Abstract
Group 10 allergens (tropomyosins) have been assumed to be a major cause of cross-reactivity between house-dust mites (HDMs) and other invertebrates. Despite all of the published data regarding the epidemiology, percent IgE binding and level of sensitization in the population, the role of tropomyosin as a cross-reactive allergen in patients with multiple allergy syndrome still remains to be elucidated. Homology between amino acid sequences reported in allergen databases of selected invertebrate tropomyosins was determined with Der f 10 as the reference allergen. The 66.9 and 54.4% identities were found with selected crustacean and insect species, respectively, whereas only 20.4% identity was seen with mollusks. A similar analysis was performed using reported B-cell IgE-binding epitopes from Met e1 (shrimp allergen) and Bla g7 (cockroach allergen) with other invertebrate tropomyosins. The percent identity in linear sequences was higher than 35% in mites, crustaceans, and cockroaches. The polar and hydrophobic regions in these groups were highly conserved. These findings suggest that tropomyosin may be a major cause of covariation of sensitization between HDMs, crustaceans, and some species of insects and mollusks.
Keywords: Cross-reactivity of tropomyosins, group 10 allergens, HDM allergens, homology, IgE-binding epitopes, multiple allergy syndrome, tropomyosins
The role of house-dust mites (HDMs) as a major source of multiple allergens that sensitize and induce rhinitis, asthma, or atopic dermatitis in a large portion of the population is well established.1–4 They belong to class Arachnida. Most notable are the species from families Pyroglyphidae, Glycyphgidae, Echymopodidae (HDMs), Acaridae, and Chortoglyphidae (storage mites),5 reported for the first time in 1964 by Voorhorst and coworkers.6 There are >24 groups of dust-mite allergenic proteins7 (Table 1).
Table 1.
Major allergen groups in mites

Df = Dermatophagoides farinae; Dp = Dermatophagoides pteronyssinus; Em = Euroglyphus maynei; Po = Psoroptes ovis; Ss = Sarcoptes scabii; Gd = Glycyphagus domesticus; Ld = Lepidoglyphus destructor; Tp = Tyrophagus putrescentiae; Bt = Blomia tropicalis; MW = molecular weight.
Cross-reactivity is said to have occurred when an antibody, originally raised against one allergen, binds to a similar allergen from another source.8 The incidence of cross-reacting allergens has often been reported in epidemiological studies or clinical observations.9–11 Cross-reactivity between allergens may cause “covariation of sensitization,” i.e., a higher observed frequency of sensitization to two or more allergens than the expected frequency. Our understanding of the cross-reactivity between HDM allergens and other allergens has markedly increased, but it is still limited; thus, it is of clinical interest to know whether or how HDM sensitization changes patients' reaction to allergens from other sources. HDM sensitization has been suspected to cause or worsen food allergy (snails and crustaceans), inhalation allergy (other mites and cockroach), and local skin reactions (scabies),12 for snails, crustaceans, cockroaches, silverfish, chironomids, and various other mites, a covariation of sensitization to HDM often exists.13 Cross-reactivity of shrimp with other crustaceans and nonedible arthropods such as cockroaches or dust mites is caused by the similarity of tropomyosin in these organisms.14 We used the bioinformatics approaches and allergenic databases to identify and study molecular similarities of tropomyosin allergen family as a potential cause of cross-reactivity and covariation of sensitization in multiple allergy syndrome. The prevalence of tropomyosin allergy has been reported by many researchers. A comprehensive review of the studies is presented in Table 2.15–27
Table 2.
A comprehensive review of prevalence studies of tropomyosin sensitization

Table format adopted from Ref. 27.
HDMs = house-dust mites; r = recombinant. For additional abbreviations, see Appendix A.
HDM GROUP 10 ALLERGENS: TROPOMYOSINS
Among the allergen groups of HDM, group 10 allergen is a muscle protein: tropomyosin.28 It is present in all eukaryotic cells associated with the thin filament in muscle and microfilament in many nonmuscle cells. Besides its role in the contractile activity of these cells it also helps in regulation of cell morphology and motility. Tropomyosin (Pfam code PF00261) is one of the few groups of Pfam database proteins that comprise of a large number of reported allergens, most of which are from invertebrate sources,14 hence considered in literature as a pan-allergen.29 Several tropomyosin isoforms have been found in different species, tissues, and cell varieties.29
The purified natural tropomyosin on SDS-PAGE has an average molecular weight (MW) of 37 kDa,17 having high frequency of glutamate, glutamine, arginine, and methionine and lowest mean frequency for cysteine and proline. Mature proteins from group 10 have 284 amino acids. The predicted isoelectric point ranges from 4.3 to 4.5. Each polypeptide is an α-helix; two parallel α-helical tropomyosin molecules form a coiled–coil structure containing two sets of seven alternating actin-binding sites.14,31 Sequence identity within the eight mite tropomyosins is 84.2%, which is higher than any other allergen (Fig. 1), whereas 75% sequence homology to other arthropods with high immunologic cross-reactivity to shellfish and other invertebrate tropomyosins has also been reported.14 Being a calcium-binding protein, tropomyosin can be purified from crude mite extract by eluting with CaCl2 buffer (0.5 M solution of CaCl2 in 0.02 M Tris-HCl) in p-aminobenzamidine column.32
Figure 1.
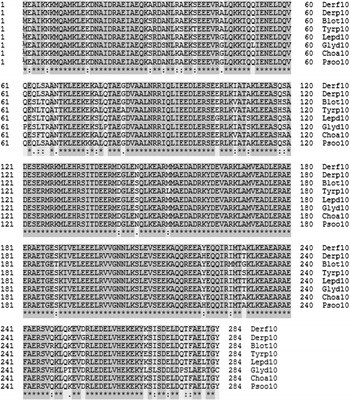
Amino acid sequence alignment: comparison of Der f 10 allergen with major group 10 allergens from mite species using the clustalo algorithm. With 240 identical (*), 26 conserved (:) and 6 semiconserved (.) positions the identity is 84.2% (UniProt FASTA). For abbreviations refer to Appendix A.
Many reports suggest tropomyosin to be an important component of immune and allergic reactions.16,18,19,24,32–37 Der f10 was the first allergen to be reported in the HDM tropomyosin group. The allergen gave a high IgE-binding frequency (80.6%), comparable with that of Der f1 (90.3%) and Der f2 (74.2%). Forty-six percent of patients tested had positive skin reactions to Der f10.23 Two recombinant Blomia tropicalis tropomyosins have been reported, with IgE-binding frequencies of 29 and 20%.18 Der p10 has a derived MW of ∼33 kDa consisting of a 15-residue signal peptide.31
Native Der p10 (nDer p10) showed 0% IgE-binding frequency,38 whereas in another study 16.7% positive reactivity was reported.39 Recombinant Der p10 (r Der p10) gave 5.6% IgE-binding frequency in HDM allergy patients.31 Another study indicated that rDer p10 was recognized by 15.2% of HDM-allergic patients.40 Tyrophagus putrescentiae tropomyosin (Tyr p10) shared 64–94% amino acid sequence identity with previously known allergenic tropomyosins. Recombinant Tyr p10 showed 12.5% IgE-binding reactivity.41 In sheep scab mite Psoroptes ovis tropomyosin homolog Pso o 10 was found to have an MW of 38 kDa. It is among the three most immunodominant allergens in sheep, having allergenicity and structure similar to other mite tropomyosins. One expressed sequence tag of P. ovis, named Pso-tropo-1 (accession no. BQ834874), showed 98% homology to Dermatophagoides farinae tropomyosin.42 A study of recombinant tropomyosin allergen of Lepidoglyphus destructor (rLep d 10) revealed 13% IgE-binding frequency.19
Positive covariation of sensitization between HDM and Sarcoptes scabiei is known through studies in which higher prevalence of HDM sensitization was reported in scabies patients compared with controls43,44 and HDM-sensitized patients with no history of scabies were seen to have a positive skin test to S. scabiei more frequently than the controls.45 In animal subjects (rabbits) 71% protection from scabies infection was observed after IgE immunization with HDM.46 Although tropomyosin allergen in S. scabiei has not been reported, Tarigan47 described an allergen of ∼35-kDa MW (SDS-PAGE) causing hypersensitivity in sensitized animal subjects. Additional investigations are needed to confirm this protein to be tropomyosin and its role in cross-reactions between HDM and S. scabiei.
