Abstract
Context:
Magnetic resonance imaging (MRI) affords high-resolution visualization of the soft tissue structures (menisci, ligaments, cartilage, etc) and bone marrow of the knee.
Evidence Acquisition:
Pertinent clinical and research articles in the orthopaedic and radiology literature over the past 30 years using PubMed.
Results:
Ligament tears can be accurately assessed with MRI, but distinguishing partial tears from ruptures of the anterior cruciate ligament (ACL) can be challenging. Determining the extent of a partial tear is often extremely difficult to accurately assess. The status of the posterolateral corner structures, menisci, and cartilage can be accurately evaluated, although limitations in the evaluation of certain structures exist. Patellofemoral joint, marrow, tibiofibular joint, and synovial pathology can supplement physical examination findings and provide definitive diagnosis.
Conclusions:
MRI provides an accurate noninvasive assessment of knee pathology.
Keywords: Magnetic Resonance Imaging, knee, ACL, PCL, meniscus
Magnetic Resonance Imaging Sequences
There are many variations and combinations of pulse sequences that can be used to image the knee with magnetic resonance imaging (MRI) (Table 1). Ligaments, tendons, and menisci all have highly ordered collagen ultrastructure, interfering with the movement of hydrogen molecules by magnetization by conventional sequences; thus, they are uniformly low in signal (dark or hypointense) on traditional MRI pulse sequences. Degeneration or tearing of these structures interferes with the collagen uniformity, manifesting as increased signal intensity in the affected areas. Proton density sequences are used primarily to evaluate the menisci, given their high sensitivity in the detection of fibrocartilage signal. A fluid-sensitive sequence (ie, fat-saturated T2-weighted or inversion recovery sequence) is most useful in detecting acute injury by allowing visualization of marrow and soft tissue edema; at least 1 plane of imaging should employ a fluid-sensitive sequence. The 3.0T magnets have substantially more signal-to-noise ratio than 1.5T magnets and allow for higher resolution protocols by which to image the structures in the knee.
Table 1.
Typical sequences.
| Axial fast spin echo (FSE) | Proton density or fat-saturated T2-weighted sequence |
| Coronal FSE | Proton density and/or fat-saturated T2-weighted sequence |
| Sagittal FSE | Proton density with/without fat saturation and fat-saturated T2-weighted ± T1-weighted sequence |
Anterior Cruciate Ligament
Acute or subacute anterior cruciate ligament (ACL) ruptures (ie, full-thickness tears) nearly always have associated marrow edema in the posterior margin of the lateral tibial plateau and/or in the lateral femoral condyle, the location of which is dependent on the extent of knee flexion at the time of injury. Both ruptures and partial-thickness tears can have marrow edema in these locations. Marrow edema may also be seen in the posterior margin of the medial tibial plateau as well as less frequently in the medial femoral condyle, reflecting a so-called contrecoup injury occurring upon relocation of the tibia following anterior tibial translation.20
Primary signs of ACL rupture include full-thickness fluid signal traversing its fibers. Not infrequently, acute ruptures manifest as enlargement of the ligament with amorphous high signal on fluid-sensitive sequences. Occasionally, avulsions at its insertions occur, most frequently at the tibial insertion; tibial avulsions occur most frequently in skeletally immature patients and in patients during motor vehicle accidents. There is usually thin, avulsed cortex and/or bone attached to the ACL with a corresponding defect at the tibia. At the femoral insertion, avulsions are most easily visualized on axial sequences whereby fluid signal replaces the normal insertion site.
Importantly, the ACL fibers should always be parallel or make an acute angle (less than 10°-15°) with the roof of the intercondylar notch. Not uncommonly, an acutely or chronically torn ACL will be posteriorly bowed or have an abnormal horizontal orientation on sagittal images (Figure 1). Additionally, waviness or undulation of the ligament’s fibers in the coronal plane is often a clue to an acute or chronic tear. Anterior tibial translation using the midportion of the lateral compartment on MRI is an important secondary sign for both acute and chronic ruptures; in a small study of 27 patients with acute ACL tears and 33 patients with chronic ACL tears, anterior tibial translation of 5 mm or more had a 93% specificity for tears and translation of 7 mm or more a specificity of 100%39 (Figure 2).
Figure 1.
(A) Sagittal fat-saturated T2-weighted image shows a full-thickness midsubstance ACL rupture (black arrow) with abnormal posterior curvature, intermediate signal of the proximal fibers, and focal abnormal buckling of the distal fibers (white arrows). (B) Sagittal fat-saturated T2-weighted image shows a normal ACL with flat anterior margin and normal thin, linear interspersed high signal paralleling its fibers; linear high signal not paralleling the fibers would reflect a tear. ACL, anterior cruciate ligament.
Figure 2.
Sagittal fat-saturated T2-weighted image shows marked anterior tibial translation in the setting of chronic ACL rupture. ACL, anterior cruciate ligament
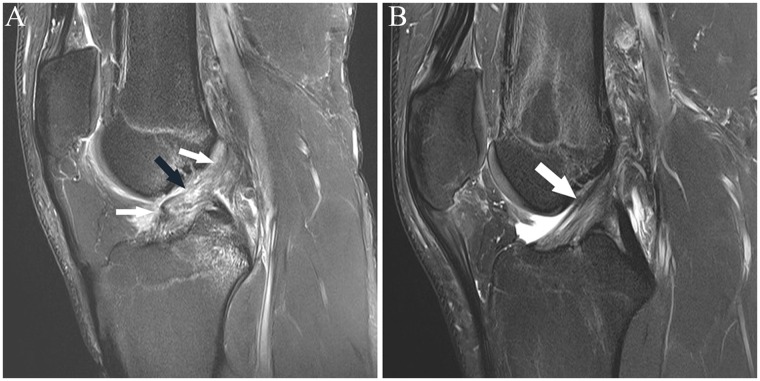
The distinction between full-thickness and partial-thickness ACL tears can be more difficult (Figure 3). Most secondary signs (eg, hemarthrosis, bone contusions in the lateral compartment, proud bone emanating from the sites of prior transchondral impaction) are not helpful as these can be seen in both partial-thickness and full-thickness tears. Acute partial tears may manifest as fluid signal replacing the disrupted fibers. They may also be visualized as intermediate signal (ie, gray on fluid-sensitive sequences) with distortion of the shape—enlargement, thinning, or abnormal contour—within a portion of its fibers. Not infrequently, the true extent of a tear is hindered by amorphous intermediate signal surrounding a tear, reflecting interstitial edema and/or hemorrhage; this could reflect a rupture or partial tear. The axial plane is helpful in delineating partial from complete tears in the proximal and midportion of the ligament; the distal fibers usually are more difficult to assess accurately on standard axial imaging due to volume averaging and less delineation of its bundles distally. Axial images clearly allow for identification of the discrete proximal and mid anteromedial and posterolateral bundles. Acute distal partial tears are most easily visualized on sagittal, or particularly coronal, sequences, whereby the same criteria as described previously are used.
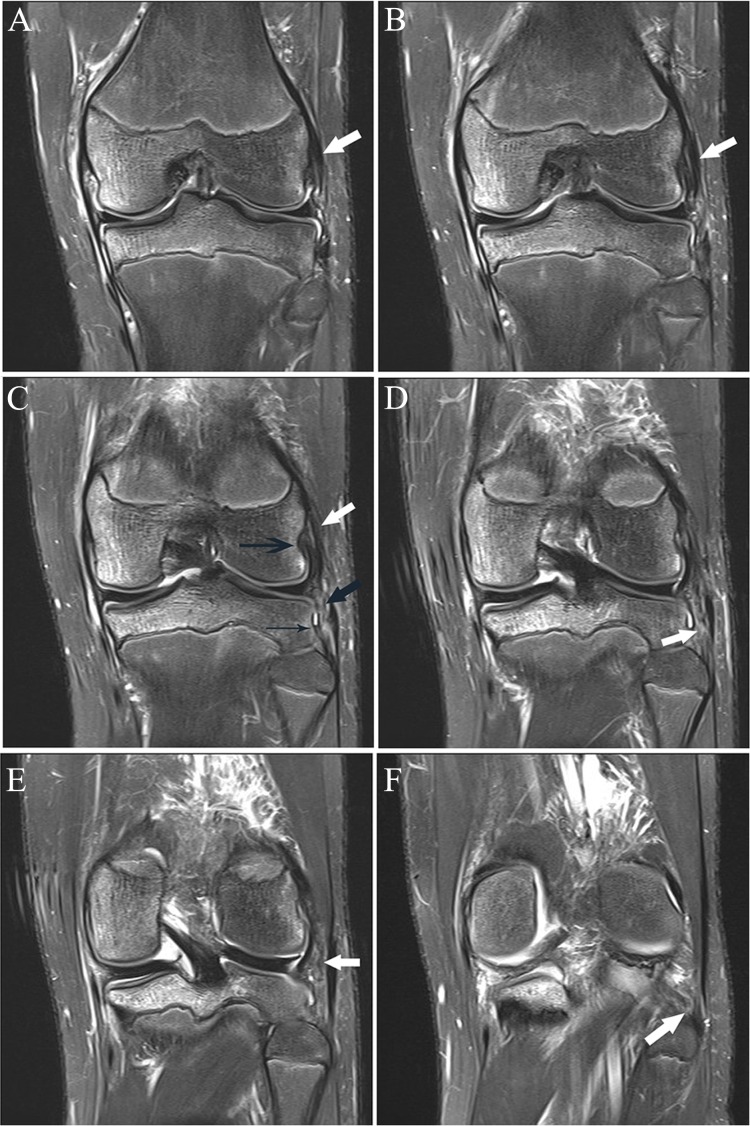
Figure 3.
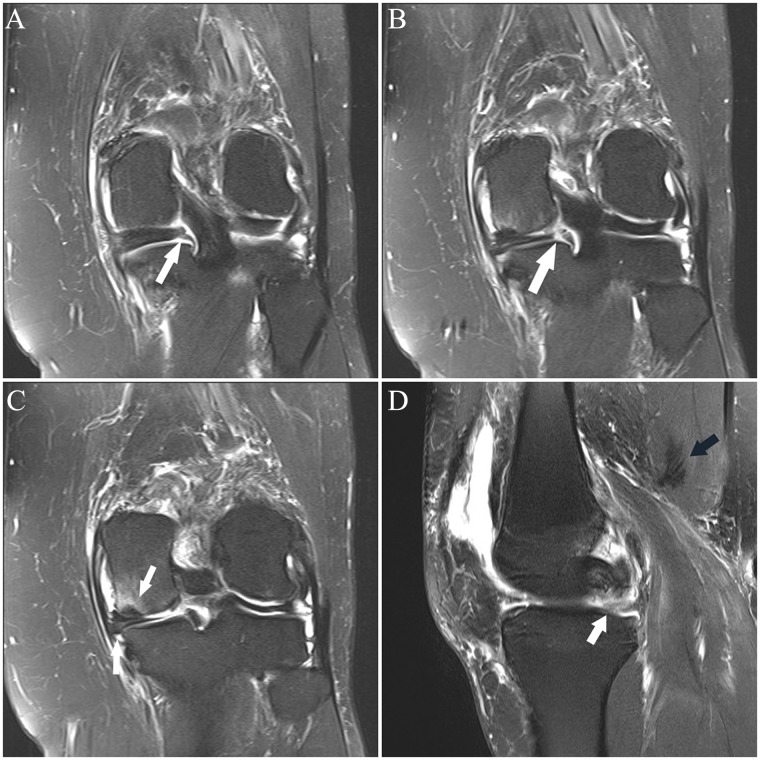
(A and B) Sagittal fat-saturated T2-weighted images show abnormal signal and shape of the midsubstance of the ACL, consistent with tear (arrow in A), with associated abnormal relative horizontal orientation of the distal fibers. In B, there is diffuse abnormal intermediate signal without clear delineation of the midsubstance fibers, which have minimal posterior convexity (arrow). Coronal images from posterior to anterior (C-G) show abnormal fraying and intermediate signal of the proximal posterolateral bundle fibers (arrow in C); in D, there is joint fluid in the normal location of the posterolateral bundle fibers at the sidewall (black arrow), with normal-appearing anteromedial bundle fibers (white arrow); in E, there is vertical fluid signal reflecting tear in the posterolateral bundle (black arrow) and abnormal intermediate signal in the anteromedial bundle (white arrow); in F, there is abnormal fluid in the posterolateral bundle (black arrow) and abnormal intermediate signal and lack of delineation of the fibers of the anteromedial bundle (white arrow);
In a retrospective study of 51 patients with an arthroscopically confirmed partial ACL tear by Van Dyck et al,40 the accuracy for diagnosis of partial tear ranged between 25% and 53% for 2 blinded musculoskeletal radiologists; 16% and 23% of the partial tears were diagnosed as ruptures by MRI by the 2 radiologists. In a separate prospective 3.0 T MRI study of 172 patients with arthroscopic correlation by the same group,42 132 of which had an intact ACL, 17 with a partial tear, and 23 with a rupture, 5 of 40 ACL (13%) tears were not correctly identified as ruptures or partial tears.
