Abstract
The diseases of myelin are among the most prevalent and disabling conditions in neurology. These diseases include both the vascular and inflammatory demyelinating disorders of adulthood, as well as the childhood leukodystrophies and cerebral palsy. These fundamentally glial disorders may be amenable to treatment by glial progenitor cells (GPCs), which give rise to astroglia and myelin-producing oligodendrocytes. Given the development of new methods for generating and isolating human GPCs, the myelin disorders may now be compelling targets for cell-based therapy. In addition, the efficient engraftment and expansion of human GPCs in murine hosts has led to the development of human glial chimeric mouse brains, which provides new opportunities for studying the species-specific roles of human glia in cognition, as well as in disease pathogenesis.
Oligodendrocytes are the sole source of myelin in the adult central nervous system (CNS), and their loss or dysfunction is at the heart of a wide variety of child and adult diseases. In children, the hereditary leuko-dystrophies accompany cerebral palsy as major sources of neurological morbidity. In adults, oligodendrocytic loss and demyelination contribute to diseases as diverse as multiple sclerosis (MS), white matter stroke, and spinal cord injury (1). In addition, demyelination is also noted in degenerative disorders as varied as normal aging and Alzheimer’s disease, and oligodendrocytic pathology has been associated with disorders as diverse as amyotrophic lateral sclerosis (2) and schizophrenia (3). As a result, the demyelinating diseases are especially attractive targets for cell-based therapeutic strategies. Several recent studies have supported the readiness with which axons can remyelinate after either congenital or acquired demyelination, if provided with myelinogenic cells (4, 5). Glial progenitor cells (GPCs)—also referred to as either oligodendrocyte progenitor cells or NG2 cells (6)—have thus become promising reagents by which to restore myelin to demyelinated regions of the diseased or injured CNS.
GPCs in Vivo
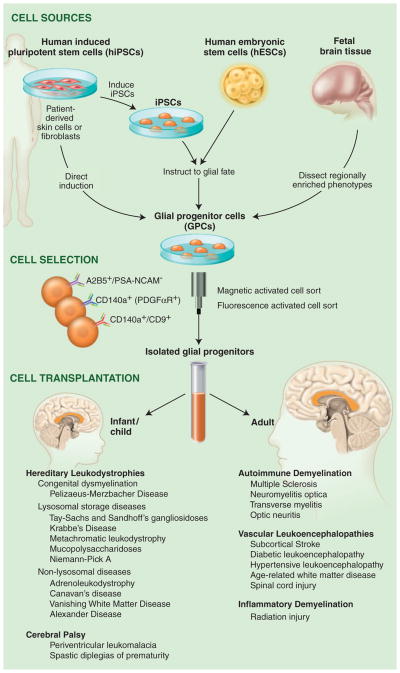
Glial progenitor cells arise from neural stem cells of the ventricular subependyma and disperse widely throughout the CNS, pervading both gray and white matter (7). In vivo, GPCs can generate both major macroglial phenotypes, astrocytes and oligodendrocytes, in a context-dependent fashion. Human glial progenitors comprise roughly 3% of all cells in the adult forebrain and may be isolated by surface antigen–targeted sorting techniques (7, 8); by this means, their gene expression patterns, dominant signaling pathways, and homeostatic self-renewal mechanisms have all been studied in detail (9, 10). In addition, both fetal and adult human GPCs have been found to efficiently generate myelinogenic oligodendrocytes upon transplantation (11). In practice, the relatively limited mitotic competence of adult GPCs and the scarcity of appropriate tissue samples limit the availability of adult GPCs for therapeutic purposes. Rather, fetal brain tissue, embryonic stem cells (ESCs), and induced pluri-potential stem cells (iPSCs) encompass the more feasible sources of transplantable human GPCs (Fig. 1).
Fig. 1.