TROPOMYOSIN ALLERGEN GROUPS IN OTHER INVERTEBRATES
In mollusks and other arthropods such as shrimp, lobster, crayfish, crabs, locusts, flies, and silverfish, tropomyosin has been classified as group 1 allergen29,48,49 and group 7 in cockroaches.29,49 There is evidence of tropomyosin being an important allergen in crustaceans such as spiny lobster (Panulirus stimpsoni, Pan s1), lobster (Homarus americanus, Hom a1),50,51 North Sea shrimp (Crangon crangon, Cra c1),52 sand shrimp (Metapenaeus ensis, Met e1),52 crab (Charyabdis feriatus, Cha f1),51 etc. In mollusks tropomyosin has been characterized in squid (Todarodes pacificus, Tod p1),53 snails (Turbo cornutus, Tur c1),54 and oyster (Crassotrea gigas, Cra g1).55 Among the insects, tropomyosin was identified in cockroaches20: Blatella germanica (Bla g7) and Periplaneta americana (rPer a7); chironomids, e.g., Chironomus kinesis (Chi k10)31 and silver fish, i.e., Lepisma saccharina (Lep s1).22
CROSS-REACTIVITY OF TROPOMYOSIN ALLERGENS IN HDM AND CRUSTACEA
A large variety of shellfish are used for human consumption. Litopenaeus vannamei is the most widely cultured shrimp species in the world.56 The Food and Agriculture Organization/World Health Organization (WHO) has categorized crustaceans such as shrimp, lobster, crab, etc. among the major allergenic foods.57 Research has indicated that the major allergen of shellfish is tropomyosin.33 Among 13 allergens studied from Penaeus aztecus Pen a1 was found to show 82% binding frequency.58 Lin and coworkers59 studied 10 patients with allergy to a shrimp species: Parapenaeus fissurus. A 39-kDa protein (Par f1) was found to give positive immunoblot in 70% of the patients.
Sequence homology of M. ensis (Met e1) and Pen i1 with allergens from other sources was studied by Reese and colleagues.29 They found 98% homology of both of the allergens with Hom a1 (Atlantic lobster), 98% with Pan s1 (spiny lobster), 82% Per a7 (American cockroach), and 81% with Der p10 (HDM). Hom a I from the H. americanus and Pan sI from P. stimpsoni showed deduced amino acid sequence homology to shrimp tropomyosin. The major allergens of the crab Charybdis feriatus (Cha f1) and lobsters (Pan s1 and Hom a1) also show significant homology to Met e1.50 Pan s1, Hom a1, and Met e1 were found to be the major immunogenic allergens in patients with shrimp allergy.60 It is the most dominant allergen in shrimp and other crustaceans, with a prevalence of sensitization varying from 72 to 100%.61 In 1998 Rao and colleagues identified two shared IgE-binding B-cell epitopes corresponding to 47–63 and 150–158 of the deduced amino-acid sequence of M. ensis and Penaeus indicus. The thermal stability and IgE binding of tropomyosin in raw and boiled shrimp extracts were compared using L. vannamei. The boiled tropomyosin had a lower stability and percent IgE binding than the protein from raw shrimp.62,63 Iparraguirre and colleagues showed that tropomyosin is involved in covariation of sensitization to crustaceans in mite-allergic patients.64 A 20-kDa novel protein has also been reported as a source of cross-reactivity between shrimp and HDM.65
In an epidemiological study with 48 patients allergic to “shellfish” 82% appeared to be sensitized to HDM as well.66 In a study of 17 HDM allergy patients receiving immunotherapy, 3 were IgE+ against shrimp and two of them had IgE against tropomyosin.61 One hundred times higher inhibition of IgE binding of boiled shrimp (C. crangon) extract was observed with mite extract (Dermatophagoides pteronyssinus) when compared with tropomyosin-depleted shrimp extract.37 The IgE-binding capacity of German cockroach (B. germanica) extract was totally abolished by boiled Atlantic shrimp (Pandalus borealis) extract, indicating strong cross-reactivity of shrimp allergens to cockroach sensitization.67
In a group of 55 dust-mite–allergic patients tropomyosin-specific IgE for shrimps (rPen a1, nPen i1, andnPen m1), HDM (rDer p10), and German cockroach (nBla g7) were measured. Two recombinant allergens, rTyr p10 and rPer a7, were used to investigate the cross-reactivity. The basophil histamine release assay showed that 11/13 patients were sensitive to tropomyosin, 8/13 to rDer p10, and 7/13 to nBla g7 and nPen m1. Immunodot study showed that there were 8/13 patients sensitive to rPer a7 and 7/13 patients sensitive to rTyr p10. Tropomyosin-specific IgE was detected in 23.6% of Der p–sensitive patients.68 Shrimp-allergic patients showed 52–88% inhibition of IgE binding to oyster extracts by shrimp extracts using radioallergosorbant test inhibition of serum pool from four oyster-sensitive individuals.69,70 Inhibition of IgE binding to shrimp extract by clam extract showed similar inhibitory potency as shrimp.71
A recent study described that among 93 HDM allergy patients (identified through skin-prick test, allergen-specific IgE, and intranasal provocation) only 4 (4.3%) patients' sera had IgE antibodies to HDM tropomyosin (Der p 10), 2 of those 4 patients (50%) showed symptoms of allergy (itching/swelling of oral mucosa and bronchial obstruction) after consumption of shrimp, indicating that cross-reactivity to tropomyosin in HDM-allergic patients in southern Bavaria, Germany, is rarer than suspected.72
CROSS-REACTIVITY OF TROPOMYOSIN ALLERGENS IN HDM AND MOLLUSCA
During the past 10 years available information about tropomyosin allergens has been limited to those of crustaceans but much remains to be investigated on the mollusks tropomyosin. Bivalves including clams and oysters are widely eaten as food in many parts of the world such as Europe, China, and Korea.
