Summary
Major strigolactones produced by rice (Oryza sativa L.) and tobacco (Nicotiana tabacum L.) were purified and their stereochemical structures were determined definitely by comparing with optically pure synthetic standards for spectroscopic data.
Key words: germination stimulant, Orobanche, rice, root parasitic plant, Striga, strigolactone, tobacco
Abstract
Major strigolactones (SLs) produced by rice (Oryza sativa L. cv. Nipponbare) and tobacco (Nicotiana tabacum L. cv. Michinoku No. 1) were purified and their stereochemical structures were determined by comparing with optically pure synthetic standards for their NMR and CD data and retention times and mass fragmentations in ESI–LC/MS and GC–MS. SLs purified from root exudates of rice plants were orobanchol, orobanchyl acetate, and ent-2’-epi-5-deoxystrigol. In addition to these SLs, 7-oxoorobanchyl acetate and the putative three methoxy-5-deoxystrigol isomers were detected by LC–MS/MS. The production of 7-oxoorobanchyl acetate seemed to occur in the early growth stage, as it was detected only in the root exudates collected during the first week of incubation. The root exudates of tobacco contained at least 11 SLs, including solanacol, solanacyl acetate, orobanchol, ent-2’-epi-orobanchol, orobanchyl acetate, ent-2’-epi-orobanchyl acetate, 5-deoxystrigol, ent-2’-epi-5-deoxystrigol, and three isomers of putative didehydro-orobanchol whose structures remain to be clarified. Furthermore, two sorgolactone isomers but not sorgolactone were detected as minor SLs by LC–MS/MS analysis. It is intriguing to note that rice plants produced only orobanchol-type SLs, derived from ent-2’-epi-5-deoxystrigol, but both orobanchol-type and strigol-type SLs, derived from 5-deoxystrigol were detected in tobacco plants.
Introduction
In the rhizosphere, strigolactones (SLs) released from plant roots induce seed germination of devastating root parasitic weeds, witchweeds (Striga spp.), broomrapes (Orobanche and Phelipanche spp.), and Alectra spp., and spore germination and hyphal branching of beneficial arbuscular mycorrhizal (AM) fungi. Therefore, SLs initiate both parasitic and symbiotic interactions between plants and these obligate heterotrophs (Bouwmeester et al., 2007; Xie et al., 2010). In addition to their roles in the rhizosphere, SLs have been shown to function in planta as a novel class of plant hormones regulating shoot branching (Gomez-Roldan et al., 2008; Umehara et al., 2008). An emerging line of evidence suggests that SLs are involved in the regulation of root architecture (Koltai, 2011; Ruyter-Spira et al., 2011), photomorphogenesis (Tsuchiya et al., 2010), secondary growth (Agusti et al., 2011), and rhizobia colonization (Soto et al., 2010; Foo and Davies, 2011). Furthermore, SLs may inhibit growth of breast cancer cells (Pollock et al., 2012).
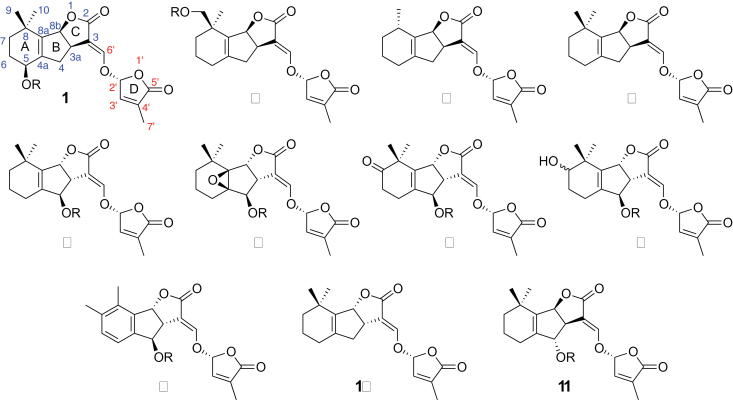
All natural SLs contain a tricyclic lactone core (the ABC-part) which connects via an enol ether bond to a butenolide moiety (the D-ring) (Figure 1). The common structural feature, the C–D part, is preserved in all natural SLs which carry different substituents on the A- and B-rings (Zwanenburg et al., 2009; Xie et al., 2010). To date, more than 20 SLs have been characterized from root exudates of various plant species. The substituents on the A- and B-rings are methyl, hydroxyl, and acetyloxyl groups and their combinations, and epoxy- and oxo-SLs have also been identified (Xie et al., 2010). In addition, methoxy-SLs have been detected in rice (Jamil et al., 2011a, 2011b).
Figure 1.
Structures of Natural Strigolactones.
These natural SLs can be classified into two groups according to the orientation of the C-ring. Strigol (1, R = H), sorgomol (2, R = H), sorgolactone (3), and 5-deoxystrigol (4) are strigol-type SLs, as they have a β-oriented C-ring. Strigol-type SLs appear to be derived from 5-deoxystrigol. The SLs carrying an α-oriented C-ring belong to orobanchol-type SLs. This group includes orobanchol (5, R = H), fabacol (6, R = H) (X. Xie et al., unpublished results), 7-oxoorobanchol (7, R = H), 7-hydroxyorobanchol (8, R = H), and solanacol (9, R = H), and are derived from ent-2’-epi-5-deoxystrigol (10) whose spectroscopic data have not yet been reported. Since rice (Oryza sativa L.) has been reported to produce orobanchol (5, R = H), orobanchyl acetate (5, R = Ac) and (ent)-2’-epi-5-deoxystrigol (Umehara et al., 2008 , 2010; Jamil et al., 2011a, 2011b), this plant is a good source for orobanchol-type SLs.
In a previous study, we found that the root exudates of tobacco (Nicotiana tabacum L.) contained at least five different germination stimulants including three SLs, solanacol (9, R = H), orobanchol (5, R = H), and 2’-epi-orobanchol (Xie et al., 2007). However, these assignments should be reexamined, since several novel SLs have subsequently been characterized (Xie et al., 2010), and, furthermore, the structure of orobanchol has recently been revised (Ueno et al., 2011; Vurro and Yoneyama, 2012).