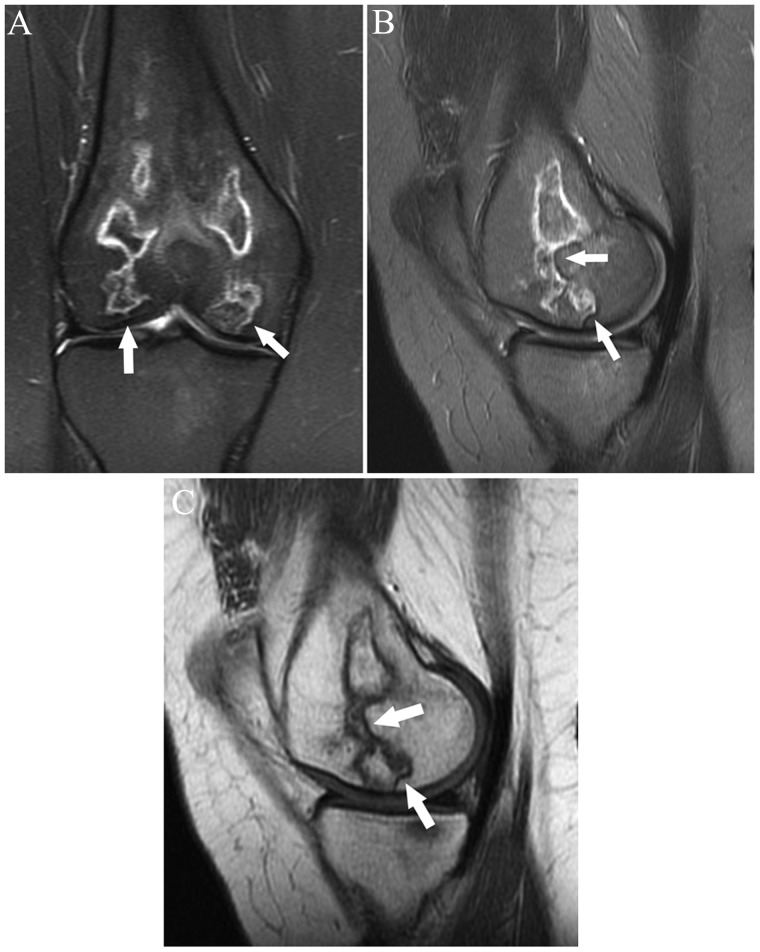
Remote partial tears, like remote ruptures, can manifest as an abnormal contour of a portion of the ligament. Some fibers may be scarred to the posterior cruciate ligament (PCL) or notch (Figure 4). Partial-thickness ACL tears, particularly chronic ones, often manifest as thin fibers (of similar signal to the remainder of its intact fibers) within the ligament that clearly have a more horizontal orientation with respect to the remainder of the normally oriented ligament (Figure 5).
Figure 4.
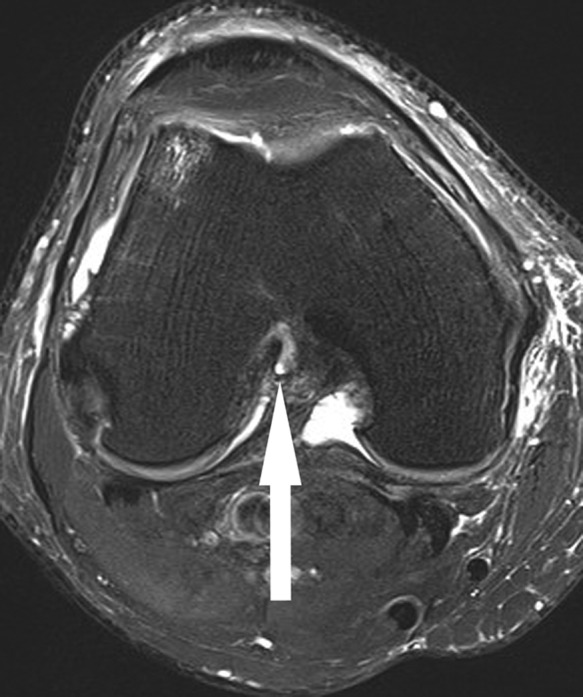
Axial fat-saturated T2-weighted image shows focal scarring of fibers of the proximal ACL to the PCL (arrow), reflecting remote partial tear. ACL, anterior cruciate ligament; PCL, posterior cruciate ligament.
Figure 5.
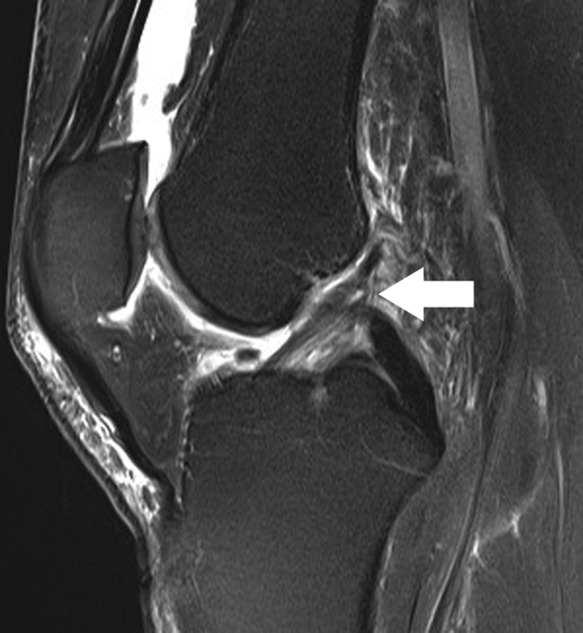
Sagittal fat-saturated T2-weighted image shows abnormal, nearly vertical course of fibers in the proximal posterolateral bundle of the ACL (arrow), reflecting prior partial tear. Superficial patellofemoral chondral loss and small mineralized body in the intercondylar notch just anterior to the ACL are incidentally noted. ACL, anterior cruciate ligament.
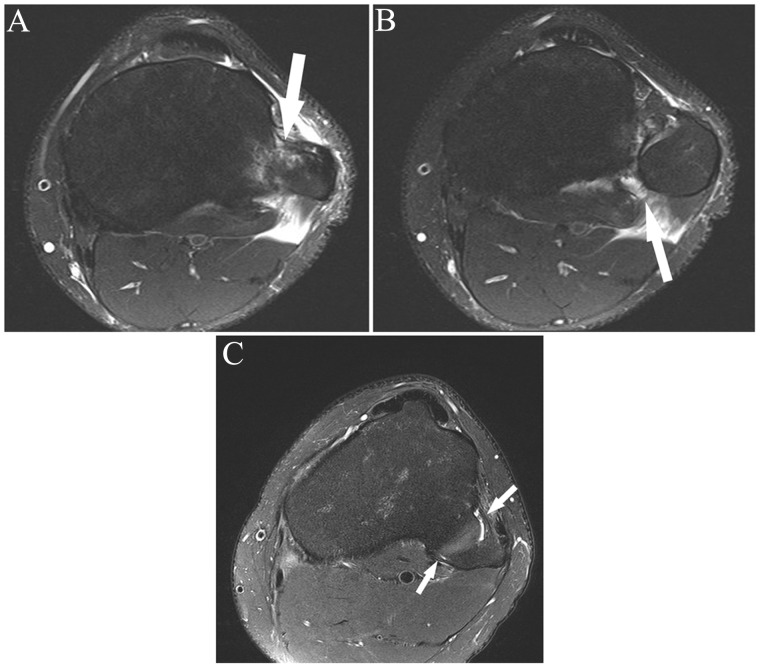
While distinguishing partial from complete cruciate ligament tears is challenging, determining the anatomical extent of a partial tear is often exceedingly difficult (Figure 6). Partial tears involving 50% or more of the ACL fibers have been shown to be a harbinger of subsequent full-thickness tears.28 A suitable strategy is to separate partial tears as either low-grade (less than 50% fiber disruption) or high-grade (more than 50% fiber disruption) (Figure 7). In a study of 97 stable and unstable ACL tears, Van Dyck et al41 found that more than 5 mm of anterior tibial translation was found only in unstable tears that required reconstruction, although the sensitivity was only 23%. Note that anterior tibial translation can be impeded in full-thickness tears by bucket-handle meniscus tear.8 Additionally, anterior tibial translation can be prevented by torn ACL fibers flipped anteriorly into the notch. In the setting of a partial tear noted on MRI, the only definitive way to determine if a tear is stable or unstable by physical examination is a pivot shift test.12
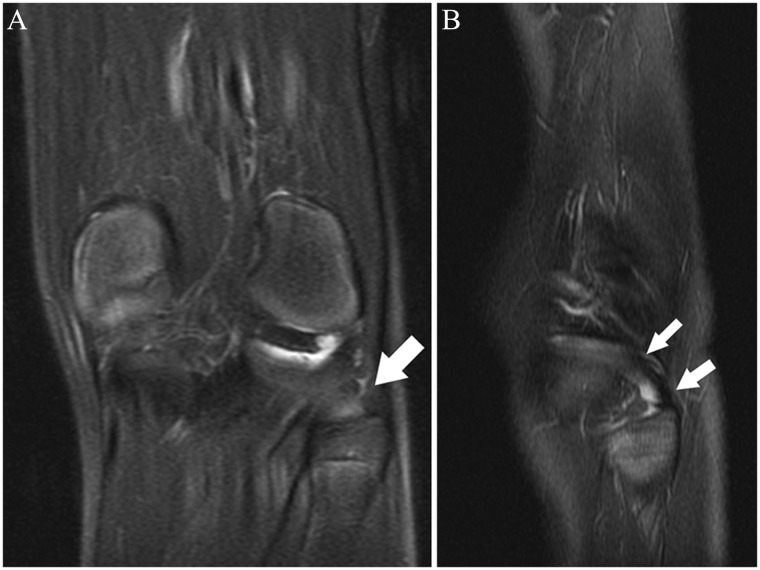
Figure 6.