Sequence alignments show that tropomyosin of clam (Sinonovacula constricta) has a 65–72% homology with other mollusks tropomyosin.73 In Europe, where snails are consumed as food, cross-reactivity between HDM and snail allergens has been reported.74–76 Covariation of sensitization to snail was observed in 31% of HDM-allergic children in a randomly selected population, where 50% of these children had never eaten snail.77 The eating of snails by HDM–snail-allergic patients may lead to severe symptoms: asthma, anaphylactic shock, generalized urticaria, and/or facial edema.61,78,79
On the other hand, there are many studies suggesting allergens other than tropomyosin as the basis of cross-reactivity.76,80,81 Several allergic fractions with a wide MW range (15–250 kDa) in gastropod allergy were observed. D. pteronyssinus extract inhibited the IgE binding to a 75-kDa protein, possibly Der p4.82 B-cell epitopes of C-terminal region of Tur c1, the tropomyosins of the snail T. cornutus, are different from those identified in Pen a1.83 An immunologic study of snail allergy identified two protein bands, one at 55 kDa and the other at 95 kDa, eliminating Der p10 as a possible cause of sensitization in patients.84
There are many reports of cross-reactivity between HDM and other mollusks species but no evidence of tropomyosin as the cross-reactive allergen is recognized.13,54,61,70,82–84
CROSS-REACTIVITY OF TROPOMYOSIN ALLERGENS IN HDM AND INSECTS
Invertebrates that infest homes and come into close contact with humans are major causative agents of allergy in susceptible individuals. The prevalence of cockroach-specific IgE antibodies has been found to be second only to that of antibodies specific to the HDM and constitute a significant risk factor for acute asthma.85 Four cockroach species, the German (B. germanica; 36.2%), the American (P. americana; 33.3%), the Japanese (Periplaneta japonica; 1.1%), and the dusky brown (Periplaneta fuliginosa; 1.7%) cockroach, have been found to infest Korean homes.86 The dusky brown cockroach was a native of the southeastern United States, Japan, and Southeast Asia but now its distribution has increased worldwide because this species infests shipping containers aboard airplanes, cargo ships, and semitruck trailers.87
In the B. germanica Bla g720 and in P. americana Per a7 are tropomyosin.21 Recently, tropomyosin from the P. fuliginosa Per f 7 was characterized and cloned to test its cross-reactivity with tropomyosins of B. germanica Bla g7 and D. farinae Der f10. The IgE-binding reactivity of the P. fuliginosa extract was inhibited 79.4% by B. germanica extract and 63.3% by D. farinae extract. Native tropomyosin inhibited the binding of IgE to the P. fuliginosa, B. germanica, and D. farinae extracts by 65.0, 51.8, and 39%, respectively.21
Covariation of sensitization between cockroaches and HDMs was reported in some studies,88,89 whereas other studies omitted possibilities of any link between the two organisms.90–92 The clinical importance of the cross-reactivity between cockroaches and HDMs is unknown. Many studies have emphasized a need for developing better diagnostic tests that are more specific and safer for the patients.93,94
Humans are also exposed to other insects such as L. saccharina (silverfish) and chironomids (flies). Allergenicity of tropomyosin from L. saccharina (Lep s1) in rLep s195 and from midge (C. kinesis) Chi k1023 has been reported. Nine allergens with 22 isoforms have been listed in the Allergome database for midge (Chironomus thummi).95 There are conflicting reports about covariation of sensitization to HDM and silverfish as well as chironomids. Although some studies have reported positive results,89,96,97 there are studies rejecting the idea of covariation between HDMs and these arthropods.98,99
The role of tropomyosin as a cross-reactive allergen was studied in Brazil. The sera from 119 Ascaris lumbricoides–infested children and 112 patients from cockroach allergy were reacted with tropomyosin monoclonal antibody. A significantly strong correlation was found for IgE antibodies to tropomyosin from A. lumbricoides and P. americana in the results. The authors proposed further cohort studies to establish clinical relevance of the findings.100
ANALYSIS OF TROPOMYOSIN ALLERGEN FAMILY
Methodology
Amino acid sequences of the tropomyosin proteins from selected species of mites and other invertebrates to which humans are exposed through air and food were searched and aligned in the National Centre for Biotechnology Information Blast (protein) database. Using Der f10 as the reference sequence, analysis with each invertebrate group was performed on the website101 to obtain a pairwise comparison of Der f10 with each sequence in the groups. Group alignment was performed in UniProt FASTA using Clustalo (clustal omega) algorithm.102
Results
The results of this analysis are summarized in Tables 3 and 4. Analysis of tropomyosin in mites show a close evolutionary relationship (refer to E10000 values in Table 3). The sequence homology indicators show a close relationship among the members of this group. Tropomyosin from HDMs is evolutionarily closer to each other than to the storage mites (Fig. 2). Comparing Der f10 pairwise with the members of phylum Crustacea showed percent identity in the range between 81 and 83.5%. Among phylum Insecta silverfish and midges seem to be distantly related to mites, showing comparatively low percent homology and higher E (10000) score. Species of the phylum Mollusca generally had a low percent homology (all values below 66%).
Table 3.
Analysis of tropomyosin allergens from mite species

The analysis was performed using Der f 10 as reference sequence (UniProt No. A7XZI8/NCBI No. ABU97468.1/BAA04557.1); protein length 284 at www.fasta.bioch.virginia.edu/fasta_www2/fasta_www.cgi with default settings.
MW = molecular weight; M.M = molecular mass; NCBI = National Centre for Biotechnology Information. For additional abbreviations, see Appendix A.
Table 4.
Analysis of tropomyosin allergens from invertebrate species reported in database

All analysis was done using Der f 10 as reference sequence (UniProt No. A7XZI8/NCBI No. ABU97468.1/BAA04557.1) protein length 284 at http://fasta.bioch.virginia.edu/fasta_www2/fasta_www.cgi) with default settings.
MW = molecular weight; NCBI/EMBL = National Centre for Biotechnology Information/European Molecular Biology Laboratory. For additional abbreviations, see Appendix A.
Figure 2.
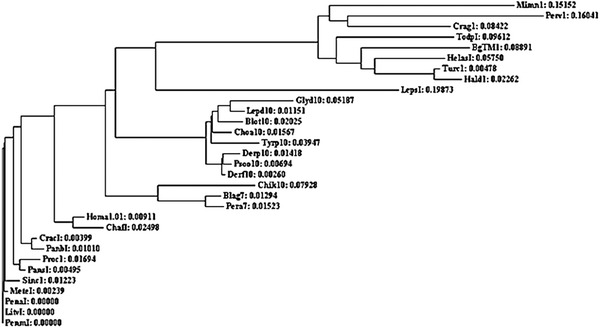
Phylogenetic tree based on aligned tropomyosin proteins from invertebrates.103 For abbreviations, see Appendix A.
There was 84.2% sequence identity (240/284) among HDMs (Fig. 1). Taking Der f10 and Der p10 sequences as a reference from mites, clustalo was performed with each group. The comparison revealed 66.9, 54.4, and 20.4% identical positions with Crustacea, Insecta, and Mollusca, respectively (Figs. 3–5).
Figure 3.

Amino acid sequence alignment: comparison of Der f 10 and Der p 10 allergens with major tropomyosin allergens from crustacean species using the clustalo algorithm. With 190 identical (*), 37 conserved (:), and 7 semiconserved (.) positions the identity is 66.9% (UniProt FASTA). For abbreviations, see Appendix A.
Figure 4.