Glial progenitor cell sources, phenotypes and clinical targets. GPCs may be directly sorted from tissue or generated from either hESCs or hiPSCs and then immunoselected based on their expression of either the A2B5 epitope or CD140a/PDGFαR. The CD140a phenotype includes all potential oligodendrocytes, whereas the tetraspanin CD9 identifies a pro-oligodendrocytic fraction (10). The choice of tissue-, hESC-, or iPSC-derived GPCs depends on whether allogeneic or autologous grafts are desired. Whereas autologous grafts of iPSC-derived GPCs might obviate the need for immunosuppression, their generation may take months, and their use in the hereditary leukodystrophies would first require correction of the underlying genetic disorder in the donor cell pool. At present, such genetic disorders of myelin may be better approached with allografted tissue- or hESC-derived GPCs.
Optimizing Cellular Agents for Treating Myelin Disorders
Disorders of myelin require extensive tissue repair and, in the case of the pediatric leuko-dystrophies, even whole neuraxis myelination. Although endogenous glial progenitors can remyelinate demyelinated lesions to some degree, the mitotic exhaustion and functional depletion of endogenous glial progenitors that may occur in acquired demyelination ultimately limits the extent and usefulness of spontaneous remyelination (12), thus necessitating the introduction of exogenous glial progenitors as therapeutic vectors. Yet to be safe and effective as therapeutic vectors, transplantable GPCs must be reliably deliverable in both purity and quantity (1). The surface antigen–based purification of human GPCs, on the basis of their selective expression of gangliosides recognized by monoclonal antibody A2B5, first allowed the isolation of these cells and their assessment in animal models of congenital hypomyelination (5, 13). These studies revealed that fetal GPCs efficiently myelinated both the brain and the spinal cord, emigrated more widely and engrafted more efficiently than did adult cells, and exhibited context-dependent differentiation as astrocytes or oligodendrocytes, suggesting their usefulness in a broad array of myelin disorders (Fig. 2, A to C).
Fig. 2.
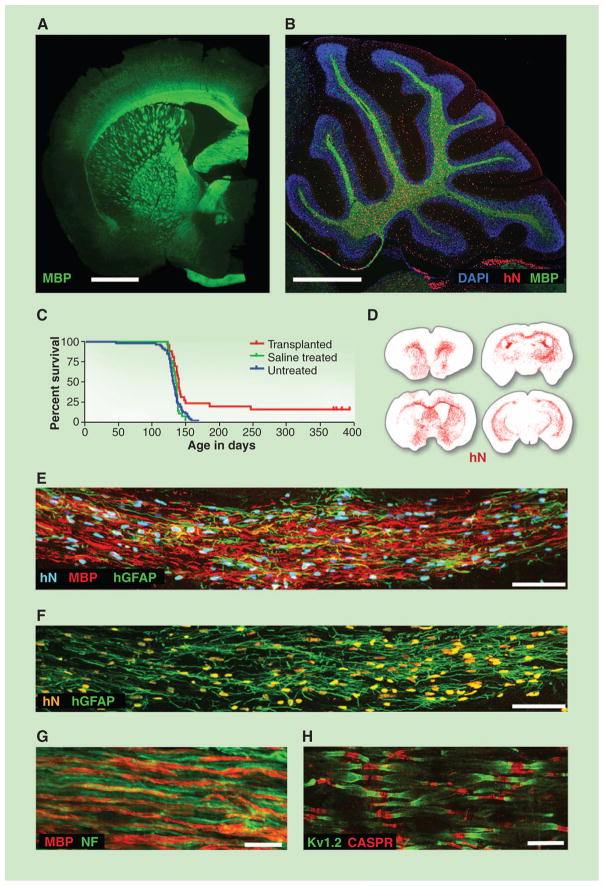
Glial progenitor cell graft-mediated myelination of a dysmyelinated host. (A) A 13-month-old shiverer (shi/shi) × rag2−/− mouse, neonatally xenografted with A2B5+/PSA-NCAM− hGPCs. MBP, myelin basic protein (shown in green). Shiverer mice do not express MBP; all immunolabeled myelin is of donor origin. (B) Sagittal view through the cerebellum. All cells were stained with 4′,6-diamidino-2-phenylindole (blue); donor cells were identified by human nuclear antigen (hN, red) and MBP (green). (C) Kaplan-Meier plot comparing survival of neonatally engrafted shiverer × rag2−/− mice to untreated and saline-injected controls. (D) CD140a-selected GPCs transplanted into neonatal shiverer × rag2−/− mice expanded and migrated extensively, rendering the brains chimeric. Red dots indicate individual human cells (hN); sacrificed at 3 months. (E and F) Coronal sections of a neonatally engrafted shiverer brain at 3 months, revealing donor-derived myelination (MBP, red) and astrocytic infiltration [human glial fibrillary acidic protein (GFAP), green] of