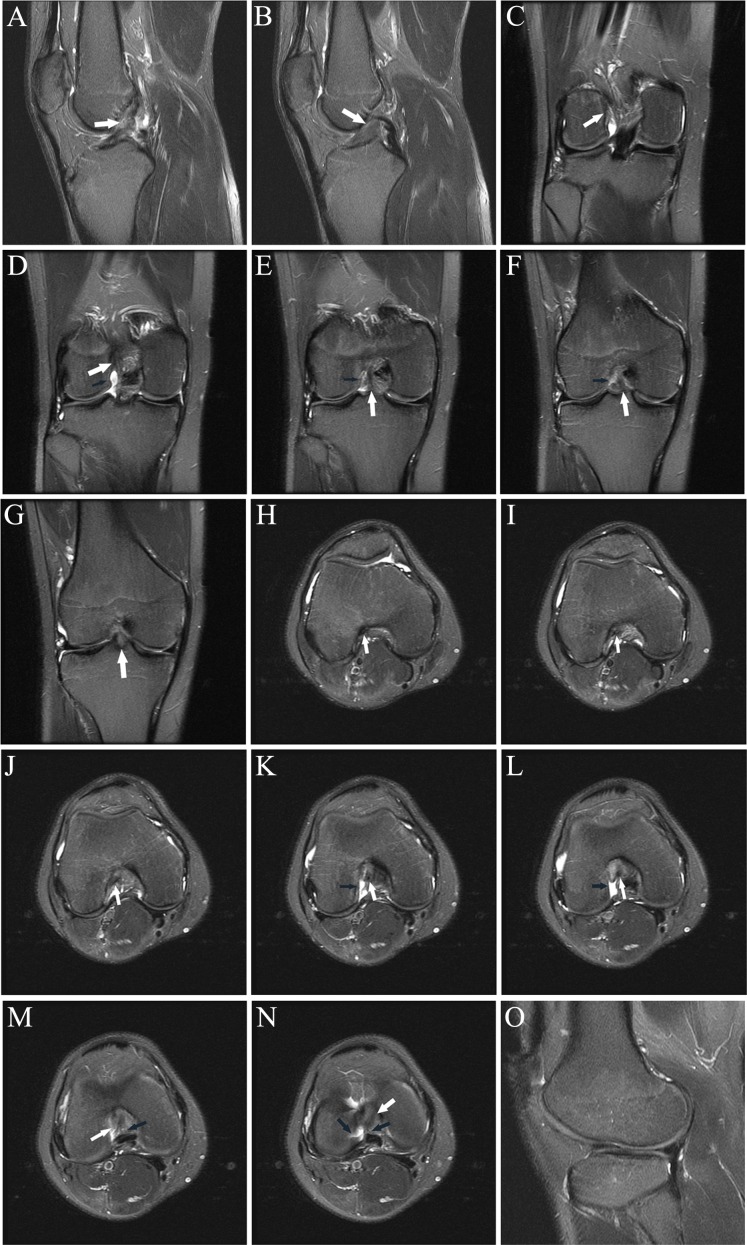
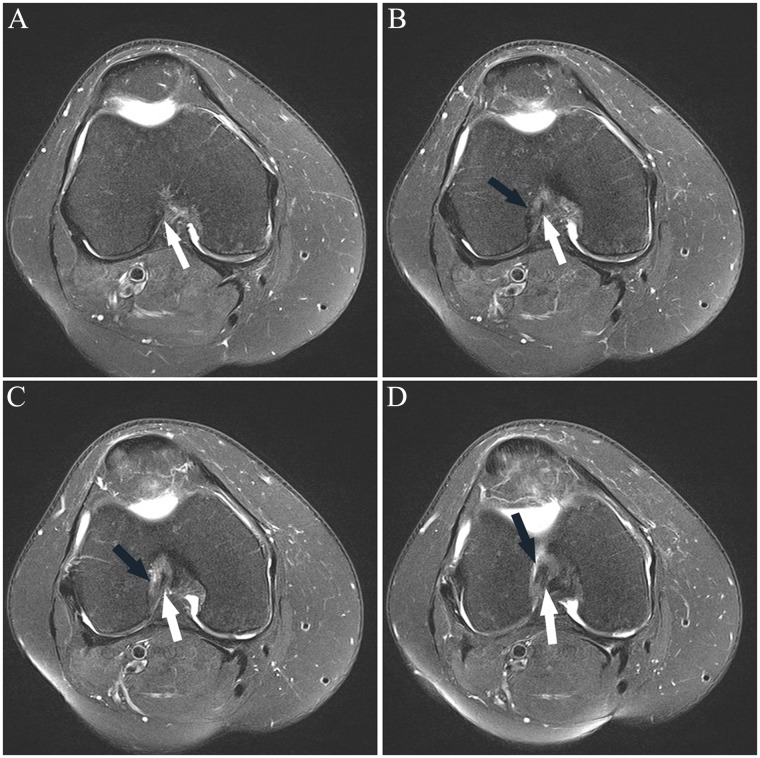
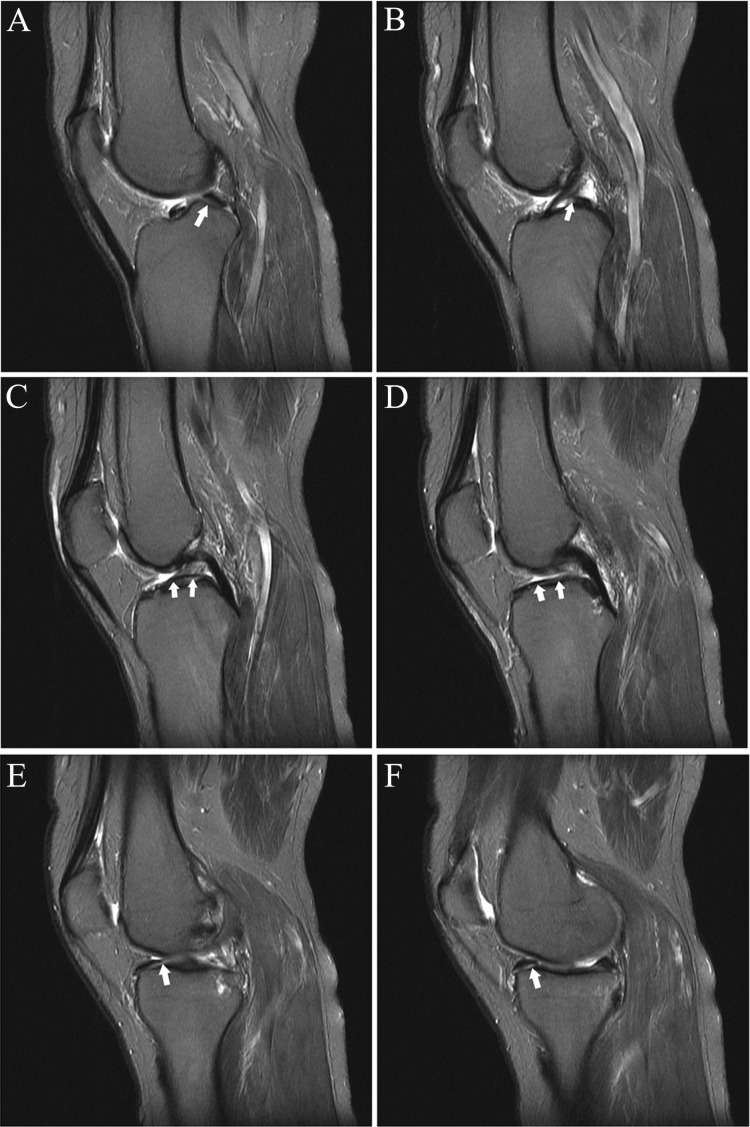
Consecutive axial fat-saturated T2-weighted images (proximal to distal) show remote partial ACL tear. At arthroscopy 4 months previously, the partial tear was noted but the ACL was not reconstructed; partial medial meniscectomy was performed. At the time of this MRI, there was an “abnormal” Lachman with firm endpoint and a 2-plus anterior drawer. (A) Normal proximal anteromedial bundle (arrow) was present. (B) The black arrow shows abnormal curvilinear contour and intermediate signal within and partially obscuring the normal low signal anterior fibers at the femoral insertion of the posterolateral bundle; the anteromedial bundle (white arrow) is normal. (C) Note the abnormal intermediate signal and subsequent poor definition of the posterolateral bundle fibers (black arrow); the anteromedial bundle the (white arrow) is normal. (D) Normal posterolateral bundle (black arrow) and anteromedial bundle (white arrow). The remainder of the ACL (not shown) was normal. ACL, anterior cruciate ligament; MRI, magnetic resonance imaging.
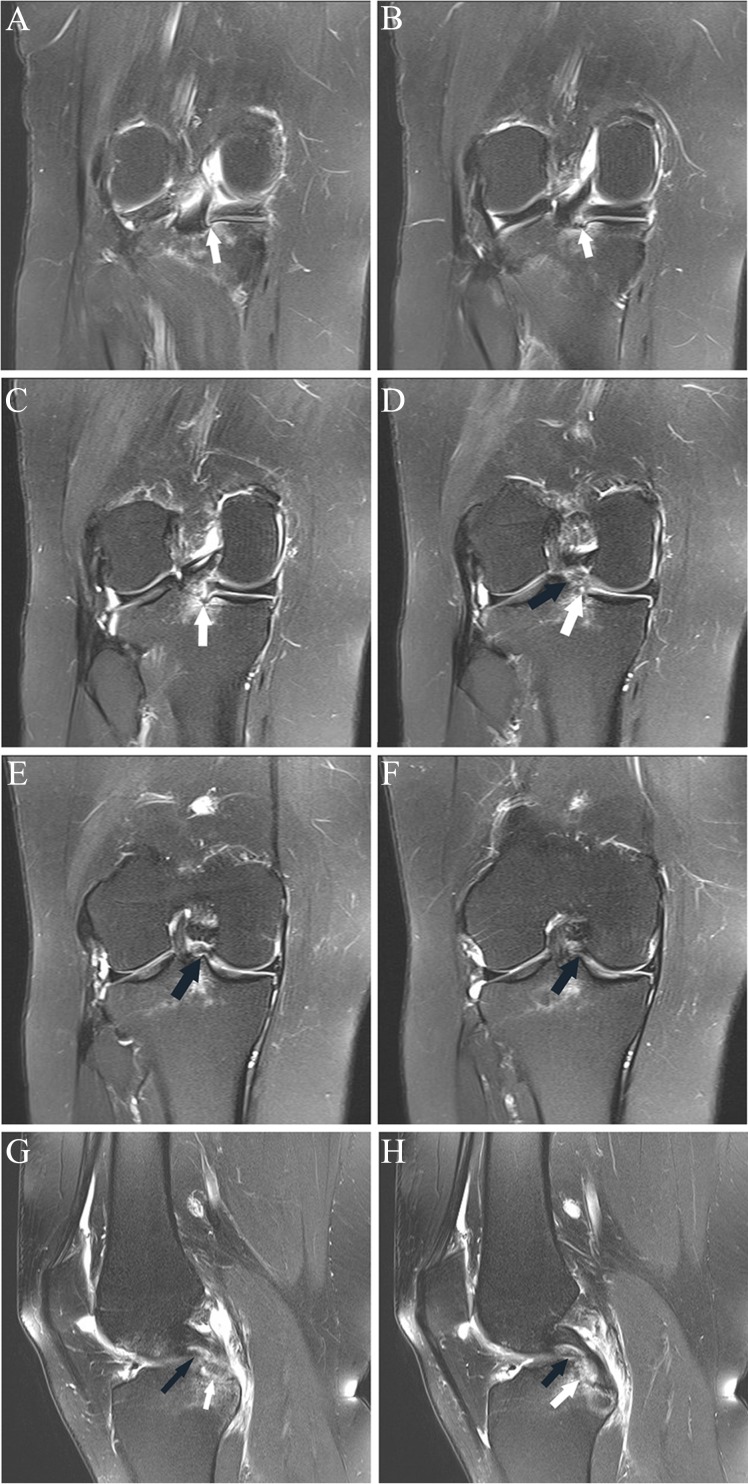
Figure 7.