Amino acid sequence alignment: comparison of Der f 10 and Der p 10 allergens with major tropomyosin allergens from insect species using the clustalo algorithm. With 155 identical (*), 60 conserved (:), and 16 semiconserved (.) positions the identity is 54.4% (UniProt FASTA). For abbreviations, see Appendix A.
Figure 5.
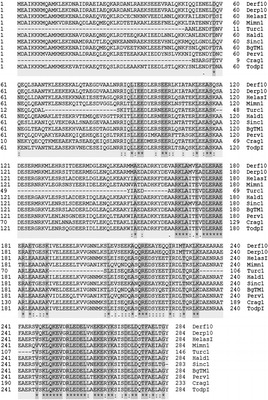
Amino acid sequence alignment: comparison of Der f 10 and Der p 10 allergens with major tropomyosin allergens from mollusk species using the clustalo algorithm. With 58 identical (*), 36 conserved (:), and 7 semiconserved (.) positions the identity is 20.4% (UniProt FASTA). For abbreviations, see Appendix A.
COMPARISON OF IgE-BINDING EPITOPES
Methodology
Some of the reported IgE-binding epitopes from invertebrate tropomyosins23,60 were aligned with the selected species of mites and other invertebrates to determine sequence similarities, polar and hydrophobic regions,which contribute in the final tertiary folding of a protein molecule. According to WHO guidelines, sequence identity >35% is a realistic cutoff value to achieve sequence specificity.14
Small areas of amino acid sequence that bind to the IgE from allergy patients' sera constitute the IgE-binding epitopes. It has been purposed that sequence and structural homology in IgE-binding epitopes may account for cross-reactivity among allergens belonging to the same protein family (Pfam).14 Most of the IgE-binding epitopes that have been reported, to date, are linear or continuous allergen-specific motifs (ASMs), identified by experimental techniques such as tryptic digestion.96
Rao and colleagues reported two IgE-binding B-cell epitopes from shrimp species P. indicus and M. ensis.59 These sequences were aligned with the selected invertebrate tropomyosins in UniProt alignment tool and striking similarities were observed.
Results
Peptide 1 “MQQLENDLDQVQESLLK” corresponding to 47–63 amino acids showed 47% identity with other crustaceans and mites, and a low percent identity value of 29.4 and 5.8% with insect and mollusks, respectively, was observed. It was further noted that polar and hydrophobic regions coincided more among crustacea and mites (Fig. 6).
Figure 6.
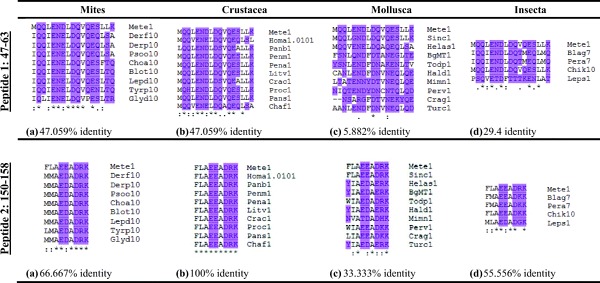
IgE-binding epitope (peptide 1, 47–63, and peptide 2, 150–158) from Met e 1 aligned with species of invertebrates (a, mites; b, crustacea; c, mollusks; and d, insects). Conserved polar regions have been highlighted (UniProt FASTA). For abbreviations, see Appendix A.
Peptide 2 “FLAEEADRK” (150–158 of the deduced amino acid sequence) was found to be more species specific; identical sequences were common within the species (Fig. 6). The “peptide 2” sequence was 100% identical in crustaceans; however, analysis with insect and mite tropomyosins indicated 55.5 and 66.6% identity. Furthermore, in mollusks there was 33.33% sequence identity (below 35% WHO limits), but S. constricta tropomyosin Sin c 1 did give a high percent identity at both peptides 1 and 2 sites. Surprisingly, the polar and hydrophobic regions of the peptide 2 sequences were 100% similar in crustaceans, insects, and mites and in mollusks the first amino acid place in the sequence showed variation in polar and hydrophobic groups. Peptide 2 may be a good candidate for the development of an immunotherapy drug for shrimp allergy.60
A similar analysis was performed with five reported B-cell IgE-binding epitope regions23 from German cockroach (B. germanica) tropomyosin. In the first epitope Epi 120,22,52–65 strikingly high percent identity (75%) was seen in the mites' group 10 allergens with Bla g7 because reference sequence (Fig. 7) polarity differences were only seen at the third and tenth amino acids places in the sequences. Crustacean percent identity was below 35% and polar/hydrophobic similarities were also low. In the insect group Per a7 sequence was 100% identical to Bla g7.
Figure 7.
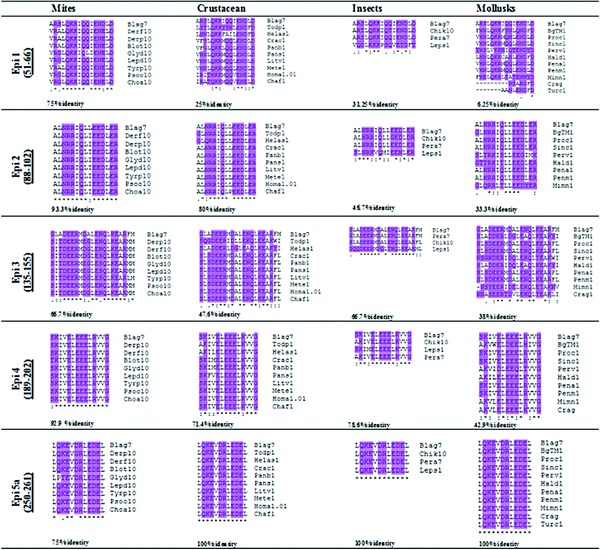
Analysis of IgE-binding epitopes of Bla g 7 (Blatella germanica tropomyosin) with selected invertebrate species. Conserved polar regions have been highlighted (UniProt FASTA). For abbreviations, see Appendix A.
Despite dissimilarities in the other two species the polar and hydrophobic amino acid positions were 100% conserved. In mollusks, again, the identity was found to be 18.75% far below the cutoff line of permissible limit.
The analysis of Epi 2 (88–102) also gave surprising results. One hundred percent identical polar and hydrophobic regions were observed with mites and an overall identity of 93.3% that was even higher than the percent identity within the insects (46.6%). The identity score with mollusks again remained low. In Epi 3 (135–155) 66.6% identity was found between Bla g7 and mite tropomyosins. There were more polar sites in the mite allergens compared with the reference sequence of epitope 3. Among the crustaceans this was a highly conserved region except for Tod p1 and Hel as 1 (refer to Appendix A for names). The group gave 47.6% identity with Bla g7 differing at the first and the last position in the amino acid sequence. In the insect analysis the silverfish (Lep s1) tropomyosin was the odd one, despite that the percent identity was 66. 6%. Epitope 4 (189–202) of Bla g7 was 92.9% identical to the mite group. Only in Blo t 10 the first polar amino acid was substituted by another (polar) amino acid, but polar/hydrophobic regions were 100% conserved. High percent identities were also observed with crustaceans and insects. Analysis of individual sequences shows that some species of Crustacea, Insecta, and Mollusca share 100% similarity in Epi 4 region. Epi 5 (250–281) constitutes a long sequence with three sub epitopes 5a, b, and c. The 5a region (250–261) was highly conserved in all groups giving a 100% identity (excluding Gly d 10). Analysis of the whole length of this fragment also gave significantly high percent identity values (all >35%). These two regions seem to be good candidates for the development of some common diagnostic test marker of cross-reactivity.