the corpus callosum. (G) Myelin (MBP, red) produced by CD140a-selected hGPCs ensheathes mouse neurofilament-positive axons (NF, green), at 3 months. (H) Reconstituted nodes of Ranvier in the cervical spinal cord of a transplanted and rescued 1-year-old shiverer × rag2−/− mouse, showing paranodal Caspr protein and juxtaparanodal voltage-gated potassium channel protein Kv1.2, symmetrically flanking each axonal node. Untransplanted shiverer brains do not have organized nodes of Ranvier and, hence, cannot support saltatory conduction by central axons (Caspr, red; Kv1.2, green). Scale bars: (A) and (B), 1 mm; (E), 50 μm; (F) and (G), 10 μm; (H), 5 μm. (A) and (B), from (54); (C) and (H), from (5); (D) to (G), from (10).
Yet despite the attractiveness of fetal GPCs as potential therapeutic vectors, they remain finite in both initial number and expansion competence, necessitating their periodic reacquisition from new donor tissues. As a result, recent efforts have focused on the production of myelinogenic GPCs from human ESCs (hESCs) and iPSCs. After the first report of myelination in the injured spinal cord by mouse ESCs (14), oligodendrocytes derived from hESCs were similarly directed to generate myelin in vivo (15, 16). But although the findings were ground-breaking, these studies did not isolate GPCs or oligodendrocytes before transplant, nor did they follow animals over the time frames required to ensure the stability of the engrafted cells. This is cause for concern because any incidentally transplanted hESCs may retain the potential for undifferentiated expansion after implantation (17). Given this concern for tumorigenesis, stringent purification of lineage-restricted GPCs may be needed to ensure their safe use. Still, this point remains controversial. In 2009, the U.S. Food and Drug Administration approved a phase 1 safety trial evaluating the use of hESC-derived GPCs in spinal cord injury without such purification (18); although the trial was halted in 2011, its sponsor reported that its cessation was not related to safety. As a result, the need for pretransplant isolation of terminally differentiated phenotypes remains unsettled.
Human ESC-based therapy suffers also from the possibility of allograft rejection and, hence, the need for immunosuppression in graft recipients. Enthusiasm has thus developed for the use of autologous grafts of myelinogenic GPCs derived from human induced pluripotential cells (hiPSCs), potentially—though not assuredly (19)—obviating the need for immune suppression. These cells are generated by the reprogramming of somatic cells to a pluripotent ground state by the forced expression of a set of transcription factors that instruct stem cell phenotype (20). iPSCs were first generated from mouse (21) and human (22) fibroblasts and have since been differentiated into a variety of phenotypes, including neurons (23), astrocytes (24), and oligodendrocytes (25). Methods established for generating GPCs from hESCs have proven effective with hiPSCs as well and yield GPCs that are highly myelinogenic in vivo (26). This capability allows us to reasonably anticipate the use of iPSC-derived oligodendrocytes for autologous treatment, especially for nongenetic vascular, traumatic, and inflammatory demyelinations.
