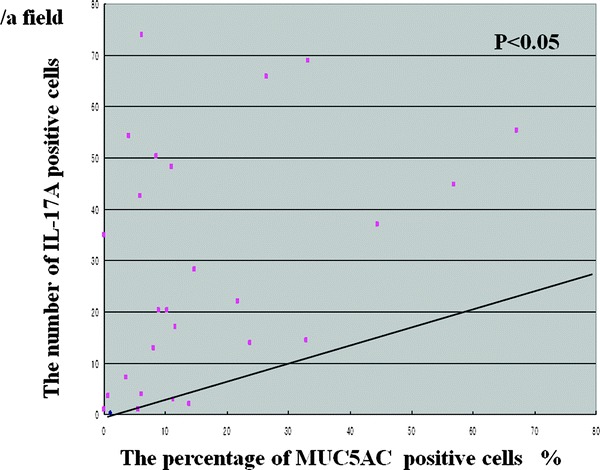
Relationship between the number of MUC5AC and IL-17A+ cells.
The mean number of MCP-1+ cells in ECRS (20 ± 31/ field) was more than twice that in non-ECRS (10 ± 5). However, the difference between ECRS and non-ECRS or the control group was not significant. No correlation between CD68 and MCP-1+ cells was observed.
The mean number of neutrophils (positive for neutrophil elastase) did not show significant differences between ECRS (10 ± 14/a field) and non-ECRS (14 ± 11/a field). The mean number of IL-8+ cells in ECRS (11 ± 17/a field) was greater than that in non-ECRS (6 ± 5/a field), but not significantly.
DISCUSSION
Poston2 reported that, in asthmatic patients, the total number of macrophages infiltrating the airway mucosa was increased and suggested that lung macrophages may have a central role in the chronic immune-mediated inflammatory response seen in the airway mucosa of asthmatic patients. Previously, we examined whether severe epithelial damage would be associated with infiltrating eosinophils in ECRS.12 Our results showed that the number of CD68+ macrophages in the subepithelia was significantly greater in ECRS than in non-ECRS. Macrophages as well as eosinophils are able to produce many cytotoxic agents such as oxidants, e.g.,13,14 and metalloproteinases.6 Based on the aforementione reports and our results, the remodeling in ECRS may be associated with infiltrating macrophages as well as eosinophils. Concerning the infiltrating macrophages in our experiment, we suggest two theories as follows. First, macrophages were phagocytotic for wastes in nasal mucosa because of inflammation as a native function. Second, eosinophils might release some chemokines for macrophages.
The present study was consistent with our previous report,15 in which a significant increase in the mean number of IL-17A+ cells in ECRS was observed in comparison with non-ECRS. The expression of IL-17A+ cells indicated inflammatory cells including both eosinophils and lymphocytes. We revealed a significant relationship between eosinophils and IL-17A+ cells and suggested that eosinophils could product IL-17A. Makihara8 reported that IL-17A expression in CRS could be found in eosinophils, macrophages, and lymphocytes by double staining. Moreover, our study could show a significant correlation between CD68 and IL-17A+ cells in all cases. Thus, macrophages as well as eosinophils are a possible source of IL-17A in ECRS. ECRS is characterized by enriched amounts of mucin-producing cells and eosinophilic mucus secretion. The key factor in the mucus production in ECRS is still unknown. In the present study, IL-17A+ cells were significantly correlated with MUC5AC+ cells, which suggest that IL-17A plays a critical role in mucus secretion. Chen9 reported that IL-17A could induce mucin gene expression (such as MUC5AC) in the airways and that there was a significant correlation between MUC5AC and IL-17A+ cells. Therefore, IL-17A in the subepithelia is likely to stimulate the MUC5AC expression of epithelia in ECRS as well as airways.
IL-8 can induce neutrophilic inflammation in both upper and lower airways.16 Silvestri17 reported that patients with severe asthma showed significantly higher amounts of IL-8 than mild–moderate asthmatic patients or controls, and increase the numbers of neutrophils. Yoshihara18 reported that epithelial damage is caused by enhanced IL-8 and the activity of neutrophils during acute exacerbations of pediatric asthma. Cundall5 described that neutrophil-derived MMP-9 was increased in severe asthma. However, neither neutrophils nor IL-8 showed significant differences between ECRS and non-ECRS in our study.
In conclusion, IL-17A, macrophages and MUC5AC are the key factors in the processes of ECRS and would be more significant than neutrophils or IL8.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Seminario MC, Geich GJ. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immuno 6:860–864, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Poston RN, Chanez P, Lacoste JY, et al. Immunohistochemical characterization of the cellular infiltration in asthmatic bronchi. Am Rev Respir Dis 145:918–921, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Wenzel SE, Schwartz LB, Langmack E, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 160:1001–1008, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Togaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int 56:331–340, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cundall M, Sun Y, Miranda C, et al. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol 112:1064–1071, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Martney JM, Gustafson CE, Bowler RP, et al. Thromboxane receptor signaling is required for fibronectin-induced matrix metalloproteinase-9 production by human and murine macrophages and is attenuated by the Arhgef1 molecule. J Biol Chem 286:44521–44531, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makihara S, Okano M, Fujiwara T, et al. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol 126:397–400, 400.e1–e11, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Thai P, Zhao YH, et al. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 278:17036–17043, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Vento SI, Ertama LO, Hytönen ML, et al. Nasal polyposis: Clinical course during 20 years. Ann Allergy Asthma Immunol 85:209–214, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Fokkens W, Lund V, Bachert C, et al. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy 60:583–601, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Saito T, Kusunoki T, Yao T, et al. Relationship between epithelia 1 damage or basement membrane thickness and eosinophilic infiltration polyps with chronic rhinosinusitis. Rhinology 47:275–279, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Vendrow AE, Hakim ZS, Madamanchi NR, et al. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Aterioscler Thromb Vasc Biol 27:2714–2721, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Ezeamuzie CI, Taslim N. Reactive oxygen species mediate phorbol ester-stimulated cAMP response in human eosinophils. Eur J Pharmacol 543:174–180, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Saito T, Kusunoki T, Yao T, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol 151:8–16, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki H, Ikeda K. Mode of action of long-term low-dose macrolide therapy for chronic sinusitis in the light of neutrophil recruitment. Curr Drug Targets Inflamm Allergy 1:117–126, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Silvestri M, Bontempelli M, Giacomelli M, et al. High serum levels of tumor necrosis factor-alpha and interleukin-8 in severe asthma: Makers of systemic inflammation. Clin Exp Allergy 36:1373–1381, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Yoshihara S, Yamada Y, Abe T, et al. Association of epithelial and signs of neutrophil mobilization in the airways during acute exacerbations of paediatric asthma. Clin Exp Immunol 144:212–216, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]