DISCUSSION AND CONCLUSION
Potential cross-reactive allergens often have very similar sequences.104,105 Thus, to determine potential cross-reactivity among allergens, the initial step is to decide the degree of similarity between them.14 Proteins with primary and tertiary structure homology and identical IgE-binding epitopes show allergenic cross-reactivity. Smith et al. showed that the allergenic cross-reactivity between Der p 2, Der f 2, and Eur m 2 occurred because of conserved antigenic surface, whereas the lack of cross-reactivity with Lep d 2 and Tyr p 2 may be a result of the multiple amino acid substitutions across the protein surface.106 Cross-reactivity is frequently observed in taxonomically related mite species. High structural homology present between allergenic proteins from apparently unrelated sources of exposure seem to play an important role in IgE-mediated poly sensitization and covariation of sensitization. These proteins have been referred to in literature as “pan-allergens.” The muscle protein tropomyosin is one example that accounts for most of the allergenic cross-reactivity in mites with other invertebrates.24 The authors proposed that exposure and sensitization to a particular food allergen may ultimately lead to sensitization to certain aeroallergens, thus, resulting in the development of sensitization to both foods and inhalants in the same patients. Cross-reactivity among tropomyosins in different invertebrate species has also provided explanation for some unusual observations of reactivity in the absence of sensitization.
Bioinformatics approaches and allergenic databases are now strong tools that help to identify molecular similarities of allergen to provide explanation for clinically observed cross-reactivity and covariation of sensitization.107–111 Furmonaviciene and Shakib analyzed three-dimensional structures of group 1 allergens showing a common motif to study their common allergenic properties.112 The frequency of charged and polar residues is an important factor in determining the allergenicity of the molecules.113 Tropomyosins are highly conserved in invertebrates (Table 4) and are among the major allergenic proteins causing a significant proportion of invertebrate allergies.23 Tropomyosin has been suggested to be the cross-reacting allergen between shellfish, insects, and mites. Our analysis has provided a detailed picture of group 10 allergens from mites and a comparison with selected invertebrate species. Based on this analysis we conclude that tropomyosins from mites, many crustaceans that are eaten as food, and some domestic insects such as cockroaches are structurally very similar. They share similar IgE-binding epitopes and therefore may be a cause of clinically reported cross-reactivity. Although our analysis was based on linear sequence of IgE-binding epitopes, a significant similarity in polar and hydrophobic regions may help in predicting ASMs.
Percentage homology does not necessarily indicate similarity in percentage IgE-binding frequency. Der f 10 shows 98% homology with Der p 10. Aki et al. showed 81% IgE binding in Der f 10,26 and Weghofer and colleagues found 0% IgE-binding frequency with nDer p 10 in 10 patients and a higher reactivity (9–18%) was reported with r Der p 10 30. An allergenic recombinant Der p 10 was shown to share >65% identity with other invertebrate tropomyosins and showed cross-reactivity with shrimp antitropomyosin.31 The storage mite allergens Blo t 10 and Lep d 10 show 95.8% identity with Der f 10 (Table 3) but their recombinant allergens r Blo t 10 and r Lep d 10 only gave 2918 and 13%19 IgE-binding frequency, respectively. Very little data are available testing any cross-reactivity among the group 10 allergens in mite species. Although at individual levels these allergens do not show high IgE binding, their striking structural similarities might provide some clue for covariation of sensitization.
Sequence similarity per se does not mean that the proteins will cross-react; it is therefore necessary to investigate and study the tertiary structural features of the allergenic proteins and their nonallergenic counterparts to get an insight to allergen cross-reactivity. Tertiary folding in an allergen molecule leads to the formation of IgE-binding epitopes; thus, similarity in folding may result in cross-reacting allergens. A parallel input from experimental data through functional proteomics and motif-based prediction through bioinformatics is required to sketch a better picture of allergenic cross-reactivities. Although there is enough evidence of clinically relevant cross-reactivity between HDMs and at least some species of shrimps and insects being caused by tropomyosin, the enormity of the problem still remains to be determined. Bioinformatics database and tools available today may be helpful in identifying homologies and predicting tertiary structure of allergenic proteins belonging to biochemically and functionally similar protein families, thus characterizing the cross-reactive epitopes for their common diagnosis and development of common immunotherapies.
Tropomyosins are highly conserved in invertebrates and are among the major allergenic proteins causing a significant proportion of invertebrate allergies. Our analysis has provided a detailed picture of group 10 allergens from mites and a comparison with selected invertebrate species. Based on this analysis we conclude that tropomyosins from mites, many crustaceans that are eaten as food, and some domestic insects such as cockroaches are structurally very similar. They share similar IgE-binding epitopes and therefore may be a cause of clinically reported cross-reactivity. Although our analysis was based on linear sequence of IgE-binding epitopes, a significant similarity in polar and hydrophobic regions may help in predicting ASMs.
ACKNOWLEDGMENTS
This review has been compiled under the research project, “House Dust Mite Species and Allergen Levels in Pakistan: Molecular Characterization and a Phylogenetic Analysis.”