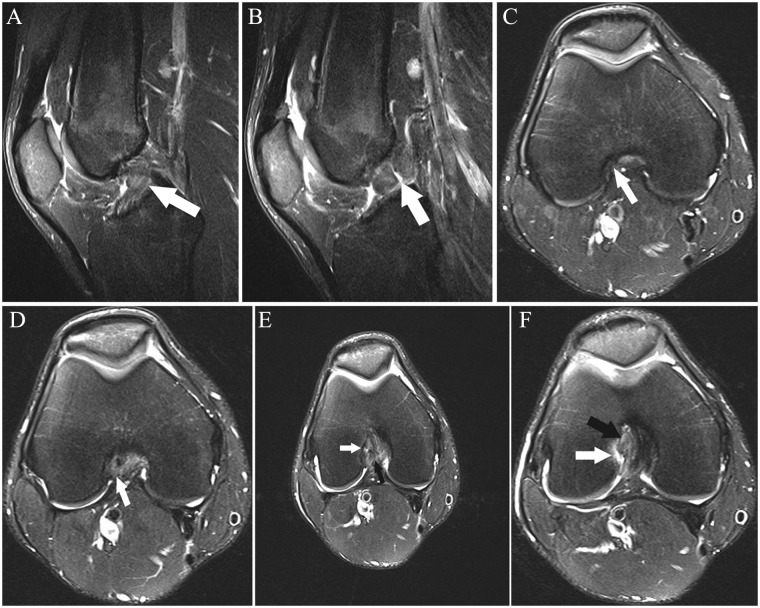
(A and B) Sagittal and (C-F) axial fat-saturated T2-weighted images show high-grade partial ACL tear. One month prior to the MRI, the patient sustained a knee injury playing soccer. At the time of this MRI, the patient had no pain or subjective instability while running; Lachman, anterior drawer, pivot shift, and KT-1000 measurements were all normal. Three weeks following this MRI, the patient had a twisting injury with positive Lachman and anterior drawer immediately following the injury, consistent with ACL rupture; the ACL was subsequently reconstructed. (A) Abnormal thickening and intermediate signal with undulation of the ACL fibers, most prominent in its midportion (arrow). (B) Abnormal thickening and intermediate signal of the mid fibers with prominent slit-like fluid signal (arrow), reflecting tear. (C) Mild abnormal undulation at the femoral insertion of the anteromedial bundle (arrow). (D) Abnormal intermediate signal and poor delineation of the anteromedial bundle fibers (arrow) is present. (E) Focal fluid signal (white arrow) traversing a portion of the posterolateral bundle at its femoral insertion; there are abnormal disorganized fibers and focal abnormal intermediate signal in the proximal anteromedial bundle (black arrow). (F) Focal disruption of the posterolateral bundle fibers, replaced by fluid signal (white arrow), and abnormal intermediate signal in the anteromedial bundle fibers (black arrow). ACL, anterior cruciate ligament.
The evaluation of the reconstructed ACL on MRI follows the same criteria for tear as the native ACL. Acute ruptures usually have associated secondary findings (eg, marrow contusions, hemarthrosis), and the diagnosis is usually more straightforward. Remote tears of the graft are usually more difficult to diagnose as at least some of its fibers may have intermediate signal that makes distinguishing tear from plastic deformation more difficult. In the setting of a remote tear, close scrutiny can reveal torn fibers scarred to the PCL or notch, although this does not differentiate partial tears from ruptures. Anterior tibial translation of more than 5 mm has been shown in a small study of 15 patients to have 90% specificity in differentiating torn from intact grafts; however, it had low specificity in distinguishing partial tears from ruptures as it was present in 57% of partial tears and, surprisingly, only 25% of ruptures.17
Posterior Cruciate Ligament
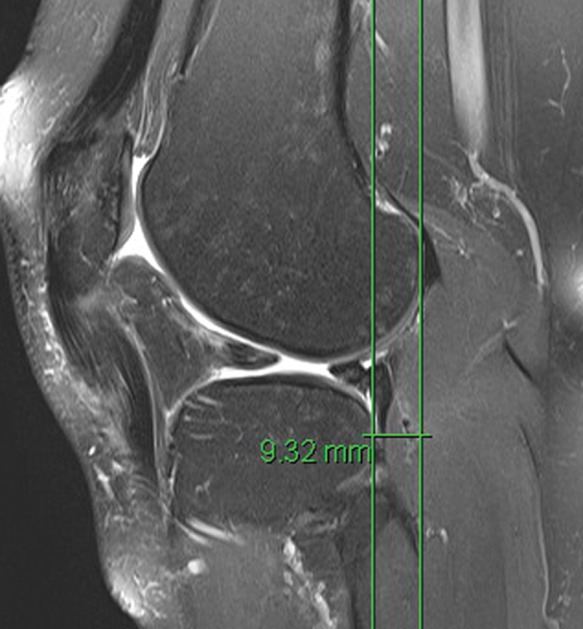
The presence of a marrow edema pattern in the anterior aspect of the medial femoral condyle and/or the medial tibial plateau suggests a recent hyperextension injury and should provoke a thorough evaluation of the PCL and the posterolateral corner structures. When the PCL ruptures, unlike the ACL, it rarely “explodes” and becomes amorphous on MRI, except, in extreme trauma (eg, multiligament knee). Acute PCL ruptures may present as full-thickness fluid signal traversing the ligament, often slitlike. In a minority of patients, the PCL can avulse off the tibia or femur. More commonly, a PCL rupture, both acute and chronic, manifests as focal or diffuse thickening of the ligament, usually with focal or diffuse intermediate intrasubstance signal on short echo time sequences (eg, proton density- and T1-weighted sequences). In a retrospective study of 34 patients with both acute and chronic PCL ruptures with surgical confirmation, Rodriguez et al32 found that the vast majority manifested as abnormal thickening of the mid to distal ligament and intermediate signal on proton density sequences; they found a specificity of 92% for rupture if the thickness of the mid to distal ligament was 7 mm or more and 99% specificity if it was 9 mm or more.
Like the ACL, partial-thickness PCL tears can be diagnosed using MRI. There may be focal thin fluid signal traversing transversely or obliquely in a portion of the ligament. Alternatively, there can be focal intermediate signal within a portion of the ligament; this portion is often focally enlarged. Additionally, longitudinal partial tears occur and manifest on MRI as thin or thick linear, elongated fluid within the ligament (Figure 8). These linear partial tears, or longitudinal splits, can manifest as PCL insufficiency.7
Figure 8.
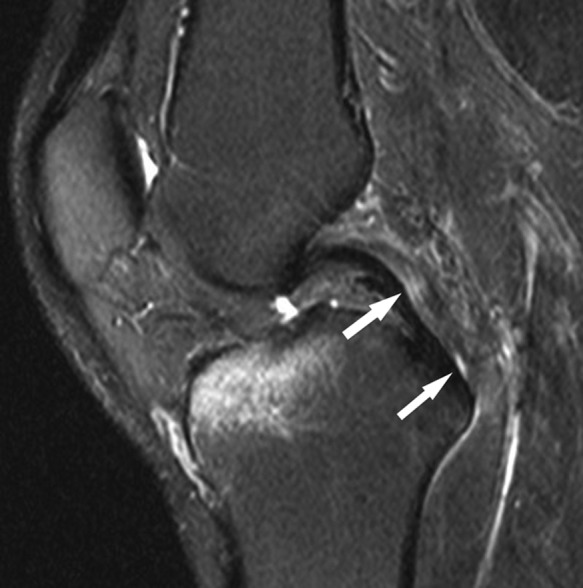
Sagittal fat-saturated T2-weighted image shows thin, elongated fluid signal in the central aspect of the PCL (arrows), representing a linear longitudinal intrasubstance tear. The posterior half of the PCL is diffusely intermediate in signal, reflecting diffuse interstitial injury. Note the marrow edema pattern in the anterior central aspect of the medial tibial plateau, reflecting recent contusion. PCL, posterior cruciate ligament.
Chronic PCL ruptures can infrequently manifest as persistent discontinuity, although the signal intensity on short echo time sequences should still remain hyperintense to normal ligament. More commonly, chronic ruptures on MRI manifest as an irregular, wavy ligament, often focally thinned, with focal or more diffuse intermediate signal (Figure 9) (see Table 2 for a list of imaging “pearls” and potential pitfalls).
Figure 9.
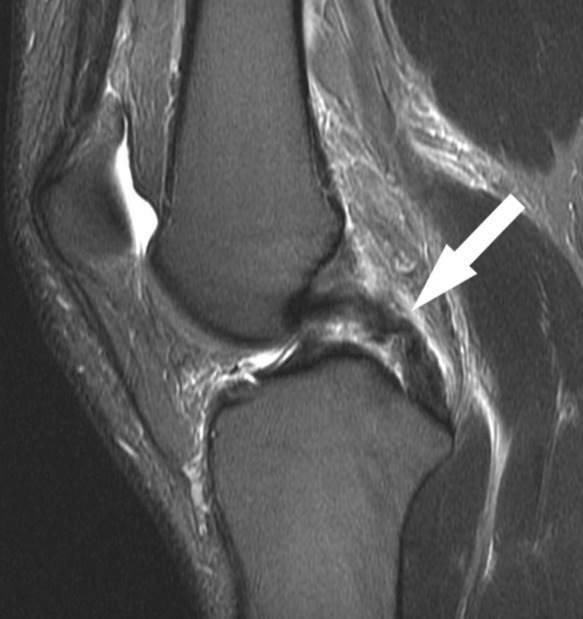
Sagittal fat-saturated T2-weighted image shows diffuse heterogenous intermediate signal and prominent thinning and undulation of the midportion of the PCL (arrow), reflecting chronic PCL tear. PCL, posterior cruciate ligament.
Table 2.
Pearls and pitfalls.
| Posterior meniscus root tears | Always scrutinize the posterior horns of the menisci to their insertions on sagittal and coronal images |
| Popliteus tendon tears | Always assess the popliteus tendon on both coronal and axial images; occasionally, focal distal ruptures may only be definitely visualized on axial images |
| Chronic PCL tears | These tears can be diagnosed when the ligament is either diffusely thickened or irregular in shape and contour, usually with intermediate intrasubstance signal; note that heavily T2-weighted images may obscure the abnormal intermediate signal in chronic tears, unlike proton density–weighted images |
| Meniscus tears | Linear subchondral edema on fat-saturated images is often a clue to an adjacent meniscus tear |
PCL, posterior cruciate ligament.