Importantly though, iPSC-derived GPCs share many of the risks of those derived from hESCs, including aberrant differentiation and tumorigenesis (27). In addition, iPSCs retain epigenetic marks of the cells from which they derive (28), so that their cell type of origin may influence their differentiation competence (29). Indeed, iPSCs may differ from one another in their lineage competence, even when sourced from the same individual and tissue, making their standardization difficult. Recent studies have reported the direct induction of neurons from fibroblasts (30), and one may anticipate the development of analogous strategies of direct induction of glial progenitors and oligodendrocytes as well. By thereby avoiding the need for pluripotential intermediates, such direct induction of GPCs may accelerate the production of transplantable cells while mitigating their risk of tumorigenesis.
Pediatric Disease Targets of GPC-Based Therapy
Tens of thousands of children in the U.S. suffer from diseases of myelin loss, including metabolic demyelinations, such as adrenoleukodystrophy; lysosomal storage disorders, such as metachromatic leukodystrophy, the neuronal ceroid lipofuscinoses, gangliosidoses, and Niemann-Pick and Krabbe’s diseases; hypomyelinating diseases, such as Pelizaeus-Merzbacher disease; myelinoclastic disorders, including vanishing white matter disease, Alexander’s disease, and Canavan’s disease (31); and, most commonly, periventricular leukomalacia and cerebral palsy (32). Their mechanistic heterogeneity notwithstanding, all of these conditions include the prominent loss of oligodendrocytes and myelin, highlighting their attractiveness as potential targets for cell replacement (see Fig. 1, bottom).
In some metabolic disorders of myelin, such as Krabbe’s disease, oligodendrocytes are essentially bystanders, killed by toxic metabolites generated by cells deficient in one or more critical enzymes (31). In others, such as Alexander’s disease and vanishing white matter disease, myelin loss may be caused by astroglial pathology (33, 34). Because glial progenitor engraftment is both widespread and associated with astrocytic and oligodendrocytic production, GPCs would seem an especially promising vehicle for dispersing astrocytes and oligodendrocytes throughout otherwise diseased and/or enzyme-deficient brain parenchyma. The lysosomal storage disorders present especially attractive targets in this regard, because wild-type (WT) lysosomal enzymes may be released by donor cells and taken up by deficient host cells through the mannose-6-phosphate receptor pathway. A relatively small number of donor glia may then provide sufficient enzymatic activity to correct the underlying catalytic deficit and storage disorder of a much larger number of host cells (35).
The cell-based rescue of enzymatically deficient host cells by WT donor neural stem cells (NSCs) was first noted in a mouse model of mucopolysaccharidosis type VII, in which neonatally implanted cells restored lost enzymatic function to the recipient forebrain (36). Human NSCs also achieved substantial enzyme replacement in the β-hexosaminidase–deficient mouse with Sandhoff disease, with corresponding functional benefits (37). In the same vein, NSCs engineered to overexpress sphingomyelinase, engrafted into sphingomyelinase-deficient Niemann-Pick type A mice, yielded substantial reductions in mis-accumulated sphingomyelin (38). Similarly, when NSCs were engrafted into a mouse model of neuronal ceroid lipofuscinosis (NCL), the cells dispersed broadly and ameliorated the lipofuscin misaccumulation of these animals (39). On that basis, a clinical trial to assess the use of human NSC allografts in treating infantile and late infantile NCL was undertaken (40). This phase 1 safety trial did not address therapeutic end points, but its initiation speaks to the efforts that may be anticipated in developing NSCs and GPCs as vehicles for intracerebral enzyme replacement in the metabolic leukodystrophies.