APPENDIX A
List of Invertebrate Species and Their Tropomyosin Allergens Used in This Article

Footnotes
Funded by Pakistan Science Foundation (PSF), Islamabad, Pakistan under Grant PSF/C-IBGE/Med (318)
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Platts-Mills TA, Vervloet D, Thomas WR, et al. Indoor allergens and asthma: Report of the Third International Workshop. J Allergy Clin Immunol 100:2–24, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Arlian LG, Geis DP, Vyszenski-Moher DL, et al. Antigenic and allergenic properties of the storage mite Tyrophagus putrescentiae. J Allergy Clin Immunol 74:166–172, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Arlian LG, Morgan MS, Neal JS. Dust mite allergens: Ecology and distribution. Curr Allergy Asthma Rep 2:401–411, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Leung R, Jenkins M. Asthma, allergy and atopy in southern Chinese school students. Clin Exp Allergy 24:353–358, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Arlian LG, Platts-Mills TAE. The biology of dust mites and the remediation of mite allergens in allergic disease. J Allergy Clin Immunol 107(suppl):S406–S413, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Voorhorst R, Spieksma FTM, Varekamp H, et al. The house-dust mite (Dermatophagoides pteronyssinus) and the allergens it produces: Identity with the house-dust allergen. J Allergy 39:325–339, 1967 [Google Scholar]
- 7. Yong TS, Jeong KY. Household arthropod allergens in Korea. Korean J Parasitol 47(suppl):S143–S153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vestergaard HS, Bindslev-Jensen C, Poulsen LK. Specific IgE to wheat in patients with grass pollen allergy. EAACI 94(suppl 2):445, 1994 [Google Scholar]
- 9. Park JW, Ko SH, Yong TS, et al. Crossreactivity of Tyrophagus putrescentiae with Dermatophagoides farina and Dermatophagoides pteronyssinus in urban areas. Ann Allergy Asthma Immunol 83:533–539, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Gafvelin G, Johansson E, Lundin A, et al. Cross-reactivity studies of a new group 2 allergen from the dust mite Glycyphagus domesticus, Gly d 2, and group 2 allergens from Dermatophagoides pteronyssinus, Lepidoglyphus destructor, and Tyrophagus putrescentiae with recombinant allergens. J Allergy Clin Immunol 107:511–518, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Hales BJ, Shen HD, Thomas WR. Cross-reactivity of T-cell responses to Dermatophagoides pteronyssinus and D. farinae. Studies with group 1 and 7 allergens. Clin Exp Allergy 30:927–933, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Ortolani C, Bruijnzeel-koomen C, Bengtsson U, et al. Controversial aspects of adverse reactions to food. Allergy 54:27–54, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Sidenius KE, Hallas TE, Poulsen LK, et al. Allergen cross-reactivity between house-dust mites and other invertebrates. Allergy 56:723–733, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Ivanciuc O, Garcia T, Torres M, et al. Characteristic motifs for families of allergenic proteins. Mol Immunol 46:559–568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weghofer M, Thomas WR, Kronqvist M, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest 38:959–965, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Asturias JA, Eraso E, Arilla MC, et al. Cloning, isolation, and IgE-binding properties of Helix aspersa (brown garden snail) tropomyosin. Int Arch Allergy Immunol 128:90–96, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Aki T, Kodama T, Fujikawa A, Miura K, et al. Immunochemical characterisation of recombinant and native tropomyosins as a new allergen from the house dust mite, D. farinae. J Allergy Clin Immunol 96:74–83, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Yi FC, Cheong N, Shek PCL, et al. Identification of shared and unique immunoglobulin E epitopes of the highly conserved tropomyosins in Blomia tropicalis and Dermatophagoides pteronyssinus. Clin Exp Allergy 32:1203–1210, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Saarne T, Kaiser L, Rsool O, et al. Cloning and characterization of two IgE-binding proteins, homologous to tropomyosin and alpha tubulin, from the mite Lepidoglyphus destructor. Int Arch Allergy Immunol 130:258–265, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Jeong KY, Lee J, Lee IY, et al. Allergenicity of recombinant Bla g 7, German cockroach tropomyosin. Allergy 58:1059–1063, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Asturias JA, Gómez-BayÓn N, Arilla MC, et al. Molecular characterization of American cockroach tropomyosin (Periplaneta americana Allergen 7) cross reactive allergen. J Immunol 162:4342–4348, 1999 [PubMed] [Google Scholar]
- 22. Bartella B, Butteroni C, Puggioni EMR, et al. Immunological characterization of a recombinant tropomyosin from new indoor source, Lepisma saccharina. Clin Exp Allergy 35:483–489, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Jeong KY, Yum HY, Lee IY, et al. Molecular cloning and characterization of tropomyosin, a major allergen of Chironomus kiiensis, a dominant species of nonbiting midges in Korea. Clin Diagn Lab Immunol 11:320–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pascual CY, Crespo JF, San Martin S, et al. Cross-reactivity between IgE-binding proteins from Anisakis, German cockroach, and chironomids. Allergy 52:514–520, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Lin RY, Shen HD, Han SH. Identification and characterization of a 30 kd major allergen from Parapenaeus fissurus. J Allergy Clin Immunol 92:837–845, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Yang AC, Arruda LK, Santos AB, et al. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J Allergy Clin Immunol 125:872–878, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Bessot JC, Metz-Favre C, Rame JM, et al. Tropomyosin or not tropomyosin, what is the relevant allergen in house dust mite and snail cross allergies? Eur Ann Allergy Clin Immunol 42:3–10, 2010 [PubMed] [Google Scholar]
- 28. Huntley JF, Machell J, Nisbet AJ, et al. Identification of tropomyosin, paramyosin and apolipophorin vitellogenin as three major allergens of the sheep scab mite, Psoroptes ovis. Parasite Immunol 26:335–342, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Reese G, Ayuso R, Lehrer SB. Tropomyosin: An invertebrate pan-allergen? Int Arch Allergy Immunol 119:247–258, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Schevzov G, Whittaker SP, Fath T, et al. Tropomyosin isoforms and reagents. Bioarchitecture 1:135–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asturias JA, Arilla MC, Gomez-Bayon N, et al. Sequencing and high level expression in Escherichia coli of the tropomyosin allergen (Der p 10) from Dermatophagoides pteronyssinus. Biochim Biophys Acta 1397:27–30, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Erban T. Purification of tropomyosin, paramyosin, actin, tubulin, troponin and kinases for chemiproteomics and its application to different scientific fields. PLoS One 6:e22860, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daul CB, Slattery M, Reese G, et al. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol 105:49–55, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Goetz DW, Whisman BA. Occupational asthma in a seafood restaurant worker: Cross-reactivity of shrimp and scallops. Ann Allergy Asthma Immunol 85:461–466, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Lehrer SB, Ayuso R, Reese G. Seafood allergy and allergens: A review. Mar Biotechnol 5:339–348, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Shanti KN, Martin BM, Nagpal S, et al. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE binding epitopes. J Immunol 151:5354–5363, 1993 [PubMed] [Google Scholar]