To avoid confusion, we would like to add a short explanation for the revision of the structure for orobanchol (5, R = H). Orobanchol was first isolated as a novel germination stimulant for the root parasitic plant Orobanche minor (Yokota et al., 1998). A structure was proposed for this stimulant by comparing with synthetic standards for their NMR and retention times of the corresponding TMS ethers in GC–MS (Mori et al., 1999). However, NMR spectra of orobanchol and its 2’-epi isomer were almost identical and the retention times in GC–MS were virtually the same. An incorrect structure was thus assigned for orobanchol, as it was supposed to have the ABC-ring configuration similar to that of strigol. We have provided an orobanchol standard, which had been purified from red clover root exudates for many researchers upon their request, and there have been many reports on the identification of orobanchol, by using the standard, from plant root exudates, plant tissues, etc. Accordingly, we propose to call this stimulant orobanchol and to revise its structure as shown in Figure 1.
Hence, in the present study, major SLs produced by rice (Oryza sativa L. cv. Nipponbare) and tobacco plants (Nicotiana tabacum L. cv. Michinoku No. 1) were purified and their stereochemical structures were determined by comparing them with optically pure synthetic standards for their NMR and CD data and retention times and mass fragmentations in ESI–LC/MS and GC–MS.
RESULTS AND DISCUSSION
Characterization of Strigolactones Produced by Rice
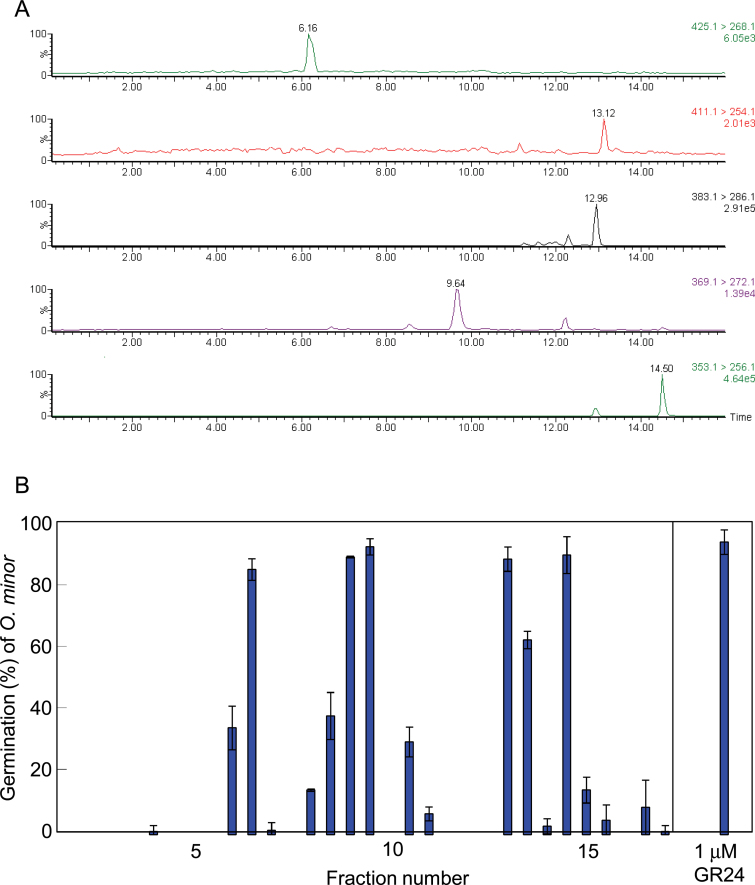
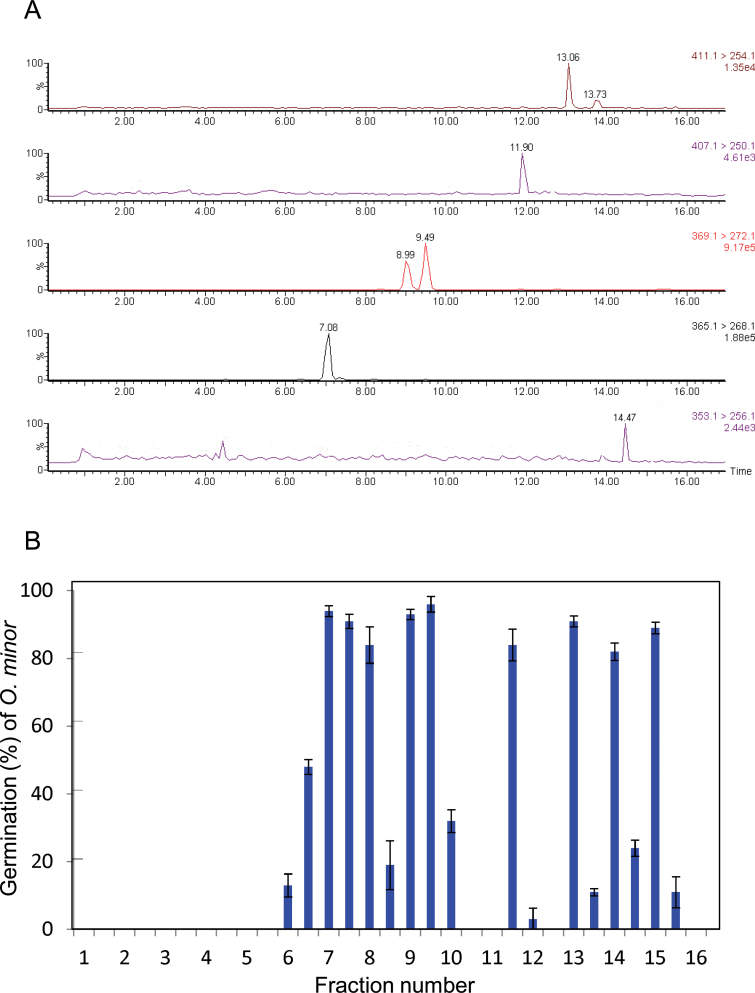
The ethyl acetate extract of root exudates collected from rice plants during the first week of hydroponic culture was found to contain 7-oxoorobanchyl acetate (7, R = Ac) and three methoxy-SLs in addition to the three SLs, orobanchol, orobanchyl acetate, and 2’-epi-5-deoxystrigol, by LC–MS/MS analysis and germination stimulation activities on O. minor seeds were accompanied by these peaks (Figure 2). Then, ethyl acetate extract of root exudates collected from about 1000 rice seedlings was purified by bioassay-guided fractionations to obtain pure SLs.
Figure 2.
Characterization of Strigolactones Exuded by Rice Plants.(A) Five-channel multiple reaction monitoring (MRM) chromatogram of rice root exudate, where the transitions of m/z 425 > 268, 411 > 254, 383 > 286, 369 > 272, and 353 > 256 were monitored for 7-oxoorobanchyl acetate, orobanchyl acetate, methoxy-5-deoxystrigol, orobanchol, and 5-deoxystrigol and their isomers, respectively.(B) Distribution of germination stimulation activity on Orobanche minor after reversed-phase high-performance liquid chromatography (RP–HPLC) separation of the root exudate. Data are means ± SE (n = 3).