Shelbourne et al35 found that patients treated nonoperatively for isolated PCL tears usually have good subjective results. In this study in which 133 patients completed questionnaires and over half had final physical examinations at a mean of 5.4 years, they found no significant increase in arthrosis as compared with a control group. In an MRI study of PCL tears, Shelbourne and colleagues36 found that 19 of 22 patients with PCL ruptures and all patients with partial tears had a continuous ligament on MRI at a mean of 3.2 years after the initial MRI, concluding that most PCL tears heal in continuity. A pitfall for this supposition, however, is that imaging the prior PCL tear with more heavily T2-weighted images will result in apparent low signal intensity; the same ligament when imaged on proton density–weighted sequences will typically appear persistently hyperintense.
In a study of 48 patients with an acute tear of the PCL with a minimum follow-up of 1 year, Akisue et al2 found that 36 (75%) patients on follow-up imaging showed a slack but continuous low signal ligament. In these patients, the degree of total anteroposterior tibial translation was significantly less (7.6 ± 3.2 mm) as compared with the 12 patients that had disrupted PCL fibers (11.4 ± 4.4 mm). Twenty-nine of the 33 patients who had a continuous low signal PCL on follow-up imaging had a firm endpoint on posterior drawer testing; 8 of 15 patients who had a disrupted PCL had no firm endpoint. This suggests that those patients whose PCL tears heal in continuity on MRI have less instability.
Posterolateral Corner
Posterolateral corner (PLC) injuries are most commonly seen in the setting of PCL and ACL ruptures. They are relatively rare in isolation. While the PLC consists of many structures, the keystones of PLC stability, including both varus and external tibial rotation stability, include the fibular collateral ligament (FCL), the popliteus tendon (PT), and the popliteofibular ligament (PFL).25,43,44 The mean load to failure is significantly greater for the PT than the FCL and PFL, each less than the failure load of the ACL and PCL; the failure load of the FCL and PFL are not significantly different.23 There should be a heightened suspicion for PLC injuries when a bone contusion is present in the anterior aspect of the medial femoral condyle and/or medial tibial plateau, reflecting a hyperextension-varus injury mechanism. Note that as in all contusions sustained in various injury mechanisms, the resultant marrow edema pattern on MRI may be relatively innocuous in the presence of an underlying ligament rupture or high-grade injury (Figure 10).
Figure 10.
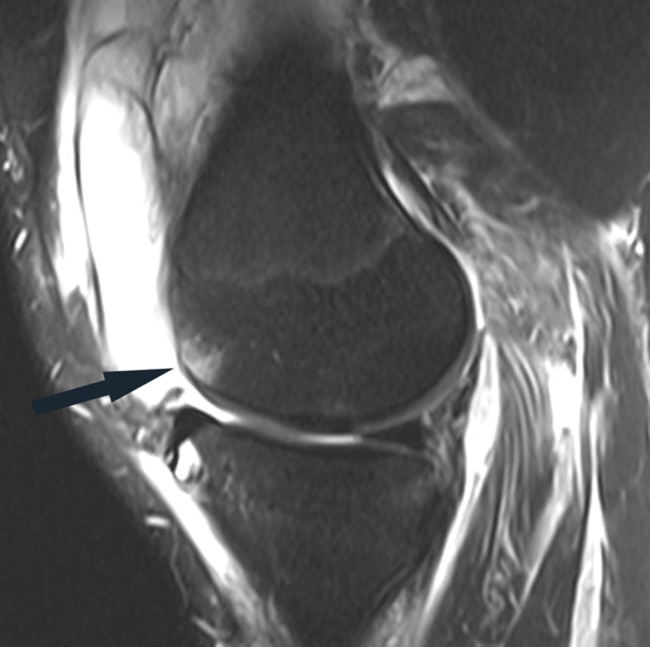
Sagittal fat-saturated T2-weighted image in a patient with acute grade III posterolateral corner injury shows a relatively small marrow edema pattern in the anterior aspect of the medial femoral condyle (arrow), reflecting a hyperextension-varus injury mechanism. Note the large joint effusion and prominent soft tissue edema.
The posterolateral corner structures are best assessed using both coronal and axial sequences. Partial-thickness tears manifest as intermediate or fluid signal within a portion of the structure. Disruptions are seen when intermediate or fluid signal traverses the entire width of the structure; often the involved structure is slightly retracted and has a lax, wavy contour. The FCL can tear anywhere in its extent, from its femoral insertion to the fibular insertion (Figure 11); it most commonly avulses off the fibula, with or without a bone avulsion fragment.24 Isolated tears of the FCL are unusual9 and thus, if seen on MRI, a search should be made for injury to other components of the PLC.
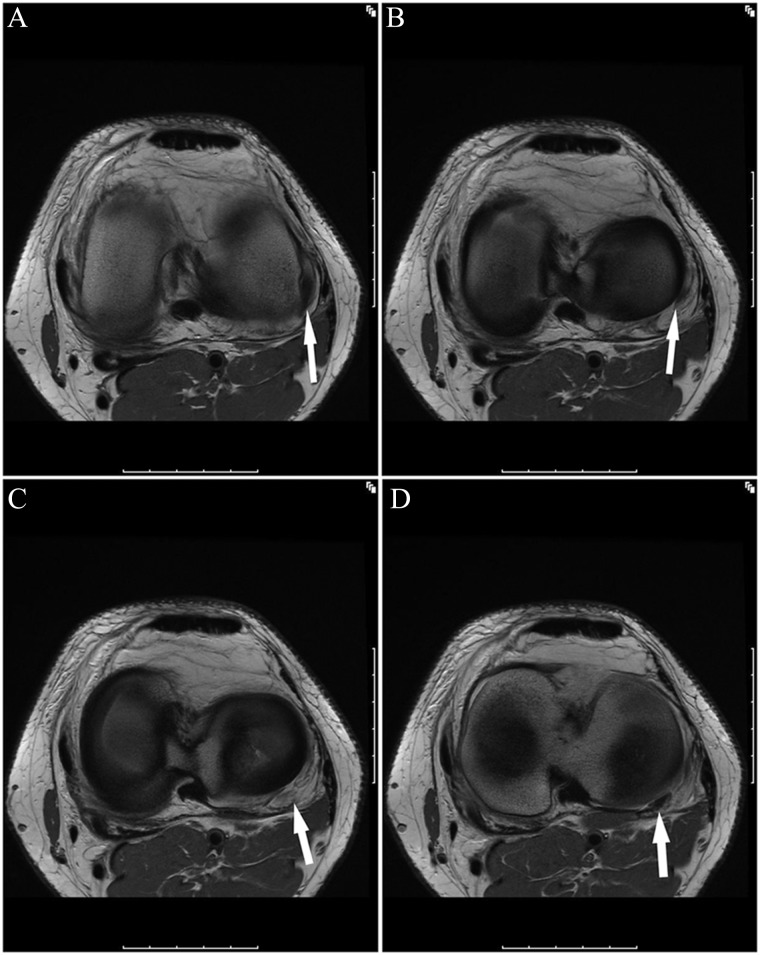
Figure 11.
(A-E, anterior to posterior) Sequential coronal images in a patient who sustained a football injury 1 week prior. (A and B) Normal proximal FCL (arrow) with homogenous low signal; marrow edema pattern predominating in the medial knee with fluid superficial to the MCL likely reflects recent valgus blow/contusion. (C) Normal posterior portion of the proximal FCL (large white arrow) with abnormal intermediate signal and mild edema surrounding the partially visualized mid and distal FCL (large black arrow); note that marrow edema pattern about the insertion of the intact popliteus tendon (intermediate-size black arrow) and the lateral capsule insertion on the tibia (lateral capsular ligament; very thin black arrow) can be associated with tears of these structures and should provoke greater scrutiny, particularly of the popliteus tendon. (D) Focal rupture of the distal fibers of the FCL (arrow) with abnormal intermediate signal proximal and distal to the tear site.
The PFL, arising just distal to the popliteus muscle-tendon junction, can likewise tear anywhere along its length. It is a difficult structure to adequately assess because of the relatively frequent occurrence of lack of clear visualization on coronal images due to volume averaging with adjacent structures. A good anatomical reference for localizing the ligament on coronal images is the inferolateral geniculate artery; the artery is located just lateral and posterior to the ligament. When the ligament is difficult to visualize clearly on coronal images, visualization often is better on sagittal images or, occasionally, on axial images (Figure 12). Not surprisingly, in an MRI study of 20 patients with grade III PLC injuries,24 the sensitivity and specificity of diagnosing a tear of the PFL was 67% and 68%, respectively. In this study, a coronal oblique sequence was used in its evaluation; perhaps using sagittal and axial images would have increased the sensitivity for the detection of tears.
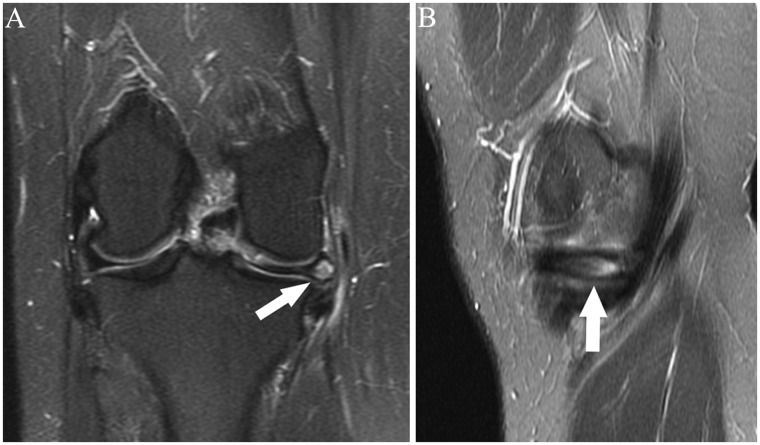
Figure 12.
(A) Coronal and (B) sagittal fat-saturated T2-weighted images show the popliteofibular ligament (arrows). The ligament is incompletely visualized in A. The intact ligament is visualized in its entirety in B.
The PT can avulse off the femur, tear in its midportion, or tear at the muscle-tendon junction. Like the PFL, portions of the PT may be difficult to clearly visualize on coronal images, usually distally as it traverses just posterior to the tibial plateau. As with all the posterolateral corner structures, but particularly for the PT, evaluation on axial images is recommended to ensure a tear is not missed on coronal images (Figure 13).
Figure 13.
Arrows in sequential axial proton density–weighted images from proximal to distal (A-D) mark the course of the popliteus tendon. (B and C) Focal tendon rupture is evident.
MRI has a high sensitivity for detecting acute grade III posterolateral corner injuries.24,29 However, Pacheco et al29 found that MRI detected only 4 of 15 cases when imaged more than 12 weeks after injury, although the study included all grades of PLC injury (percentages were not delineated).
Unlike the MCL, the posterolateral corner structures do not have an intrinsic healing capacity; in an in vivo model of PLC in 14 rabbits, LaPrade et al26 found that no PT rupture and only 1 FCL rupture healed at 14 weeks. In none of the specimens did a significant scar form at the rupture sites. Thus, MRI should be able to detect chronic grade III injuries, manifesting as persistent disruption.
The exact combination or number of PLC structure tears necessary to produce a grade III injury has not been elucidated. In a biomechanical study on cadavers, Gollehon et al15 found that significant varus and external rotation occurred when both the fibular collateral ligament and deep ligament complex (PT and arcuate ligament) were sectioned but not when either was sectioned alone.
Peroneal nerve palsy occurs in up to 30% of cases of acute PLC injuries.9 The common peroneal nerve and the proximal deep and superficial portions can be readily visualized on MRI. Typically, axial images are best for its evaluation, although portions of the nerve are visualized well on sagittal and coronal images. Injury to the nerve typically manifests as increased size of its fascicles and/or increased signal of its fascicles on fluid-sensitive sequences. Neurotmesis/disruption is present when there is frank disruption of its fibers, manifested in the acute phase typically by a fluid-filled gap in the nerve.