The intracerebral delivery of GPCs would seem an especially promising approach for treating those enzyme-deficiencies associated with early demyelination, which may require both enzyme replacement and structural remyelination. Metachromatic leukodystrophy (MLD), for example, is characterized by deficient expression of arylsulfatase A, which results in sulfatide misaccumulation and oligodendrocyte loss. Experimental models of MLD have responded well to GPC grafts, with broad dispersal and integration, as well as enzymatic rescue and sulfatide clearance (41). Krabbe’s disease, characterized by galactocerebrosidase deficiency and early demyelination, is another storage disorder that may prove amenable to concurrent GPC-based enzymatic repletion and myelin restoration. When children with Krabbe’s disease were transplanted with umbilical cord stem cells, they manifested slower disease progression, but the benefits of transplantation to children engrafted after symptom onset seemed minimal (42). Yet the intracerebral infiltration of umbilical cord stromal derivatives is modest, suggesting that treatment of these children with GPCs, which are able to achieve broad parenchymal dispersal as well as structural remyelination, might involve a more promising treatment strategy.
The experimental assessment of GPCs as vectors for remyelination has proceeded most aggressively in animal models of congenital hypomyelination. In an early study of cell-based myelin repair, mouse neural stem cells were transplanted into newborn shiverer mice, a hypomyelinated mutant deficient in myelin basic protein, and yielded context-dependent myelination (43). On that basis, we transplanted sorted human GPCs into neonatal shiverers, so as to assess the relative myelinogenic potential of GPCs (13). When delivered as highly enriched isolates, fetal human GPCs spread widely throughout the brain (Fig. 2, A, B, and D), developing as astrocytes and oligodendrocytes in a context-dependent fashion. The donor-derived oligodendrocytes generated ultrastructurally mature myelin that effectively ensheathed host shiverer axons and formed nodes of Ranvier (Fig. 2H), which allowed the restoration of normal trans-callosal conduction velocities in the transplanted mice (5, 13). By using a five-site injection protocol to achieve broader dispersal of GPCs, we next established cell engraftment throughout the entire neuraxis, with myelination of the spinal cord and roots, as well as the entire brain, brainstem, cerebellum, and cranial nerve roots (5). This was associated with substantially prolonged survivals in transplanted mice, with phenotypic recovery and frank rescue of a large minority (Fig. 2C). These data strongly suggested the feasibility of neonatal GPC implantation in treating childhood disorders of myelin formation and maintenance. Later studies refined the criteria for selecting myelinogenic progenitors by identifying the platelet-derived growth factor α receptor (PDGFαR) epitope CD140a as recognizing the entire population of oligodendrocyte-competent progenitors (10). CD140a-sorted GPCs proved superior to those selected on the basis of A2B5 in both their efficiency and extent of myelination and were also highly migratory; thus, CD140a-sorted GPCs have supplanted the latter as a preferred cellular vector for therapeutic remyelination (Fig. 2, D to G).
Hence, the intracerebral delivery of GPCs may prove a viable approach to the treatment of a wide variety of enzymatic and storage disorders. In practice though, individual treatment regimens will need to be tailored to specific disease phenotypes and stages. Little data are available as to the numbers or proportion of WT cells required to achieve correction of enzymatic activity and substrate clearance in any storage disorder, and these values may need to be empirically derived for every disease target. Similarly, the extent of myelination required for effective treatment remains unclear, as do the extent and duration of immunosuppression required for allograft acceptance; these parameters may also vary with disease phenotype. These caveats notwithstanding, neural stem cell implantation is already under assessment as a means of myelin replacement in Pelizaeus-Merzbacher disease (44), and we anticipate that future efforts will similarly assess the efficacy of GPC grafts in this and related disorders.
Adult Disease Targets of GPC-Based Treatment
In adults, oligodendrocytic loss contributes to diseases as diverse as hypertensive and diabetic white matter loss, traumatic spinal cord and brain injury, and MS and its variants. In addition, oligodendrocytic loss is prominent in the degenerative dementia associated with age-related white matter loss. All of these are potential targets of GPC replacement therapy, though the adult disease environment may limit this approach in ways not encountered in pediatric disease targets. For instance, the chronically ischemic brain tissue of diabetics with small vessel disease may require aggressive treatment of the underlying vascular insufficiency before any cell-replacement strategy may be considered. Similarly, the inflammatory disease environments of MS and many of the leukodystrophies present their own challenges, which need to be overcome before cell-based remyelination can succeed (12, 45). Nonetheless, current disease-modifying strategies for treating both vascular and autoimmune diseases have advanced to the point where transplant-based remyelination of adult targets may now be feasible.