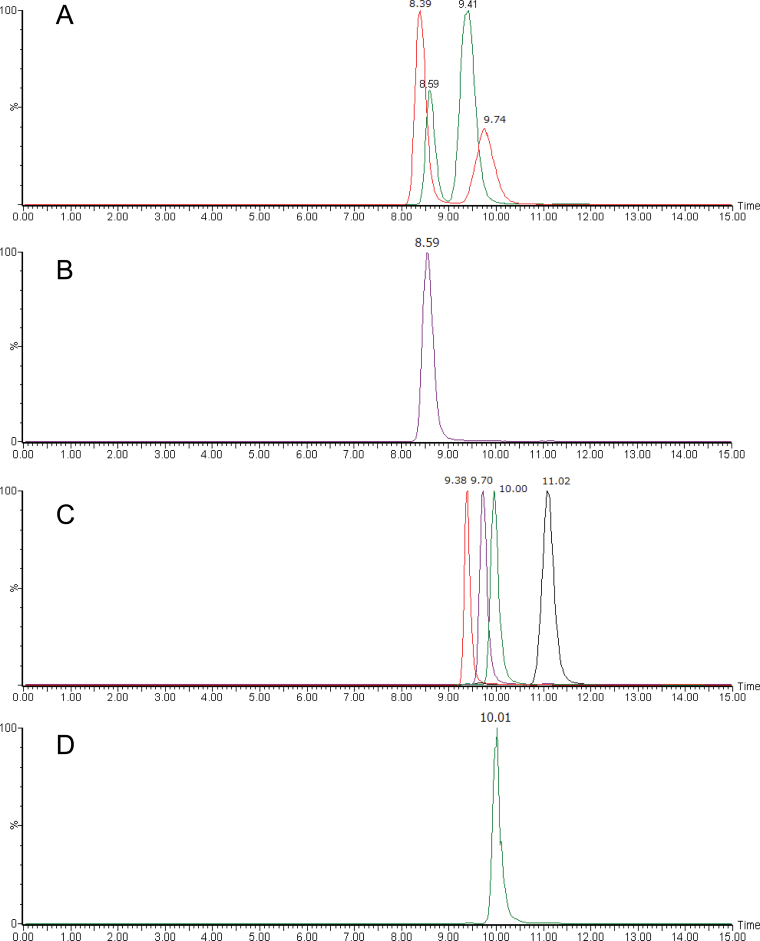
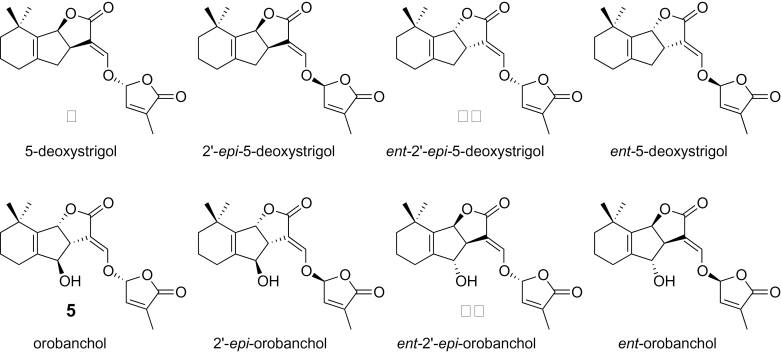
To confirm the stereochemistry of isolated SLs, purified samples were analyzed by chiral–LC–MS/MS. The four stereoisomers each (for structures, see Figure 3) of orobanchol (Figure 4A) and 5-deoxystrigol (Figure 4B) could be separated clearly by the chiral–LC–MS/MS under the conditions employed in this study. These results demonstrated that rice plant (Nipponbare) produces orobanchol (5, R = H) and ent-2’-epi-5-deoxystrigol (10) but not their stereoisomers at least at detectable levels. Similar results were obtained for stereochemistry of orobanchyl acetate while its isolated amount was not enough for 1H-NMR or CD measurement (data not shown). The assignments of stereochemistry of orobanchol (5, R = H) and ent-2’-epi-5-deoxystrigol (10) were also confirmed with their CD spectra (Supplemental Figure 1). The amount of 7-oxoorobanchyl acetate (7, R = Ac) was not enough to obtain 1H-NMR and CD spectra but we propose to revise its stereochemistry as in Figure 1 based on the stereochemistry of orobanchol (5, R = H). This would be confirmed when organic synthesis of 7-oxoorobanchyl acetate (7, R = Ac) is achieved. Consequently, rice plant (Nipponbare) has been confirmed to produce 7-oxoorobanchyl acetate (7, R = Ac), orobanchol (5, R = H), orobanchyl acetate (5, R = Ac), and ent-2’-epi-5-deoxystrigol (10) as major SLs. Since 7-oxoorobanchyl acetate (7, R = Ac) was detected only in the root exudates collected during the first week of incubation, this SL seemed to be produced in the early growth stage.
Figure 3.
Stereoisomers of 5-Deoxystrigol and Orobanchol.
Figure 4.
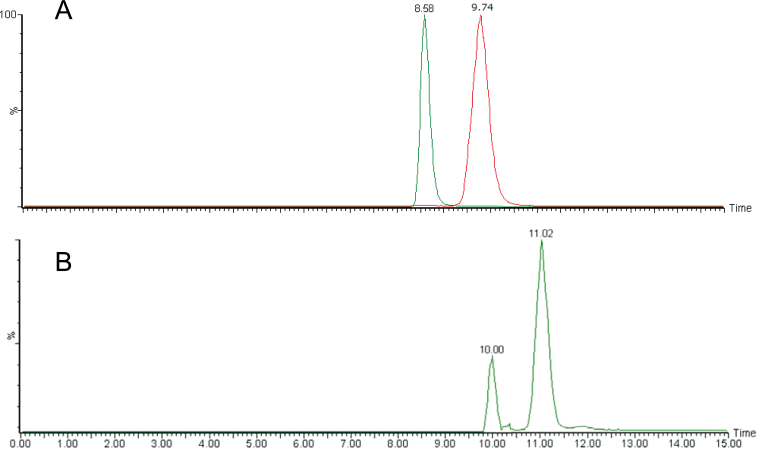
Chiral–LC–MS/MS Analyses of Orobanchol and 5-Deoxystrigol Isomers Isolated from Rice Root Exudate.(A) Synthetic standards of orobanchol (Rt: 8.59min), ent-orobanchol (Rt: 9.41min), 2’-epi-orobanchol (Rt: 8.39min), and ent-2’-epi-orobanchol (Rt: 9.74min).(B, D) Rice root exudate.(C) Synthetic standards of 5-deoxystrigol (Rt: 11.02min), ent-5-deoxystrigol (Rt: 9.70min), 2’-epi-5-deoxystrigol (Rt: 9.38min), and ent-2’-epi-5-deoxystrigol (Rt: 10.00min).