Medial Collateral Ligament
The primary planes for assessing the superficial fibers of the medial collateral ligament (MCL) on MRI are the coronal and axial planes. Like the lateral-sided structures, the axial plane should always be utilized in the evaluation of the MCL as partial-thickness tears may not be clearly visualized on coronal sequences because of volume averaging. A grade 1 injury, or sprain, of the MCL is manifested on MRI by edema about the fibers, usually adjacent to its superficial margin, in the presence of intact fibers. Grade 2 injuries are partial-thickness tears. Grade 3 injuries are full-thickness disruptions. Often when there is complete disruption, the MCL has an undulating, lax configuration. The distal MCL should always be followed distally to its insertion, medial to the pes anserine tendons. Occasionally, the MCL in distal disruptions can become superficially located with respect to the pes anserine tendons, analogous to a Stener lesion of the thumb10 (Figure 14).
Figure 14.
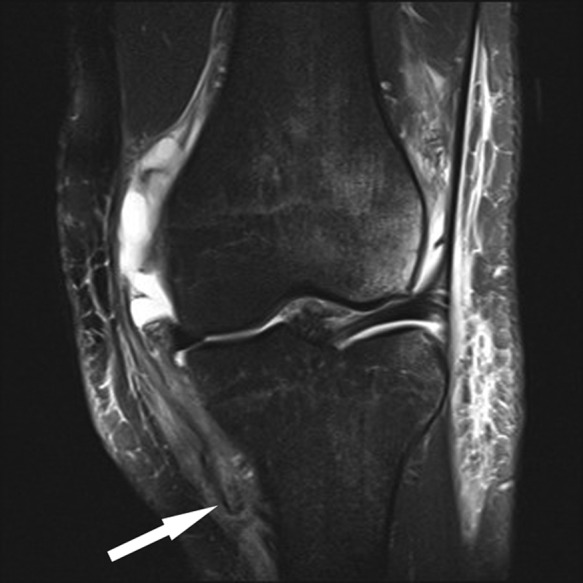
Coronal fat-saturated T2-weighted image shows the disrupted distal superficial MCL fibers (arrow) located superficial to the pes anserine tendons. Subchondral marrow edema pattern in the lateral femoral condyle reflects transchondral impaction of acute ACL rupture (not shown). ACL, anterior cruciate ligament; MCL, medial collateral ligament.
Menisci
Short echo-time sequences (eg, proton density- and T1-weighted) are the most sensitive for detection of signal in fibrocartilaginous structures, including the meniscus. The criteria for diagnosing a meniscus tear on MRI include abnormal meniscus shape and/or signal contacting the meniscus superior or inferior articular surface. The signal can either be an intermediate or fluid signal. Specificity for diagnosing meniscus tears is improved if abnormal shape and/or signal is visualized on 2 or more images, whether in the same plane or not.14
Secondary signs of meniscus tears include meniscus extrusion and subchondral edema. Linear subchondral edema is often present adjacent to a meniscus tear; if it is present, this can be a clue to an adjacent tear (Figure 15). Meniscus extrusion more than 3 mm for the medial meniscus often occurs in the setting of complex or high-grade root tears of the posterior horn, although it may be seen in the setting of marked meniscus degeneration and medial compartment osteoarthritis with Fairbank’s signs.11,21
Figure 15.
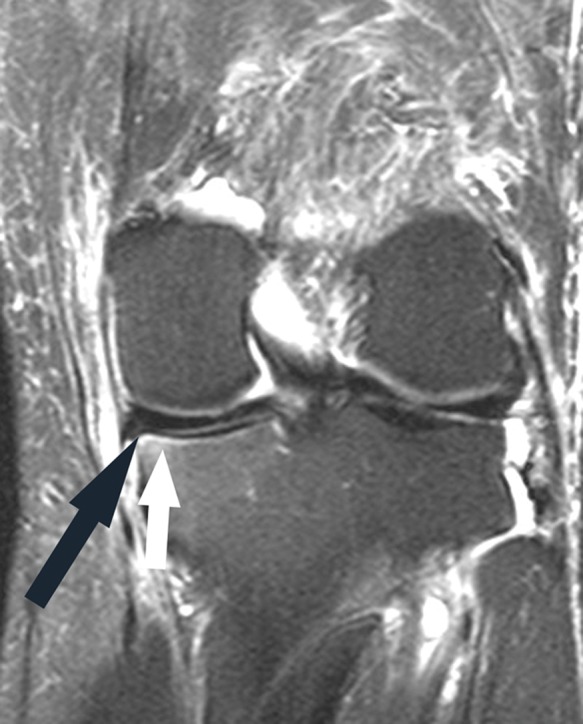
Coronal fat-saturated T2-weighted image shows linear subchondral edema in the medial tibial plateau (white arrow), most intense adjacent to a tear of the periphery of the posterior horn medial meniscus (black arrow).
If a portion of the meniscus is absent (eg, the free edge is abnormally blunted instead of the usual triangular shape), there are only 2 reasons: either there has been prior debridement/resection or the meniscus is torn, oftentimes manifesting as a displaced meniscus flap (Figure 16). Meniscal tissue does not usually macerate and resorb; therefore, in the setting of absent meniscus tissue and no prior debridement, a meniscus tear is present. A meniscus flaps or displaced meniscal tissue is nearly always the same signal (ie, isointense) to the remainder of the meniscus on all pulse sequences (Figure 17), unless there is extensive intrasubstance degeneration of the displaced tissue.
Figure 16.
(A-F) Sequential coronal fat-saturated T2-weighted images show an abnormal vertical margin of the posterior root medial meniscus (arrows in A-C), consistent with tear; (A and B) note the adjacent linear subchondral edema. (D) The far anterior portion of the posterior root has a prominent intermediate signal (white arrow). There is an abnormal low signal structure just superior and central to the posterior root (black arrows in D-F), consistent with a posterior root flap; if a structure of similar signal to the meniscus is present in an abnormal location, it is most likely a displaced meniscus flap. (G and H) Sagittal fat-saturated T2-weighted images in this case show intermediate signal in the posterior root in G (white arrow), consistent with degeneration. The posterior root is barely recognizable in H (white arrow), reflecting tear. Small, elongated low signal structure just anterosuperior to the root (black arrows in G and H) represents the displaced meniscus flap, as seen in images D through F.
Figure 17.
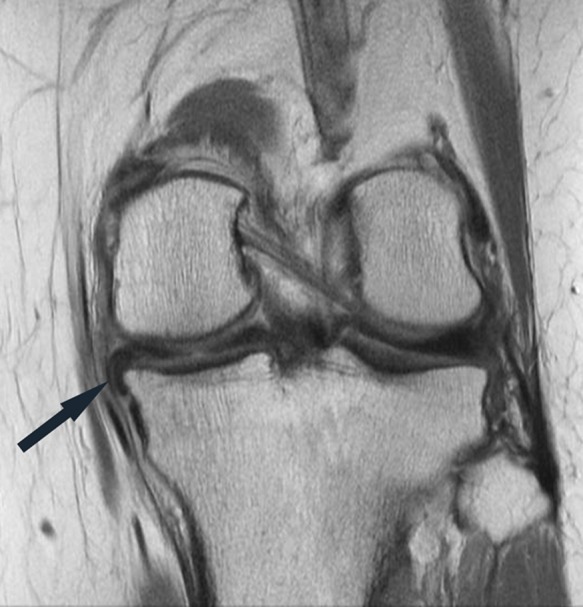
Coronal proton density–weighted image shows tissue isointense to the menisci adjacent to the posterior horn-body junction medial meniscus in the caudal gutter (arrow), reflecting a displaced meniscus flap.
A corollary is that if an isointense structure is present where there should be none, it is either ligament or, more commonly, a meniscus flap. Occasionally, an aberrant ligament extension to the meniscus or an oblique meniscomeniscal ligament may be present (Figure 18). The oblique meniscomeniscal ligament is present in less than 5% of patients and can be followed in the notch extending from the anterior horn of one meniscus to the posterior horn of the other.33 If tissue isointense to the meniscus is visualized, following its course on sequential images usually allows for detection of its origin.
Figure 18.
Sagittal fat-saturated T2-weighted images from lateral to medial (A-F) show a thin ligament connecting the posterior horn lateral meniscus to the anterior horn medial meniscus (arrows in B-E), a medial oblique meniscomeniscal ligament. The posterior horn lateral meniscus (arrow in A) and anterior horn medial meniscus (arrow in F) are normal.
Tears of the posterior root attachments of both menisci lead to significantly increased load in the respective compartments due to loss of hoop containment.3,34 Significantly increased compartment loads result in early osteoarthritis due to the resultant load transfer on the articular cartilage.16,18 Thus, it is crucial to diagnose these tears on MRI. The tears are most commonly radial splits or complex tears. They are common in middle-aged, often obese patients, particularly women in which they more commonly involve the medial meniscus.19 Brody et al5 and Laundre et al27 found a higher incidence of posterior root tears of the lateral meniscus in the setting of ACL rupture. De Smet et al13 found that posterior root tears of the lateral meniscus were 10 times more frequent in patients with ACL ruptures than in those with an intact ACL.
Posterior root tears of either meniscus can easily be missed on MRI if the roots are not closely scrutinized. In all patients, the posterior roots of both menisci should be assessed all the way to their insertions on both coronal and sagittal images (Figures 19, and 20). Not uncommonly, all or a portion of the meniscus may be nonvisualized on a sagittal image, reflecting a tear in the plane of the image. Occasionally, a posterior root tear can be confirmed in the axial plane (Figure 21). The posterior roots are vascularized and can be repaired.1,3,22
Figure 19.
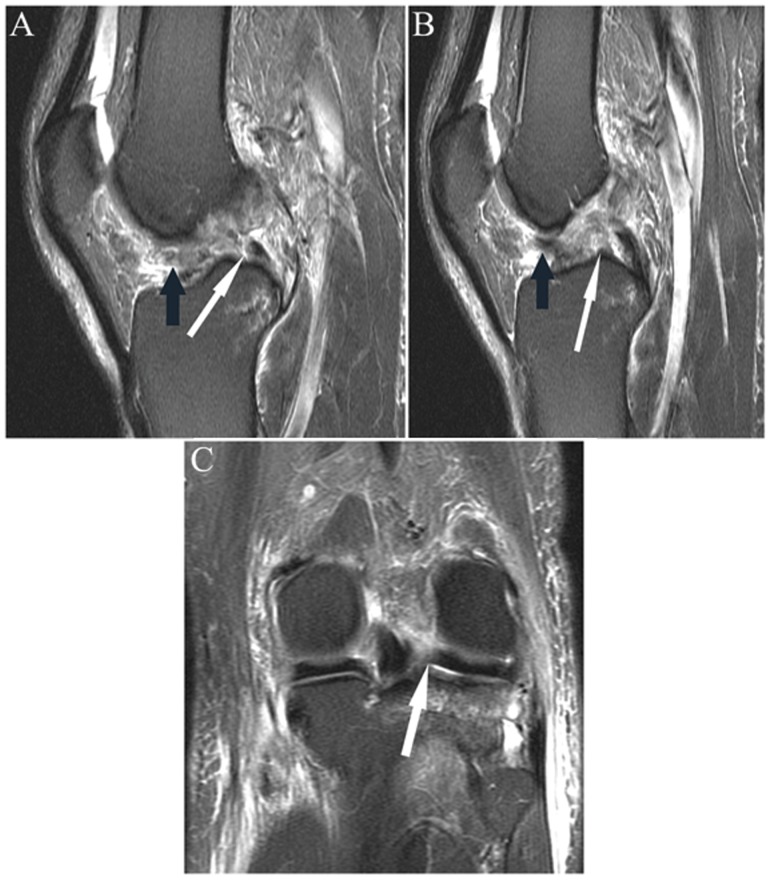
Sequential coronal fat-saturated T2-weighted images from anterior to posterior (A-C) show an abnormal contour of the posterior root medial meniscus (arrow in A) with complex fluid signal, reflecting complex tear with radial split (at tip of arrow in B). (C) Note the extrusion (ie, peripheral to the margin of the plateau) of the posterior horn (more medial arrow) with a small subchondral fracture of the medial margin of the femoral condyle (more lateral arrow). (D) Sagittal fat-saturated T2-weighted image shows abnormal contour and irregular high signal in the posterior root (white arrow), reflecting the tear; incidentally noted is focal low signal about a tendon in the anterior semimembranosus muscle (black arrow), reflecting fat in a small lipoma that was better visualized on adjacent images.