- 37. Witteman AM, Akkerdaas JH, van Leeuwen J, et al. Identification of a cross-reactive allergen (presumably tropomyosin) in shrimp, mite, and insects. Int Arch Allergy Immunol 105:56–61, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Weghofer M, Thomas WR, Pittner G, et al. Comparison of purified Dermatophagoides pteronyssinus allergens and extract by two-dimensional immunoblotting and quantitative immunoglobulin E inhibitions. Clin Exp Allergy 35:1384–1391, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Jimenez S, Puerta L, Mendoza D, et al. IgE Antibody responses to recombinant allergens of Blomia tropicalis and Dermatophagoides pteronyssinus in a tropical environment. All Clin Immun Int 19:233–238, 2007 [Google Scholar]
- 40. Resch Y, Weghofer M, Seiberler S, et al. Molecular characterization of Der p 10: A diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy 41:1468–1477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeong KY, Lee H, Lee JS, et al. Molecular cloning and the allergenic characterization of tropomyosin from Tyrophagus putrescentiae. Protein Pept Lett 14:431–436, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Kenyon F, Welsh M, Parkinson J, et al. Expressed sequence tag survey of gene expression in the scab mite Psoroptes ovis—Allergens, proteases and free-radical scavengers. Parasitology 126:451–460, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Falk ES, Dale S, Bolle R, et al. Antigens common to scabies and house dust mites. Allergy 36:233–238, 1981 [DOI] [PubMed] [Google Scholar]
- 44. Moustafa EH, el-Kadi MA, al-Zeftawy AH, et al. The relation between scabies and hypersensitivity to antigens of house dust mites and storage mites. J Egypt Soc Parasitol 28:777–787, 1998 [PubMed] [Google Scholar]
- 45. Falk ES, Bolle R. In vitro demonstration of specific immunological hypersensitivity to scabies mite. Br J Dermatol 103:367–373, 1980 [DOI] [PubMed] [Google Scholar]
- 46. Arlian LG, Rapp CM, Morgan MS. Resistance and immune response in scabies-infested hosts immunized with Dermatophagoides mites. Am J Trop Med Hyg 52:539–545, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Tarigan S. Identification and characterization of heat-stable allergens from Sarcoptes scabiei. J Ilmu Ternak Dan Veteriner 11: 52–60, 2006 [Google Scholar]
- 48. Krieger J, Raming K, Knipper M, et al. Cloning, sequencing and expression of locust tropomyosin. Insect Biochem 20:173–184, 1990 [Google Scholar]
- 49. De Witt AM, Mattsson L, Lauer I, et al. Recombinant tropomyosin from Penaeus aztecus (r Pen a 1) for measurement of specific immunoglobulin E antibodies relevant in food allergy to crustaceans and other invertebrates. Mol Nutr Food Res 48:370–379, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Mykles DL, Cotton JL, Taniguchi H, et al. Cloning of tropomyosins from lobster (Homarus americanus) striated muscles: Fast and slow isoforms may be generated from the same transcript. J Muscle Res Cell Motil 19:105–115, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Leung PS, Chen YC, Gershwin ME, et al. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J Allergy Clin Immunol 102:847–852, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Bauermeister K, Wangorsch A, Garoffo LP, et al. Novel crustacean allergens identified in north sea shrimp Crangon crangon and other crustacean species. Mol Immunol 48:1983–1992, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Miyazawa H, Fukamachi H, Inagaki Y, et al. Identification of the first major allergen of a squid (Todarodes pacificus). J Allergy Clin Immunol 98:948–953, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Ishikawa M, Ishida M, Shimakura K, et al. Purification and IgE-binding epitopes of a major allergen in the gastropod Turbo cornutus. Biosci Biotechnol Biochem 62:1337–1343, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Leung PS, Chu KH. cDNA cloning and molecular identification of the major oyster allergen from the Pacific oyster Crassostrea gigas. Clin Exp Allergy 31:1287–1294, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Ayuso R, Grishina G, Bardina L, et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol 122:795–802, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Food and Agriculture Organization/World Health Organization FAO/WHO Evaluation of allergenicity of genetically modified foods. In: Report of a Joint FAO/WHO Expert Consultation of Allergenicity of Foods Derived from Biotechnology FAO/WHO, 22–25, 2001 [Google Scholar]
- 58. Reese G, Jeoung BJ, Daul CB, et al. Characterization of recombinant shrimp allergen Pen a 1 (tropomyosin). Int Arch Allergy Immunol 113:240–242, 1997 [DOI] [PubMed] [Google Scholar]
- 59. Lin RY, Shen HD, Han SH. Identification and characterization of a 30 kd major allergen from Parapenaeus fissurus. J Allergy Clin Immunol 92:837–845, 1993 [DOI] [PubMed] [Google Scholar]
- 60. Rao PV, Rajagopal D, Ganesh KA. B- and T-cell epitopes of tropomyosin, the major shrimp allergen. Allergy 53(suppl 46):S44–S47, 1998 [DOI] [PubMed] [Google Scholar]
- 61. van Ree R, Antonicelli L, Akkerdaas JH, et al. Possible induction of food allergy during mite immunotherapy. Allergy 51:108–113, 1996 [PubMed] [Google Scholar]
- 62. Byun MW, Kim JH, Lee JW, et al. Effects of gamma radiation on the conformational and antigenic properties of a heat-stable major allergen in brown shrimp. J Food Prot 63:940–944, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Liu G-M, Cheng H, Nesbit JB, et al. Effects of boiling on the IgE-binding properties of tropomyosin of shrimp (Litopenaeus vannamei). J Food Sci 75:T1–T5, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Iparraguirre A, Rodríguez-Pérez R, Juste S, et al. Selective allergy to lobster in a case of primary sensitization to house dust mites. J Investig Allergol Clin Immunol 19:409–413, 2009 [PubMed] [Google Scholar]
- 65. Villalta D, Tonutti E, Visentini D, et al. Detection of a novel 20 kDa shrimp allergen showing cross-reactivity to house dust mites. Eur Ann Allergy Clin Immunol 42:20–24, 2010 [PubMed] [Google Scholar]
- 66. Castillo R, Carrilo T, Blanco C, et al. Shellfish hypersensitivity: Clinical and immunological characteristics. Allergol Immunopathol (Madr) 22:83–87, 1994 [PubMed] [Google Scholar]
- 67. Crespo JF, Pascual C, Helm R, et al. Cross-reactivity of IgE-binding components between boiled Atlantic shrimp and German cockroach. Allergy 50:918–924, 1995 [DOI] [PubMed] [Google Scholar]
- 68. Liao E, Lee M, Tsaii J. The tropmyosin specific IgE and its roles of crossreactivity between shrimp and dust mites. Clin Transl Allergy 1(suppl 1):70, 2011 [Google Scholar]
- 69. Hoffman DR, Day ED, Jr, Miller JS. The major heat stable allergen of shrimp. Ann Allergy 47:17–22, 1981 [PubMed] [Google Scholar]
- 70. Lehrer SB, McCants ML. Reactivity of IgE antibodies with crustacea and oyster allergens: Evidence for common antigenic structures. J Allergy Clin Immunol 80:133–139, 1987 [DOI] [PubMed] [Google Scholar]
- 71. Desjardins A, Malo JL, L'Archeveque J, et al. Occupational IgE-mediated sensitization and asthma caused by clam and shrimp. J Allergy Clin Immunol 96:608–617, 1995 [DOI] [PubMed] [Google Scholar]
- 72. Becker S, Moritz Gröger, Canis M, et al. Tropomyosin sensitization in house dust mite allergic patients. Eur Arch Otorhinolaryngol 269:1291–1296, 2012 [DOI] [PubMed] [Google Scholar]
- 73. Song J, Zhang H, Zhigang L, et al. Mango profilin: Cloning, expression and cross-reactivity with birch pollen profilin Bet v 2. Mol Biol Rep 35:231–237, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Pajno GB, Morabito L, Ruggeri C, et al. Allergie alimentaire et asthme. Bronchospasme après ingestion d'escargots chez des enfants allergiques aux acariens. Rev Fr Allergol 34:141–144, 1994 [Google Scholar]