The three putative methoxy-SLs were detected in root exudates of rice cultivar Nipponbare and also in NERICA rice cultivars (Jamil et al., 2011a, 2011b), but amounts obtained in the present study were not enough for their structural elucidation.
Characterization of Strigolactones Produced by Tobacco
As reported previously, burley tobacco Michinoku No. 1 has been shown to produce at least five germination stimulants including three SLs, solanacol (9, R = H), 2’-epi-orobanchol, and orobanchol (5, R = H) (Xie et al., 2007). Although 2’-epi-orobanchol was the first 2’-epi-SL, its stereochemistry should be reexamined, as the structure of orobanchol (5, R = H) has been revised (Ueno et al., 2011). In this study, ethyl acetate extract of root exudates collected from about 600 tobacco seedlings was subjected to bioassay-guided purifications to isolate pure germination stimulants. The crude extract appeared to contain at least seven SLs, as shown in Figure 5. LC–MS/MS analysis suggested that they were two orobanchyl acetate isomers (m/z 411 > 254), solanacyl acetate isomer (m/z 407 > 250), two orobanchol isomers (m/z 369 > 272), solanacol (m/z 365 > 268), and 5-deoxystrigol (m/z 353 > 256) (Figure 5A), and germination stimulation activities to seeds of O. minor were detected in the fractions corresponding to the retention times of these SLs (Figure 5B). The two orobanchol isomers were identified as orobanchol (5, R = H) and ent-2’-epi-orobanchol (11, R = H) by chiral–LC–MS/MS as shown in Figure 6A. The stereochemistries of the two orobanchyl acetate isomers were determined in a similar manner and they were orobanchyl acetate (5, R = Ac) and ent-2’-epi-orobanchyl acetate (11, R = Ac). The identity of solanacyl acetate (9, R = Ac) was confirmed by direct comparison of spectroscopic data and retention times and fragmentations in LC–MS and GC–MS with those of synthetic standard (Chen et al., 2010). Although separation of 5-deoxystrigol (4) from its 2’-epi-isomer on an ODS column was often not clear, particularly with crude samples, they could be separated well by chiral-HPLC as shown in Figure 4C. Since direct precursor of orobanchol (5, R = H) is not 5-deoxystrigol (4) but ent-2’-epi-5-deoxystrigol (10), the fraction after the first silica gel column chromatography (CC) containing 5-deoxystrigol was analyzed by chiral–LC–MS/MS and this tobacco cultivar was found to produce both 5-deoxystrigol (4) and ent-2’-epi-5-deoxystrigol (10) (Figure 6B). In addition to these eight SLs, small amounts of three putative didehydro-orobanchol isomers were detected by LC–MS/MS. Furthermore, two sorgolactone isomers but not sorgolactone were detected as minor SLs by LC–MS/MS analysis (data not shown).
Figure 5.
Characterization of Strigolactones Exuded by Tobacco Plants.(A) Five-channel multiple reaction monitoring (MRM) chromatogram of tobacco root exudate, where the transitions of m/z 411 > 254, 407 > 250, 369 > 272, 365 > 268, and 353 > 256 were monitored for orobanchyl acetate, solanacyl acetate, orobanchol, solanacol, and 5-deoxystrigol and their isomers, respectively.(B) Distribution of germination stimulation activity on Orobanche minor after reversed-phase high-performance liquid chromatography (RP–HPLC) separation of the root exudate. Data are means ± SE (n = 3).
Figure 6.
Chiral–LC–MS/MS Analyses of Orobanchol and 5-Deoxystrigol Isomers Isolated from Tobacco Root Exudate.(A) Combined MRM chromatogram monitoring orobanchol and its isomers. Orobanchol (Rt: 8.58min) and ent-2’-epi-orobanchol (Rt: 9.74min) were separately analyzed by chiral–LC–MS/MS and chromatograms were combined.(B) MRM chromatogram monitoring 5-deoxystrigol isomers. The peaks correspond to 5-deoxystrigol (Rt: 11.02min) and ent-2’-epi-5-deoxystrigol (Rt: 10.00min). Retention times of these stereoisomers are shown in Figure 4.
Consequently, in the present study, the burley tobacco cultivar Michinoku No. 1 has been shown to produce 11 SLs. In the previous study (Xie et al., 2007), we could not detect didehydro-orobanchol isomers (stimulant B) in the root exudates from this tobacco cultivar, possibly due to the differences in growth conditions. The stimulants A, C, D, E, and F described in the previous report (Xie et al., 2007) are solanacol (9, R = H), ent-2’-epi-orobanchol (11, R = H), orobanchol (5, R = H), solanacyl acetate (9, R = Ac), and orobanchyl acetate (5, R = Ac) (+ ent-2’-epi-orobanchyl acetate), respectively. 5-Deoxystrigol (4) and ent-2’-epi-5-deoxystrigol (10) were not detected in the previous study, as they are more lipophilic than sorgolactone, which was the most lipophilic SLs known at that time. Therefore, these SLs could have been eluted after we stopped LC–MS/MS analyses in the previous study.
In the present study, we have conducted the most comprehensive characterization of SLs produced by rice and tobacco plants. As mentioned earlier, natural SLs can be classified into two groups based on the orientation of the C-ring: orobanchol-type SLs with α-oriented C-ring and strigol-type SLs with β-oriented C-ring. It is intriguing that rice produces only orobanchol-type SLs but tobacco produces both types. In addition, it should be noted that natural SLs identified so far have 2’(R) configuration, as both 2’-epi-orobanchol (11, R = H) and 2’-epi-5-deoxystrigol (10) are ent-SLs. However, we are not sure whether plants produce only 2’(R)-SLs. They may also produce very low levels of 2’(S)-SLs. Further study is needed to determine roles of stereochemistry of SLs in their biological functions.