Interest in cell-based remyelination has been focused on MS, a debilitating disease characterized by both inflammatory myelinolysis and degenerative axonal loss. The attraction of MS as a therapeutic target derives from its high incidence and prevalence, with more than 300,000 cases in the U.S. alone. MS has been a difficult target for cell therapy, given its relapsing course and the limitations of introducing fresh cells into an inflammatory environment. Nonetheless, a new generation of immune modulators has substantially diminished disease recurrence, making cell replacement a tenable repair strategy. Natalizumab (anti-α4 integrin), alemtuzumab (anti-CD52), rituximab (anti-CD20), and fingolomod (a sphingosine-1-phosphate receptor modulator), have all been associated with significant reductions in relapse rate (46). These advances in the immunomodulatory control of MS suggest that focus may now shift from disease attenuation to the repair of demyelinated lesions.
The intracerebral delivery of GPCs into demyelinated brain may offer a feasible strategy for such myelin repair. When human GPCs were transplanted directly into lysolecithin-induced demyelinated lesions in the adult rat brain, the cells matured as oligodendrocytes and myelinated residual host axons, though with lower efficiency than in congenitally hypomyelinated brain (11). Similarly, in a new model of axon-sparing demyelination in adult cats, remyelination occurred efficiently from endogenous progenitors (4). Thus, GPCs seem to be effective cellular vectors for adult remyelination, though the complexity of the disease environment, which may include axonal loss, may make adult targets less approachable than their pediatric counterparts; this is especially true in aged patients (12, 47). Thus, any cell-based strategies for treating adult demyelination will require not only disease modification, but also rigorous stratification to define those patients with sufficient axonal preservation to benefit from this approach.
Besides the myelinated tracts of the brain, the ascending sensory and descending motor tracts of the spinal cord are frequent victims of demyelination, whether from MS, neuromyelitis optica, or segmental injuries. In efforts to remyelinate the contused rat spinal cord, implanted GPCs have been found to disperse and generate both astrocytes and myelinogenic oligodendrocytes (48). Similarly, hESC-derived oligodendrocytes can remyelinate demyelinated cord lesions (15), with functional benefit (49). As noted, a safety trial of hESC-derived GPCs transplanted into patients with high-grade thoracic cord lesions was initiated on the basis of these observations (18). Although the therapeutic potential of a solely remyelinative strategy in patients with such high-grade lesions is unclear, such GPC grafts may hold great promise in carefully selected patients with isolated segmental demyelination.
Human GPC-Engrafted Chimeric Mice as Systems for Assessing Human Glial Function
When hypomyelinated mutant mice are engrafted neonatally with human GPCs, the donor cells mature as both myelinating oligodendrocytes and fibrous astrocytes, ultimately yielding mice with substantially humanized white matter (5). Large numbers of human donor cells also remain as progenitors, which eventually predominate, displacing and ultimately replacing the endogenous mouse glial progenitor pool. This competitive advantage of human over murine glial progenitors is evident in WT and hypomyelinated mice, such that in the setting of normal glial turnover, the human GPCs also give rise to gray matter astrocytes, eventually resulting in substantial astrocytic as well as oligodendrocytic humanization of the recipient rodent brains (Fig. 2F).