Figure 20.
(A and B) Sagittal and (C) coronal fat-saturated T2-weighted sequences in a patient with acute ACL rupture. Note the focal absence of the anterior portion of the posterior horn root lateral meniscus (white arrow in A) with absence of the expected low signal root in the subsequent more central image (white arrow in B), reflecting root tear/“avulsion.” Figure C shows the tear in the coronal plane (arrow). Note fibers of the ACL displaced anteriorly in the notch (black arrows in A and B). ACL, anterior cruciate ligament.
Figure 21.
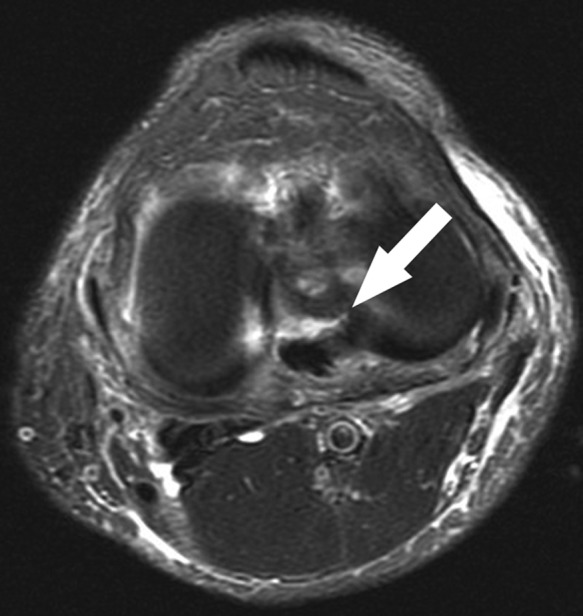
Axial fat-saturated T2-weighted sequence of the same patient as in Figure 14 shows the tear of the posterior root lateral meniscus well (arrow). Occasionally, axial images through the joint line show meniscus tears to good advantage, although frequently volume averaging with adjacent structures precludes definitive visualization. Note the multifocal thin fluid along the superficial crural fascia, reflecting shearing at the fat-fascial interface, a good clue to recent pivoting/twisting injury.
Peripheral vertical longitudinal tears of the posterior horn of either meniscus are overwhelmingly seen in the presence of an ACL tear, either acute or chronic (Figure 22). This type of tear in the setting of ACL rupture more frequently involves the lateral meniscus. These tears can occur at the insertion of the meniscofemoral ligament(s), reflecting a cleavage plane at the site of its insertion on the lateral meniscus; Park et al30 found that insertion of the meniscofemoral ligaments 16 mm or more lateral to the PCL on sagittal images had a high specificity for peripheral vertical tears of the lateral meniscus.
Figure 22.
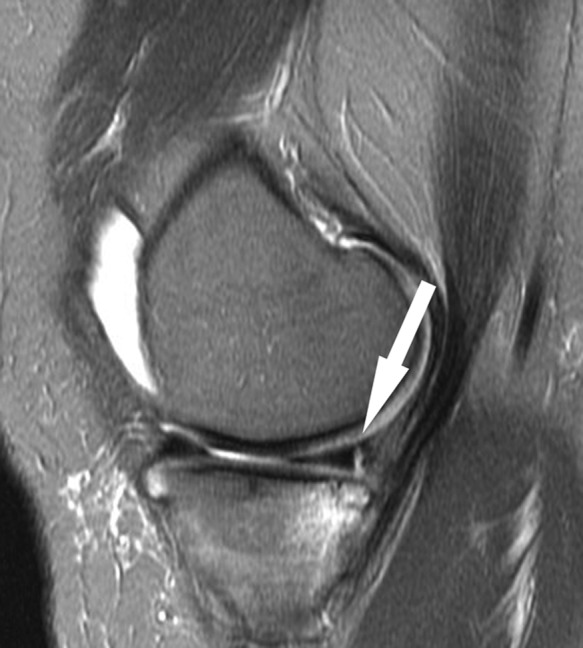
Sagittal fat-saturated T2-weighted sequence shows a vertical longitudinal tear of the peripheral vascular segment of the posterior horn medial meniscus (arrow) in the presence of acute ACL rupture (not shown). Peripheral vertical tears not infrequently are difficult to distinguish from meniscocapsular separation; the difference is that the former have tissue that is isointense to meniscus, oftentimes very thin as in this case, located just peripheral to the tear. Note the subchondral fracture of the adjacent tibial plateau, reflecting contrecoup impaction after ACL rupture. ACL, anterior cruciate ligament.
Meniscocapsular separation can be difficult to distinguish from far peripheral vertical longitudinal tears of the posterior horn of the medial meniscus. The most specific criterion is visualizing fluid, oftentimes thin, completely interposed between the posterior horn and the capsule (Figure 23). The correlate in the posterior horn of the lateral meniscus is disruption of the popliteomeniscal fascicles, which can render the posterior meniscus hypermobile. It may be difficult to distinguish between peripheral vertical tears and meniscocapsular separation in the evaluation of the posterior horn or body segment of the medial meniscus, particularly when there is incomplete fluid abutting the peripheral margin of the meniscus, simulating a peripheral vertical tear.
Figure 23.
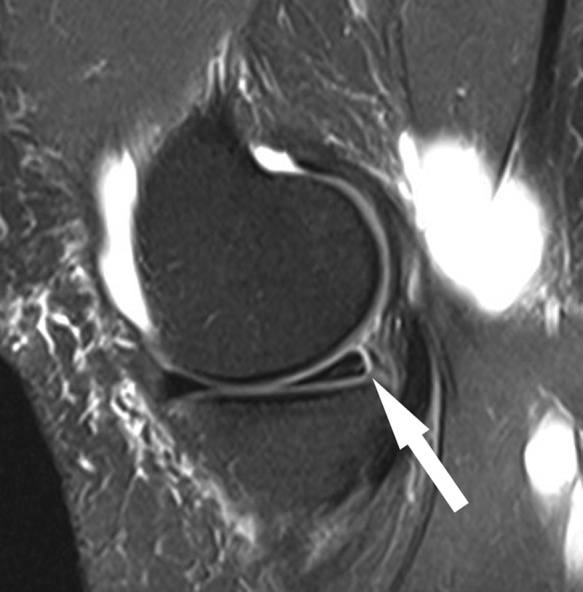
Sagittal fat-saturated T2-weighted sequence shows medial meniscocapsular separation with thin fluid interposed between the posterior horn of the medial meniscus and capsule (arrow). Globular fluid in posterior soft tissues is a popliteal cyst.
Parameniscal and intrameniscal cysts are more frequent in the medial meniscus (Figure 24).6 Parameniscal cysts are usually small fluid collections that most often communicate with a meniscus tear, usually a horizontal tear or a complex tear with a horizontal component.6 An intrameniscal cyst is seen on MRI as a globular signal within and expanding the confines of a portion of the meniscus. Infrequently, intrameniscal cysts can be symptomatic in the absence of a communicating tear; treatment in these cases includes percutaneous decompression or unroofing and debridement of the cyst and subsequent repair.4
Figure 24.
(A) Coronal and (B) sagittal fat-saturated T2-weighted images show prominent enlargement and intermediate to high signal in the body segment medial meniscus (arrows), representing a meniscal cyst. Note how it bulges the outer margin of the meniscus, slightly displacing the adjacent posterior portion of the superficial MCL. No definite meniscus tear was noted communicating with the cyst; a meniscus tear is present in the majority of these cases. MCL, medial collateral ligament.
Patellofemoral Joint
The medial patellofemoral ligament (MPFL) is the major medial soft tissue stabilizer of the patella. It is a very thin, elongated structure in its entire course (Figure 25). It has a consistent close intermingling with the aponeurosis and/or fibers of the undersurface of the vastus medialis obliquus (VMO) muscle.31,37,38 Various cadaveric dissections examining its insertion at the femur and patella have come to varying conclusions. Smirk and Morris37 found that it most commonly inserted on the posterior aspect of the medial epicondyle, roughly 10 mm distal to the adductor tubercle; they found that the vast majority inserted on the “superomedial” patella, while 8% inserted on the inferomedial patella. In a cadaveric study, Philipott et al31 found that for MPFL reconstruction to have isometry, the reconstruction needed to be 10 mm posterior to the medial femoral epicondyle and 10 mm distal to the adductor tubercle; it inserted on the proximal half of the medial patella. Tuxoe et al38 found that the majority inserted on the adductor tubercle just proximal to the insertion of the MCL and distal to the insertion of the adductor magnus tendon; it inserted on the proximal two-thirds of its medial margin. Thus, there are varying conclusions as to the ligament’s exact insertion sites on the femur and patella.
Figure 25.
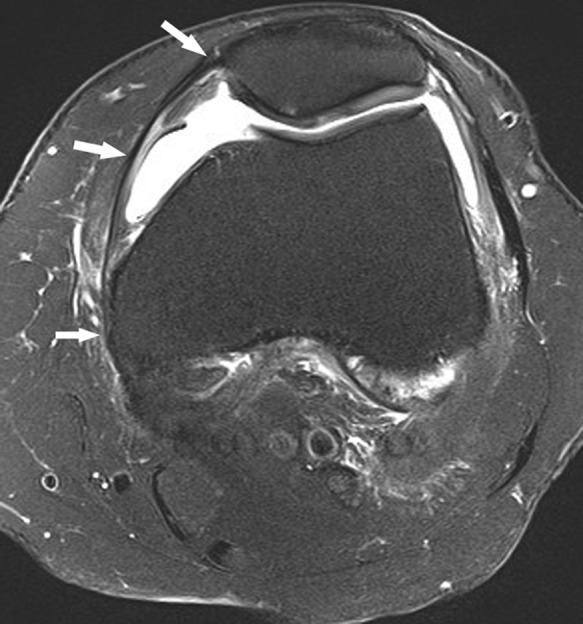
Axial fat-saturated T2-weighted image shows the course of a normal MPFL (arrows). It is unusual to see the ligament in its entirety on a single axial image. MPFL, medial patellofemoral ligament.