METHODS
Plant Material and Chemicals
Orobanche minor Sm. seeds were collected from mature plants that parasitized red clover (Trifolium pratense L.) in the Watarase basin of Tochigi Prefecture, Japan. Seeds of rice (Oryza sativa cv. Nipponbare) were obtained from a local supplier and seeds of tobacco (Nicotiana tabacum L. cv. Michinoku No. 1) were generous gifts of Japan Tobacco Inc.
The synthetic standards of four stereoisomers of orobanchol and four stereoisomers of 5-deoxystrigol were prepared according to the literature (Mori et al., 1999; Akiyama et al., 2005, 2010). Solanacol (9, R = H) and solanacyl acetate (9, R = Ac) were kindly provided by Dr François-Didier Boyer (INRA/CNRS, France). Other chemicals of analytical grade and HPLC solvents were obtained from Kanto Chemical Co. Ltd (Tokyo, Japan) and Wako Pure Chemical Industries Ltd (Tokyo, Japan).
Instrumentation
HPLC analysis and purification were conducted on a Hitachi L-2130 low-pressure gradient system and a Hitachi L-7100 high-pressure gradient system equipped with a UV and PDA detector, respectively. 1H-NMR spectra were recorded in CDCl3 (δH 7.26) on a JEOL JMN-ECA-500 spectrometer. Optical rotation of ent-2’-epi-5-deoxystrigol was measured with a JASCO P-1010 polarimeter (the amounts of other SLs purified in this study were not enough for optical rotation measurement). CD spectra were recorded with a JASCO J-720W spectropolarimeter in acetonitrile (MeCN). EI–GC/MS spectra were obtained with a JOEL JMS-Q1000GC/K9 on a DB-5 (J&W Scientific, Agilent) capillary column (5 m × 0.25 mm) using a He carrier gas (3 ml min–1). The operating conditions were the same as reported earlier (Yokota et al., 1998). ESI–LC–MS/MS analyses were performed using a Quattro LC tandem MS instrument from Micromass (Manchester, UK). Wakogel C-300 (Wako Pure Chemical Industries Ltd) was used for silica CC. The ODS (C18) columns (Mightysil RP-18, 10 × 250 mm, 10 μm; 4.6 × 250 mm, 5 μm; 2.1 × 250 mm, 5 m; Kanto Chemical Co. Ltd) and a CN column (Develosil CN-UG-5, 4.6 × 250 mm, 5 μm; Nomura Chemical Co. Ltd, Seto, Japan) were used for preparative and analytical HPLC.
Growth Conditions and Collection of Root Exudates
Rice seeds (cv. Nipponbare) were surface-sterilized in 70% ethanol for 2 min and then 1% sodium hypochlorite for 3 min. After being thoroughly rinsed with sterile distilled water, the seeds were soaked in water at 23°C for 4 d. Germinated seeds (n = 10) were transferred to a strainer (28×23×9 cm, width × length × height (W × L × H)) lined with a sheet of gauze moistened by placing it in a slightly larger container (28.5×23.5×11 cm, W × L × H) containing 1 L of tap water as the culture medium in a growth chamber with a 14/10-h photoperiod at 120 μmol photons m–2 s–1 at 30/24°C. Rice plants were grown in tap water for 10 d and then the two strainers were transferred to a larger container (53.5×33.5×14 cm, W × L × H) containing 12 L of half-strength Tadano and Tanaka medium (Tadano and Tanaka, 1980) without phosphate. Culture media were refreshed every 3 d, and root exudates released into culture medium were adsorbed by activated charcoal using circulation pumps (Akiyama et al., 2005; Yoneyama et al., 2008).
Tobacco seeds (cv. Michinoku No. 1) were surface-sterilized in 70% ethanol for 1 min and then in 1% sodium hypochlorite for 1min. Approximately 100 seedlings growing in a 5-cm layer of rock wool were transferred to a strainer (28×23×9cm, W × L × H) lined with a sheet of gauze moistened by placing it in a slightly larger container (28.5×23.5×11cm, W × L × H) containing 1 L of tap water and supplied with a 1000-fold diluted liquid fertilizer (Hyponex, Hyponex Japan, Osaka) as required in a glass house maintained at 22–28°C under natural daylight conditions. In total, six strainers were used to collect root exudates. Twenty-one days after sowing, the two strainers were transferred to a larger container (53.5×33.5×14cm, W × L × H) containing 10 L of tap water and 1 mM CaCl2. The root exudates released into culture medium were adsorbed by activated charcoal using circulation pumps as described before. The tap water media and charcoal were exchanged every 3 d.
The root exudates adsorbed on the charcoal were eluted with acetone. After the acetone was evaporated in vacuo, the residue was dissolved in 50ml of water and extracted three times with 50ml of ethyl acetate. The ethyl acetate extracts were combined, washed with 0.2M K2HPO4 (pH 8.3), dried over anhydrous MgSO4, and concentrated in vacuo. These crude extracts were stored in sealed glass vials at 4°C until use.
Germination Assay
Germination assays using O. minor seeds were conducted as reported previously (Yoneyama et al., 2008). Temperature for conditioning and germination was 23°C. Each test solution, unless otherwise mentioned, contained 0.1% (v/v) acetone.
Strigolactone Detection and Identification by LC–MS/MS
Identification of strigolactones was performed using a tandem mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ionization (ESI) source and coupled to a UPLC system (Hitachi, Japan). Chromatographic separation was achieved on an ODS column (L-Column2 ODS, 2.1×50mm, 2.0 µm; CERI, Japan) by applying a methanol–water (MeOH–H2O) gradient to the column, starting from 30% methanol and rising to 45% methanol at 3.0min, followed by a 5-min gradient to 50% methanol, followed by a 4-min gradient to 70% methanol, followed by a 3-min gradient to 100% methanol, which was maintained for 3min, before going back to 30% methanol using a 1-min gradient, prior to the next run. Finally, the column was equilibrated for 3min, using this solvent composition. The column was operated at 40°C with a flow rate of 0.2ml min–1. Separation of stereoisomers was achieved on isocratic (99% MeOH/H2O) UPLC on a chiral column (CHIRAL-PACK AD-3R, 2.1×150mm, 3.0 µm; DAICEL Corp., Japan) at a flow rate of 0.2ml min–1 and the column temperature was set to 30°C.