The result of these events is that the xeno-grafted mouse brains can become substantially humanized in their glial constituents (5). These human glial chimeras lend themselves to the investigation of questions that were never before approachable, for lack of an appropriate in vivo model of human glial function. First, what are the species-specific contributions of human glia to neural network function? Astrocytes clearly play a central role in synaptic efficiency and plasticity in mammals (50). Hominid evolution in particular has been attended by increasing astrocytic complexity, which may have contributed greatly to the evolution of higher cognitive functions in primates. Human astrocytes are larger and far more fibrous than those of infraprimate mammals and include more encompassed synapses (51). As such, is astrocytic specialization the basis for human cognitive evolution? The high degree of human glial chimerization of these brains will permit us to address this issue and should provide great opportunity for future studies of the species-specific roles of astrocytes in human cognition and disease alike. In addition, the advent of new technologies for generating glia from iPSCs suggests the potential for establishing chimeric mice with glia produced from iPSCs sourced from patients with specific neurological and psychiatric disorders, as a means of assessing the specific contributions of glia to the pathogenesis of those conditions. This approach may be of particular value in assessing the role of glia in those neural diseases that are exclusive to humans, such as schizophrenia, whose phylogenetic appearance may parallel that of human astrocytic evolution.
Thus, the disorders of glia, and of myelin in particular, stand out as especially promising initial targets for cell-based therapy of neurological disease. With the use of a common strategy for GPC implantation, a broad set of both pediatric and adult disorders of the brain and spinal cord may prove amenable to structural repair. In addition, the human glial chimeric mice that have been established as a means of evaluating these transplant strategies may provide us with exciting new models for studying the species-specific roles of human glia and their progenitors in both normal physiology and disease.
Note added in proof
As this Review went to press, two new papers appeared that address the potential utility of propagated neural stem cells, as opposed to lineage-restricted glial progenitors, for myelinating congenitally hypomyelinated brain. Uchida et al. (52) describe the production of myelin in the brains of immunodeficient shiverer mice transplanted neonatally with human neural stem cells. Gupta et al. (53) then describe the early results of delivering these cells to the brains of children with Pelizaeus-Merzbacher disease, in a phase 1 safety trial that reports a favorable safety profile in the implanted children, as well as radiographic data suggestive of donor-derived myelin production in the graft recipients.
Acknowledgments
Work discussed in the Goldman lab was supported by grants from the National Institute of Neurological Disorders and Stroke (R01NS75345 and R01NS39559), the National Multiple Sclerosis Society, the Adelson Medical Research Foundation, the Mathers Charitable Foundation, and the New York State Stem Cell Research Program (NYSTEM). S.A.G. is an inventor on the following patents held by the University of Rochester and/or Cornell University: U.S. Patent 7,524,491 B2 (“Non-human animals with human glial chimeric brains”), U.S. Patent 8,206,699 (“Myelination of congenitally dysmyelinated forebrains using oligodendrocyte progenitor cells”), and U.S. Patent 8,263,402 (“A method for isolating and purifying oligodendrocytes and oligodendrocyte progenitor cells”).
References and Notes
- 1.Roy N, Windrem M, Goldman SA. In: Myelin Biology and Disorders. Lazzarini R, editor. Elsevier; Amsterdam: 2004. pp. 259–287. [Google Scholar]
- 2.Lee Y, et al. Nature. 2012;487:443. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tkachev D, et al. Lancet. 2003;362:798. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 4.Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Proc Natl Acad Sci USA. 2009;106:6832. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Windrem MS, et al. Cell Stem Cell. 2008;2:553. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiyama A, Komitova M, Suzuki R, Zhu X. Nat Rev Neurosci. 2009;10:9. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 7.Roy NS, et al. J Neurosci. 1999;19:9986. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes MC, et al. Nat Med. 2003;9:439. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 9.Sim FJ, et al. Ann Neurol. 2006;59:763. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- 10.Sim FJ, et al. Nat Biotechnol. 2011;29:934. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Windrem MS, et al. J Neurosci Res. 2002;69:966. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 12.Franklin RJM, Ffrench-Constant C. Nat Rev Neurosci. 2008;9:839. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 13.Windrem MS, et al. Nat Med. 2004;10:93. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- 14.Brüstle O, et al. Science. 1999;285:754. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 15.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Glia. 2005;49:385. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 16.Izrael M, et al. Mol Cell Neurosci. 2007;34:310. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Roy NS, et al. Nat Med. 2006;12:1259. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 18.www.clinicaltrials.gov, NCT01217008.