- 75. Didier A, Goyeau E, Panaye JP, et al. Prévalence des tests cutanés positifs à l'escargot chez les patients présentant des manifestations allergiques respiratoires. Corrélations avec l'allergie aux acarienset incidence clinique. Rev fr Allergol 36:466–469, 1996 [Google Scholar]
- 76. Guilloux L, Vuitton DA, Delbourg M, et al. Cross-reactivity between terrestrial snails (Helix species) and house dust mite (Dermatophagoides pteronyssinus). II. In vitro study. Allergy 53:151–158, 1998 [DOI] [PubMed] [Google Scholar]
- 77. Vuitton DA, Ranc e F, Paquin ML, et al. Cross-reactivity between terrestrial snails (Helix species) and house-dust mite (Dermatophagoides pteronyssinus). In vivo study. Allergy 53:144–150, 1998 [DOI] [PubMed] [Google Scholar]
- 78. Amoroso S, Cocchiara R, Locorotondo G, et al. Antigens of Euparipha pisana (snail). I. Identification of allergens by means of in vivo and in vitro analysis. Int Arch Allergy Appl Immunol 85:69–75, 1988 [PubMed] [Google Scholar]
- 79. Fernandes J, Reshef A, Patton L, et al. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy 33:956–961, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Bessot JC, Metz-Favre C, Rame JM, et al. Tropomyosin or not tropomyosin, what is the relevant allergen in house dust mite and snail cross allergies? Eur Ann Allergy Clin Immunol 42:3–10, 2010 [PubMed] [Google Scholar]
- 81. de Maat-Bleeker F, Akkerdaas JH, van Ree R, et al. Vineyard snail allergy possibly induced by sensitization to-housedust mite (Dermatophagoides pteronyssinus). Allergy 50:438–440, 1995 [DOI] [PubMed] [Google Scholar]
- 82. Azofra J, Lombardero M. Limpet anaphylaxis: cross-reactivity between limpet and house-dust mite Dermatophagoides pteronyssinus. Allergy 58:146–149, 2003 [DOI] [PubMed] [Google Scholar]
- 83. Martins LM, Peltre G, da Costa Faro CJ, et al. The Helix aspersa (Brown garden snail) allergen repertoire. Int Arch Allergy Immunol 136:7–15, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Caiado J, Lundberg M, Pedro E, et al. Snail allergy without house dust mite sensitization. Allergol Immunopathol (Madr) 37:107–108, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Pollart SM, Chapman MD, Fiocco GP, et al. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J Allergy Clin Immunol 83:875–882, 1989 [DOI] [PubMed] [Google Scholar]
- 86. Jeong KY, Lee IY, Lee J, et al. Effectiveness of education for control of house dust mites and cockroaches in Seoul, Korea. Korean J Parasitol 44:73–79, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Appel AG, Smith LM., II Biology and management of the smokybrown cockroach. Annu Rev Entomol 47:33–55, 2002 [DOI] [PubMed] [Google Scholar]
- 88. Birnbaum J, Orlando JP, Charpin D, et al. Cockroaches and mites share the same beds. J Allergy Clin Immunol 96:561–562, 1995 [DOI] [PubMed] [Google Scholar]
- 89. Witteman AM, van den Oudenrijn S, van Leeuwen J, et al. IgE antibodies reactive with silverfish, cockroach and chironomid are frequently found in mite-positive allergic patients. Int Arch Allergy Immunol 108:165–169, 1995 [DOI] [PubMed] [Google Scholar]
- 90. Mungan D, Celik G, Sin B, et al. Characteristic features of cockroach hypersensitivity in Turkish asthmatic patients. Allergy 53:870–873, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Tandon N, Maitra SB, Saha GK, et al. Role of cockroaches in allergy to house dust in Calcutta, India. Ann Allergy 64:155–157, 1990 [PubMed] [Google Scholar]
- 92. Kang BC, Chang JL, Johnson J. Characterization and partial purification of the cockroach antigen in relation to house dust and house dust mite (D.f.) antigens. Ann Allergy 63:207–212, 1989 [PubMed] [Google Scholar]
- 93. Kang BC, Wu CW, Johnson J. Characteristics and diagnoses of cockroach-sensitive bronchial asthma. Ann Allergy 68:237–244, 1992 [PubMed] [Google Scholar]
- 94. Barletta B, Butteroni C, Puggioni EM, et al. Immunological characterization of a recombinant tropomyosin from a new indoor source, Lepisma saccharina. Clin Exp Allergy 35:483–489, 2005 [DOI] [PubMed] [Google Scholar]
- 95. Galindo PA, Lombardero M, Mur P, et al. Patterns of immunoglobulin E sensitization to chironomids in exposed and unexposed subjects. J Investig Allergol Clin Immunol 9:117–122, 1999 [PubMed] [Google Scholar]
- 96. Eriksson NE, Ryden B, Jonsson P. Hypersensitivity to larvae of chironomids (non-biting midges). Cross-sensitization with crustaceans. Allergy 44:305–313, 1989 [DOI] [PubMed] [Google Scholar]
- 97. Yamashita N, Ito K, Miyamoto T, et al. Allergenicity of Chironomidae in asthmatic patients. Ann Allergy 63:423–426, 1989 [PubMed] [Google Scholar]
- 98. Morsy TA, Saleh WA, Farrag AM, et al. Chironomid potent allergens causing respiratory allergy in children. J Egypt Soc Parasitol 30:83–92, 2000 [PubMed] [Google Scholar]
- 99. Hirabayashi K, Kubo K, Yamaguchi S, et al. Studies of bronchial asthma induced by chironomid midges (Diptera) around a hypereutrophic lake in Japan. Allergy 52:188–195, 1997 [DOI] [PubMed] [Google Scholar]
- 100. Santos AB, Rocha GM, Oliver C, et al. Crossreactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol 121:1040–1046, 2008 [DOI] [PubMed] [Google Scholar]
- 101. William R. Pearson and the University of Virginia FASTA Program. Available online at fasta.bioch.virginia.edu/fasta_www2/fasta_www.cgi; accessed March 12, 2012
- 102. UniProt Identifiers. Available online at www.uniprot.org/align; accessed March 3, 2012
- 103. Schein CH, Ivanciuc O, Midoro-Horiuti T, et al. An allergen portrait gallery: Representative structures and an overview of IgE binding surfaces. Bioinform Biol Insights 11:113–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aalberse RC, Stadler BM. In silico predictability of allergenicity: From amino acid sequence via 3D structure to allergenicity. Mol Nutr Food Res 50:625–627, 2006 [DOI] [PubMed] [Google Scholar]
- 105. Bonds RS, Midoro-Horiuti T, Goldblum R. A structural basis for food allergy: the role of cross-reactivity. Curr Opin Allergy Clin Immunol 8:82–86, 2008 [DOI] [PubMed] [Google Scholar]
- 106. Smith W, Mills KL, Hazell LA, et al. Molecular analysis of the group 1 and 2 allergens from the house dust mite, Euroglyphus maynei. Int Arch Allergy Immunol 118:15–22, 1999 [DOI] [PubMed] [Google Scholar]
- 107. Breiteneder H, Mills ENC. Structural bioinformatic approaches to understand cross-reactivity. Mol Nutr Food Res 50:628–632, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Brusic V, Petrovsky N. Bioinformatics for characterisation of allergens, allergenicity and allergic crossreactivity. Trends Immunol 24:225–228, 2003 [DOI] [PubMed] [Google Scholar]
- 109. Furmonaviciene R, Sutton BJ, Glaser F, et al. An attempt to define allergen-specific molecular surface features: A bioinformatic approach. Bioinformatics 21:4201–4204, 2005 [DOI] [PubMed] [Google Scholar]
- 110. Hileman RE, Silvanovich A, Goodman RE, et al. Bioinformatic methods for allergenicity assessment using a comprehensive allergen database. Int Arch Allergy Appl Immunol 128:280–291, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Schein CH, Ivanciuc O, Braun W. Bioinformatics approaches to classifying allergens and predicting cross-reactivity. Immunol Allergy Clin 27:1–27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Thomas K, Bannon G, Hefle S, et al. In silico methods for evaluating human allergenicity to novel proteins. International Bioinformatics Workshop Meeting Report, February 23–24, 2005. Toxicol Sci 88:307–310, 2005 [DOI] [PubMed] [Google Scholar]
- 113. Chan SL, Ong ST, Ong SY, et al. Nuclear magnetic resonance structure-based epitope mapping and modulation of dust mite group 13 allergen as a hypoallergen. J Immunol 176:4852–4860, 2006 [DOI] [PubMed] [Google Scholar]