The drying and nebulizing gas was nitrogen generated from pressurized air in an N2G nitrogen generator (Parker-Hanifin Japan, Tokyo, Japan). The nebulizer gas flow was set to approximately 100 L h–1 and the desolvation gas flow to 500 L h–1. The interface temperature was set to 400°C and the source temperature to 150°C. The capillary and cone voltages were adjusted to orobanchol and to the positive ionization mode. MS/MS experiments were conducted using argon as the collision gas and the collision energy was set to 16 eV. The collision gas pressure was 0.15 Pa. The mass spectrometer was operated in positive ESI mode. Sample injection volume was 5 µl. Multiple reaction monitoring (MRM) was used for identification of SLs in root exudates and extracts by comparing retention times with synthetic and natural SL standards.
Isolation and Identification of ent-2′-epi-5-Deoxystrigol (10) and Orobanchol (5, R = H) from Rice Root Exudates
The crude extract (398 mg) of root exudates collected from in total about 1000 seedlings of rice (Nipponbare) grown hydroponically was fractionated by silica gel CC (20 × 250mm) using a gradient of n-hexane/ethyl acetate (100:0–0:100, 10% step) to give fractions 1–11. All fractions were monitored by LC–MS/MS to detect ent-2′-epi-5-deoxystrigol (10) and orobanchol (5, R = H).
Fraction 4 (30% ethyl acetate) containing ent-2′-epi-5-deoxystrigol was concentrated (34.84 mg) and subjected to silica gel CC (15 × 100mm) using n-hexane/ethyl acetate (75:25) as the eluting solvent system. Fractions were collected every 10ml. Fractions 12–19 containing ent-2′-epi-5-deoxystrigol (10) were combined (10.43 mg) and was purified by isocratic (60% MeCN/H2O) HPLC on an ODS column (Mightysil RP-18, 10×250mm, 10 μm; Kanto Chemicals, Japan) at a flow rate of 3.0ml min–1 to give pure ent-2′-epi-5-deoxystrigol (10, 2.14mg, R t 22.7min, detection at 238 nm).
ent-2′-epi-5-Deoxystrigol:
1H-NMR (500MHz, CDCl3) δ: 1.09 (s, 3H, Me-10), 1.11 (s, 3H, 9-Me), 1.30–1.49 (m, 2H, 7-H), 1.61–1.70 (m, 2H, 6-H), 1.82–1.97 (m, 2H, 5-H), 2.02 (t, 3H, J=1.6Hz, 7′-H), 2.30 (d, 1H, J=16.8Hz, 4β-H), 2.67 (dd, 1H, J=8.8 and 16.8Hz, 4α-H), 3.55–3.59 (m, 1H, 3α-H), 5.51 (d, 1H, J=8.0Hz, 8β-H), 6.12 (m, 1H, 2′-H), 6.92 (m, 1H, 3′-H), 7.42 (m, 1H, 6′-H). [α]D 22 –159 (c 0.05, MeCN) ([α]D 32 –168 (c 0.0520, MeCN), Akiyama et al., 2010). CD (MeCN; c 0.00005) λmax (Δε) nm: 240.1 (–5.26), 215 (19.6).
Fraction 8 (70% ethyl acetate) containing orobanchol (5, R = H) was concentrated (22.17mg) and subjected to silica gel CC (15 × 100mm) using n-hexane/ethyl acetate (30:70) as the eluting solvent system. Fractions were collected every 10ml. Fractions 12–19 containing orobanchol were combined (10.43mg) and separated by preparative ODS–HPLC (Mightysil RP-18, 10×250mm, 10 µm; Kanto Chemicals, Japan) with the MeCN/H2O gradient system (30:70 to 0:100 over 40min) as eluent at a flow rate of 3.0ml min–1. The active fraction (2.92mg) eluted as a single peak at 15.4min (detection at 239 nm) was collected. This fraction was purified by isocratic (50% MeCN/H2O) HPLC on a Develosil CN column (4.6×250mm, 5 μm; Nomura Chemicals, Japan) at a flow rate of 1.0ml min–1 to give orobanchol (5, R = H, 1.03mg, R t 17.8min).
Orobanchol:
1H-NMR (500MHz, CDCl3) δ: 1.13 (s, 3 H, 8-Me), 1.14 (s, 3 H, 8-Me), 1.38–1.51 (m, 2 H, 7-CH2), 1.68–1.72 (m, 2 H, 6-CH2), 1.94–1.99 (m, 1 H, 5-H), 2.03 (t, 3 H, J = 1.5 Hz, 4′-Me), 2.11–2.16 (m, 1 H, 5-H’), 3.41 (ddd, 1 H, J = 7.3, 2.7, and 1.7 Hz, 3α-H), 4.56 (d, 1 H, J = 6.2 Hz, 4-H), 5.61 (d, 1 H, J = 7.3 Hz, 8β-H), 6.18 (t, 1 H, J = 1.5 Hz, 2′-H), 6.97 (t, 1 H, J = 1.6 Hz, 3′-H), 7.52 (d, 1 H, J = 2.7 Hz, 6′-H). CD (MeCN; c 0.00005) λmax (Δε) nm: 260.1 (–1.19), 219 (13.5).
CD data of synthetic standards of orobanchol and its stereoisomers, and orobanchol and ent-2′-epi-5-deoxystrigol purified from rice root exudate are shown in Supplemental Figures 1 and 2, respectively.
Isolation and Identification of 5-Deoxystrigol (4), ent-2′-epi-5-Deoxystrigol (10), Orobanchol (5, R = H), ent-2′-epi-Orobanchol (11, R = H), Orobanchyl Acetate (5, R = Ac), ent-2′-epi-Orobanchyl Acetate (11, R = Ac), and Solanacyl Acetate (9, R = Ac) from Tobacco Root Exudates
The crude extract (352mg) collected from in total approximately 600 seedlings of tobacco grown hydroponically was fractionated by silica gel CC (20×250mm) using a gradient of n-hexane–ethyl acetate (100:0–0:100, 10% step) to give fractions 1–11. One-thousandth of each fraction was dissolved in 50 μl of 50% MeOH, filtered, and 5 μl was injected into the HPLC connected to the tandem mass spectrometry operated in the ESI positive mode. 5-Deoxystrigol (4) and ent-2′-epi-5-deoxystrigol (10) were detected by monitoring transition of m/z 353 to 256, orobanchol (5, R = H) and ent-2′-epi-orobanchol (11, R = H) by m/z 369 to 272, and orobanchyl acetate (5, R = Ac) and ent-2′-epi-orobanchyl acetate (11, R = Ac) by m/z 411 to 272.