- 19.Zhao T, Zhang ZN, Rong Z, Xu Y. Nature. 2011;474:212. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 20.Izpisúa Belmonte JCI, Ellis J, Hochedlinger K, Yamanaka S. Nat Rev Genet. 2009;10:878. doi: 10.1038/nrg2700. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Cell. 2006;126:663. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, et al. Science. 2007;318:1917. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 23.Wernig M, et al. Proc Natl Acad Sci USA. 2008;105:5856. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu BY, Du ZW, Zhang SC. Nat Protoc. 2009;4:1614. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czepiel M, et al. Glia. 2011;59:882. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- 26.Windrem MS, et al. Soc Neurosci Abstr. 2011;228.02 [Google Scholar]
- 27.Mattis VB, Svendsen CN. Lancet Neurol. 2011;10:383. doi: 10.1016/S1474-4422(11)70022-9. [DOI] [PubMed] [Google Scholar]
- 28.Stadtfeld M, et al. Nature. 2010;465:175. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polo JM, et al. Nat Biotechnol. 2010;28:848. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pang ZP, et al. Nature. 2011;476:220. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers J. In: Myelin Biology and Disorders. Lazzarini R, editor. Elsevier; Amsterdam: 2004. pp. 663–690. [Google Scholar]
- 32.Silbereis JC, Huang EJ, Back SA, Rowitch DH. Dis Model Mech. 2010;3:678. doi: 10.1242/dmm.002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietrich J, et al. Nat Med. 2005;11:277. doi: 10.1038/nm1195. [DOI] [PubMed] [Google Scholar]
- 34.Bugiani M, et al. J Neuropathol Exp Neurol. 2011;70:69. doi: 10.1097/NEN.0b013e318203ae74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeyakumar M, Dwek R, Butters T, Platt F. Nat Rev Neurosci. 2005;6:1. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- 36.Snyder EY, Taylor RM, Wolfe JH. Nature. 1995;374:367. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 37.Lee J-P, et al. Nat Med. 2007;13:439. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 38.Shihabuddin LS, et al. J Neurosci. 2004;24:10642. doi: 10.1523/JNEUROSCI.3584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamaki SJ, et al. Cell Stem Cell. 2009;5:310. doi: 10.1016/j.stem.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 40.www.clinicaltrials.gov, NCT00337636.
- 41.Givogri MI, et al. J Neurosci. 2006;26:3109. doi: 10.1523/JNEUROSCI.4366-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escolar ML, et al. N Engl J Med. 2005;352:2069. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 43.Yandava BD, Billinghurst LL, Snyder EY. Proc Natl Acad Sci USA. 1999;96:7029. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.www.clinicaltrials.gov, NCT01391637.
- 45.Ip CW, et al. J Neurosci. 2006;26:8206. doi: 10.1523/JNEUROSCI.1921-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstock-Guttman B, Ramanathan M. Nat Rev Neurol. 2012;8:66. doi: 10.1038/nrneurol.2011.213. [DOI] [PubMed] [Google Scholar]
- 47.Ruckh JM, et al. Cell Stem Cell. 2012;10:96. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Glia. 2004;45:1. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- 49.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Stem Cells. 2010;28:152. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang J, Jiang L, Goldman SA, Nedergaard M. Nat Neurosci. 1998;1:683. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 51.Oberheim NA, et al. J Neurosci. 2009;29:3276. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchida N, et al. Sci Transl Med. 2012;4:ra136. [Google Scholar]
- 53.Gupta N, et al. Sci Transl Med. 2012;4:ra137. [Google Scholar]
- 54.Goldman SA. Arch Neurol. 2011;68:848. doi: 10.1001/archneurol.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]