Fraction 4, fractions 5 and 6, fraction 7, and fraction 8 were found to contain 5-deoxystrigol and its isomer, orobanchyl acetate and its isomer, solanacyl acetate, and orobanchol and its isomer, respectively. Each fraction was then purified successively by silica gel CC and ODS–HPLC monitored at 240nm. The peaks detected were collected and analyzed by LC–MS/MS. Identities of 5-deoxystrigol (4), ent-2′-epi-5-deoxystriol (10), orobanchol (5, R = H), ent-2′-epi-orobanchol (11, R = H), orobanchyl acetate (5, R = Ac), and ent-2′-epi-orobanchyl acetate (11, R = Ac) were confirmed by comparing retention times in ODS–HPLC–MS/MS, also by chiral–HPLC–MS/MS, and GC–MS with those of synthetic standards. The 1H-NMR and CD spectroscopic data of these compounds were identical to those reported in the literature.
Fraction 4 (30% ethyl acetate) containing 5-deoxystrigol (4) and its isomer were concentrated (10.84mg) and subjected to silica gel CC (15 × 100mm) using n-hexane/ethyl acetate (75:25) as the eluting solvent system. Fractions were collected every 10ml. Fractions 9–26 containing the two 5-deoxystrigol isomers were combined (2.43mg) and were purified by isocratic (50% MeCN/H2O) HPLC on an ODS column (Inertsil ODS-4, 4.6×250mm, 5 μm; GL Science, Japan) at a flow rate of 1.0ml min–1 to give pure 5-deoxystrigol (4, 0.87mg, R t 34.7min, detection at 238nm) and ent-2′-epi-5-deoxystrigol (10, 0.14mg, R t 32.4min, detection at 238nm).
Fractions 5 and 6 (40% and 50% ethyl acetate) containing orobanchyl acetate (5, R = Ac) and its isomer were concentrated (9.29mg) and subjected to silica gel CC (15 × 100mm) using n-hexane/ethyl acetate (35:65) as the eluting solvent system. Fractions were collected every 10ml. Fractions 8–12 and fractions 14–19 were found to contain an isomer of orobanchyl acetate and orobanchyl acetate, respectively, by LC–MS/MS analysis. Fractions 8–12 were combined (2.17mg) and were purified by a gradient with MeCN/H2O (50:50 to 100:0 over 40min) HPLC on an ODS column (Inertsil ODS-4, 4.6×250mm, 5 μm; GL Science, Japan) at a flow rate of 0.8ml min–1 to give pure ent-2′-epi-orobancyl acetate (11, R = Ac, 0.14mg, R t 21.4min, detection at 242nm). Fractions 14–19 were combined (3.73mg) and were purified by a gradient with MeCN/H2O (50:50 to 100:0 over 40min) HPLC on an ODS column (Inertsil ODS-4, 4.6×250mm, 5 μm; GL Science, Japan) at a flow rate of 0.8ml min–1 to give pure orobanchyl acetate (5, R = Ac, 0.26mg, R t 28.6min, detection at 242nm).
ent-2′-epi-Orobanchyl acetate:
1H-NMR (500MHz, CDCl3) δ: 1.14 (s, 3 H, 8-Me), 1.16 (s, 3 H, 8-Me), 1.39–1.52 (m, 2 H, 7-CH2), 1.65–1.72 (m, 2 H, 6-CH2), 1.86–1.97 (m, 1 H, 5-H), 2.30 (s, 3H, 9-Me), 2.37 (s, 3H, 10-Me), 3.42–3.44 (dd, 1H, J = 7.3, and 1.7 Hz, 3α-H), 5.62 (d, 1H, J = 7.3 Hz, 8β-H), 5.80 (s, 1H, 4-H), 6.13 (t, 1 H, J = 1.5 Hz, 2′-H), 6.97 (t, 1 H, J = 1.6 Hz, 3′-H), 7.58 (d, 1 H, J = 2.7 Hz, 6′-H).
Fraction 7 (60% ethyl acetate) containing solanacyl acetate (9, R = Ac) was concentrated (14.78mg) and subjected to silica gel CC (15 × 100mm) using n-hexane/ethyl acetate (75:25) as the eluting solvent system. Fractions were collected every 10ml. Fractions 21–29 containing solanacyl acetate (9, R = Ac) were combined (6.22mg) and separated by preparative ODS–HPLC (Inertsil ODS-4, 4.6×250mm, 5 μm; GL Science, Japan) with the MeCN/H2O gradient system (50:50 to 0:100 over 40min) as eluent at a flow rate of 1.0ml min–1. The active fraction (1.92mg) eluted as a single peak at 11.4min (detection at 238nm) was collected. This fraction was further purified by isocratic (70% MeCN/H2O) HPLC on a Develosil CN column (4.6×250mm, 5 μm; Nomura Chemicals, Japan) at a flow rate of 0.8ml min–1 to give solanacyl acetate (9, R = Ac, 0.23mg, R t 8.6min, detection at 254nm).
Solanacyl acetate:
1H-NMR (500 MHz, CDCl3) δ: 2.03 (s, 3H, 4-H), 2.05 (t, 3H, J = 1.5 Hz, 4’-Me), 2.30 (s, 3H, 9-Me), 2.37 (s, 3H, 10-Me), 3.86–3.88 (ddd, 1H, J = 7.3, 3.4 and 2.0 Hz, 3α-H), 6.17 (d, 1H, J = 7.3 Hz, 8β-H), 6.20 (t, 1H, J = 1.5 Hz, 2’-H), 6.35 (s, 1H, 4-H), 6.98 (t, 1H, J = 1.5 Hz, 3’-H), 7.14 and 7.21 (AB quartet, 2H, J = 7.8 Hz, 5-H, 6-H), 7.49 (d, 1H, J = 2.4 Hz, 6′-H). CD (MeCN; c 0.00005) λmax (Δε) nm: 221 (6.67), 241 (–6.33). GC–EIMS, 70eV, m/z (rel. int.): 384 [M]+ (1), 342 (16), 245 (4), 227 (100), 184 (25), 97 (78).
Fractions 8 and 9 (70% and 80% ethyl acetate) containing orobanchol (5, R = H) and its isomer were concentrated (15.15mg) and subjected to silica gel CC (15 × 100mm) using n-hexane/ethyl acetate (40:60) as the eluting solvent system. Fractions were collected every 5ml. Fractions 25–46 and fractions 55–61 were found to contain an isomer of orobanchol and orobanchol (5, R = H), respectively, by LC–MS/MS analysis. Fractions 25–46 were combined (3.32mg) and were purified by gradient with MeCN/H2O (30:70 to 100:0 over 40min) HPLC on an ODS column (Inertsil ODS-4, 4.6×250mm, 5 μm; GL Science, Japan) at a flow rate of 1.0ml min–1 to give pure ent-2′-epi-orobanchol (11, R = H, 0.74mg, R t 14.7min, detection at 240nm). Fractions 55–61 were combined (2.43mg) and were purified by gradient with MeCN/H2O (30:70 to 100:0 over 40min) HPLC on an ODS column (Inertsil ODS-4, 4.6×250mm, 5 μm; GL Science, Japan) at a flow rate of 1.0ml min–1 to give pure orobanchol (5, R = H 0.62mg, R t 12.1min, detection at 240nm).
ent-2′-epi-Orobanchol:
1H-NMR (500MHz, CDCl3) δ: 1.13 (s, 3 H, 8-Me), 1.14 (s, 3 H, 8-Me), 1.38–1.51 (m, 2 H, 7-CH2), 1.68–1.72 (m, 2 H, 6-CH2), 1.94–1.99 (m, 1 H, 5-H), 2.03 (t, 3 H, J = 1.5 Hz, 4′-Me), 2.11–2.16 (m, 1 H, 5-H′), 3.41 (ddd, 1 H, J = 7.3, 2.4, and 1.7 Hz, 3α-H), 4.58 (d, 1 H, J = 6.2 Hz, 4-H), 5.61 (d, 1 H, J = 7.3 Hz, 8β-H), 6.21 (t, 1 H, J = 1.5 Hz, 2′-H), 6.95 (t, 1 H, J = 1.6 Hz, 3′-H), 7.47 (d, 1 H, J = 2.7 Hz, 6′-H). CD (MeCN; c 0.00005) λmax (Δε) nm: 204.1 (–27.9), 226.1 (39.4).
SUPPLEMENTARY DATA
Supplementary data are available at Molecular Plant Online.
FUNDING
This work was supported by the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry and KAKENHI (18208010, 23380061) from the Japan Society of the Promotion of Science (JSPS).
Supplementary Material
Acknowledgments
We thank F.-D. Boyer (INRA/CNRS, France) for his kind gifts of solanacol and its derivatives. No conflict of interest declared.
References
- Agusti J., et al. (2011). Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl Acad. Sci. U S A. 108, 20242–20247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K., Matsuzaki K., Hayashi H. (2005). Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 435, 824–827 [DOI] [PubMed] [Google Scholar]
- Akiyama K., Ogasawara S., Ito S., Hayashi H. (2010). Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 51, 1104–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H.J., Roux C., Lopez-Raez J.A., Bécard G. (2007). Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 12, 224–230 [DOI] [PubMed] [Google Scholar]
- Chen V.X., Boyer F.-D., Rameau C., Retailleau P., Vors J.-P., Beau J.-M. (2010). Stereochemistry, total synthesis, and biological evaluation of the new plant hormone solanacol. Chem. Eur. J. 16, 13941–13945 [DOI] [PubMed] [Google Scholar]
- Foo E., Davies N.W. (2011). Strigolactones promote nodulation in pea. Planta. 234, 1073–1081 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V., et al. (2008). Strigolactone inhibition of shoot branching. Nature. 455, 189–194 [DOI] [PubMed] [Google Scholar]
- Jamil M., et al. (2011a). Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Res. 51, 373–385 [Google Scholar]
- Jamil M., Rodenburg J., Charnikhova T., Bouwmeester H.J. (2011b). Pre-attachment Striga hermonthica resistance of New Rice for Africa (NERICA) cultivars based on low strigolactone production. New Phytol. 192, 964–975 [DOI] [PubMed] [Google Scholar]
- Koltai H. (2011). Strigolactones are regulators of root development. New Phytol. 190, 545–549 [DOI] [PubMed] [Google Scholar]
- Mori K., Matsui J., Yokota T., Sakai H., Bando M., Takeuchi Y. (1999). Structure and synthesis of orobanchol, the germination stimulant for Orobanche minor . Tetrahedron Lett. 40, 943–946 [Google Scholar]
- Pollock C.B., Koltai H., Kapulnik Y., Prandi C., Yarden R.I. (2012). Strigolactones: a novel class of phytohormones that inhibit the growth and survival of breast cancer cells and breast cancer stem-like enriched mammosphere cells. Breast Cancer Res. Treat. 134, 1041–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C., et al. (2011). Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones?. Plant Physiol. 155, 721–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M.J., et al. (2010). First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 42, 383–385 [Google Scholar]
- Tadano T., Tanaka A. (1980). The effect of low phosphate concentrations in culture medium on early growth of several crop plants (in Japanese, translated by the authors). Jpn. J. Soil Sci. Plant Nutr. 51, 399–404 [Google Scholar]
- Tsuchiya Y., et al. (2010). A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat. Chem. Biol. 6, 741–749 [DOI] [PubMed] [Google Scholar]
- Ueno K., Nomura S., Muranaka S., Mizutani M., Takikawa H., Sugimoto Y. (2011). Ent-2’-epi-orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J. Agric. Food Chem. 59, 10485–10490 [DOI] [PubMed] [Google Scholar]
- Umehara M., et al. (2008). Inhibition of shoot branching by new terpenoid plant hormones. Nature. 455, 195–200 [DOI] [PubMed] [Google Scholar]
- Umehara M., Hanada A., Magome H., Takeda-Kamiya N., Yamaguchi S. (2010). Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 51, 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurro M., Yoneyama K. (2012). Strigolactones: intriguing biologically active compounds: perspectives for deciphering their biological role and for proposing practical application. Pest Manag. Sci. 68, 664–668 [DOI] [PubMed] [Google Scholar]
- Xie X., Kusumoto D., Takeuchi Y., Yoneyama K., Yamada Y., Yoneyama K. (2007). 2’-Epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J. Agric. Food Chem. 55, 8067–8072 [DOI] [PubMed] [Google Scholar]
- Xie X., Yoneyama K., Yoneyama K. (2010). The strigolactone story. Annu. Rev. Phytopathol. 48, 93–117 [DOI] [PubMed] [Google Scholar]
- Yokota T., Sakai H., Okuno K., Yoneyama K., Takeuchi Y. (1998). Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry. 49, 1967–1973 [Google Scholar]
- Yoneyama K., et al. (2008). Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 179, 484–494 [DOI] [PubMed] [Google Scholar]
- Zwanenburg B., Mwakaboko A.S., Reizelman A., Anilkumar G., Sethumadhavan D. (2009). Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 65, 478–491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.