The most obvious clue to acute or subacute injury of the MPFL is detection of recent lateral patellar dislocation, uniformly associated with marrow edema in the medial margin of the patella and marrow edema at the impaction site on the lateral femoral condyle. The impaction site on the lateral femoral condyle is nearly always more anterior than the typical impaction, site sustained during ACL rupture. MPFL tears can occur anywhere along its course, from its femoral to its patellar insertion (Figure 26). Frank disruptions are usually obvious, revealing disruption of its fibers; soft tissue edema adjacent to the ligament is a good clue to the site of injury. A common finding of disruption of the MPFL at the femur is fluid extending about the posterior and lateral margins of the distal VMO muscle (Figure 27). The MPFL, like all ligaments, should have uniform low signal on all pulse sequences. Intermediate or high signal present within a portion, or sometimes the entire, MPFL reflects interstitial or partial-thickness tearing.
Figure 26.
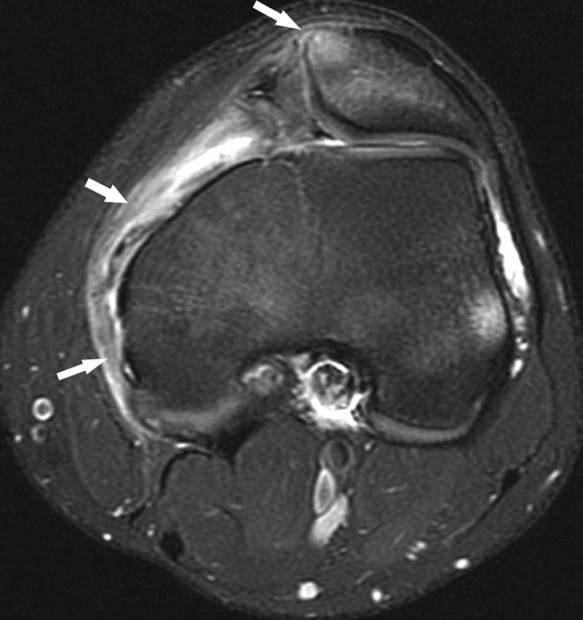
Axial fat-saturated T2-weighted image shows diffuse intermediate signal and thickening of the entire course of the MPFL, consistent with interstitial tear, as well as partial-thickness tear of the ligament at the patella, denoted by arrows. Note the marrow edema pattern in the medial patellar facet, reflecting contusion after recent dislocation. There is a shallow, dysplastic trochlea. (Focal high signal at the lateral femoral epicondyle reflects magnetic susceptibility artifact related to femoral tunnel fixation, located just proximal to image, of ACL reconstruction.) MPFL, medial patellofemoral ligament; ACL, anterior cruciate ligament.
Figure 27.
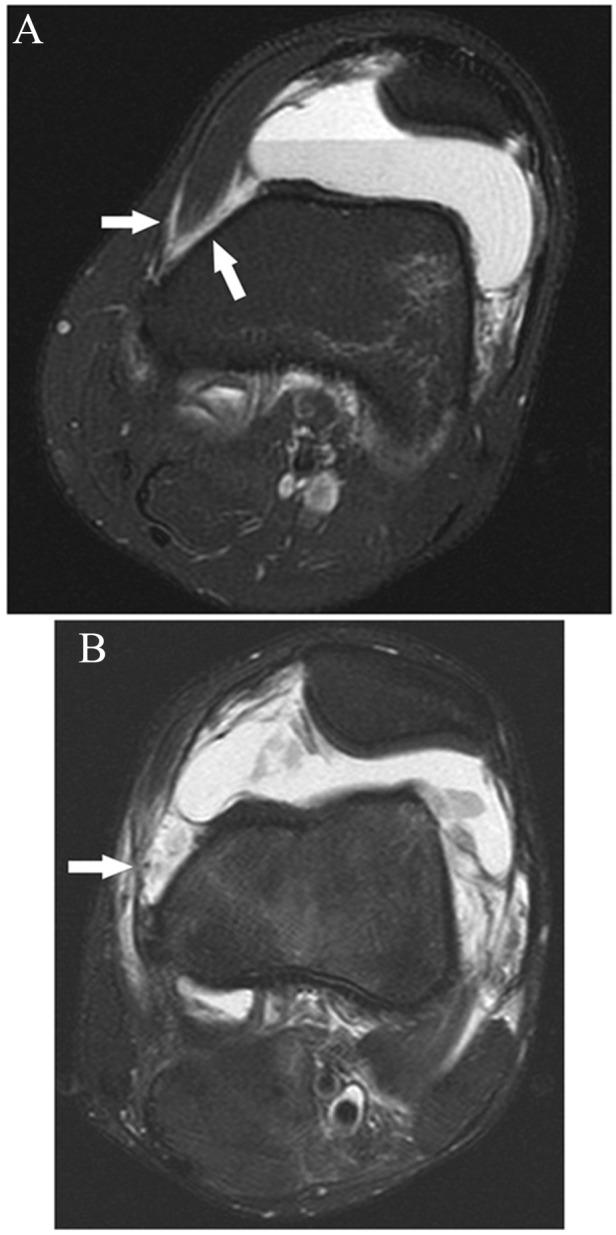
Axial fat-saturated T2-weighted images following acute patellar dislocation. Thin extracapsular fluid (arrows in A) extending posterolaterally about the VMO muscle was noted due to focal disruption of the proximal portion of the MPFL (arrow in B). VMO, vastus medialis obliquus; MPFL, medial patellofemoral ligament.
Chronic MPFL tears are more difficult to diagnose. The MPFL may be focally or diffusely attenuated or abnormally thickened and irregular in contour. Another clue to a remote tear is proliferative change at its insertions, most frequently noted at the patella.
Pathomorphology predisposing to patellar dislocation can be readily assessed with MRI. The trochlear groove-tibial tubercle (TT-TG) distance can be measured on axial images as the transverse distance between the trochlear sulcus and the apex of the tibial tubercle (Figure 28). Patella alta can be diagnosed with typical measurements on sagittal images. Trochlear dysplasia likewise can be readily assessed on axial images.
Figure 28.
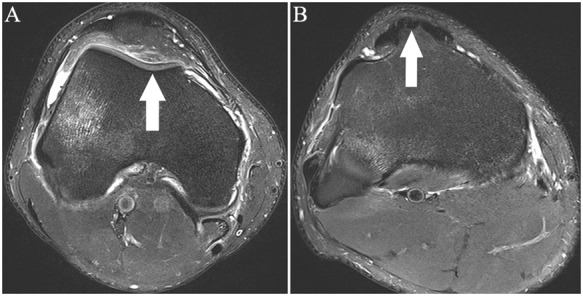
Axial fat-saturated T2-weighted images show measurement of the TT-TG distance. The trochlea groove (TG) is the deepest point of the concavity of the trochlea (arrow in A). The apex of the tibial tubercle (arrow in B) denotes TT. The transverse distance between the two points is the TT-TG distance. TT-TG, trochlear groove-tibial tubercle.
Chondral shear injuries, typically occurring over the medial patellar facet and lateral trochlea, are readily visualized, both with axial and complementary sagittal images. Importantly, determination can be made as to the presence of tidemark attached to the displaced chondral fragment(s); tidemark is present when there is uniform thin, linear low signal along 1 side of the fragment (Figure 29). This distinction is clinically relevant, as the presence of adjacent subchondral bone with the tidemark may make the displaced fragment amenable to primary repair.
Figure 29.
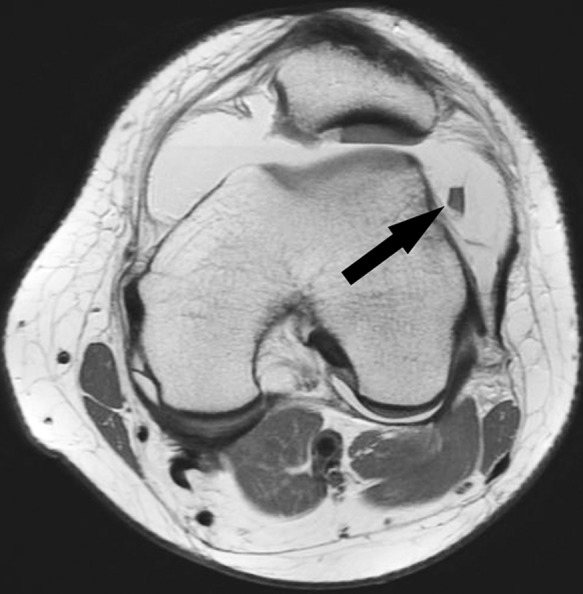
Axial proton density-weighted image following acute patellar dislocation shows a full-thickness chondral shear of the central portion of the lateral patellar facet. Arrow denotes the displaced chondral fragment; note the thin low signal margins on both sides of the fragment, one reflecting tidemark and the other lamina splendens (the most superficial layer of hyaline cartilage), as is seen in the intact lateral patellar facet cartilage. Note the fibrillation and full-thickness fissure of the cartilage over the medial facet and large joint effusion containing a faint fluid-fluid level, reflecting hemarthrosis.
Unique to the pediatric and adolescent population are patellar sleeve avulsions. These appear on MRI as marrow edema in the inferior pole of the patella with variable chondral or osteochondral displacement of the inferior pole (Figure 30). The extent of displacement of cartilaginous avulsions, obviously occult on radiographs, can be accurately assessed on MRI using sagittal and occasionally coronal images.
Figure 30.
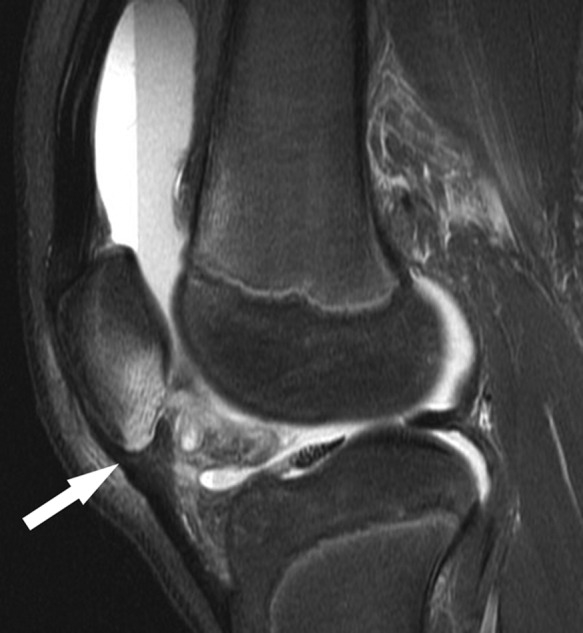
Sagittal T2-weighted image shows marrow edema pattern in the inferior pole of the patella and high signal in the nondisplaced overlying cartilage (arrow), reflecting a nondisplaced patellar sleeve injury. Note the large hemarthrosis.
Cartilage
Chondral defects can be accurately assessed with MRI, particularly with higher field strength magnets and optimal pulse sequence selection. Volume averaging not infrequently poses challenges in not only determining the depth but also the extent of chondral lesions. Therefore, it is recommended to routinely assess the cartilage in each joint in 2 planes (eg, the patellofemoral joint cartilage and the cartilage over the posterosuperior margin of the femoral condyles in both sagittal and axial planes); frequently, chondral defects are more accurately evaluated in 1 of the 2 planes. Full-thickness defects with exposed bone commonly have adjacent subchondral edema on fluid-sensitive sequences. Chondral shear injuries typically have well-shouldered margins that are easily visible on MRI. Chondral delamination, typically due to shear injury, is visualized when thin fluid signal parallels the tidemark and often heralds a displaced flap (Figure 31).
Figure 31.
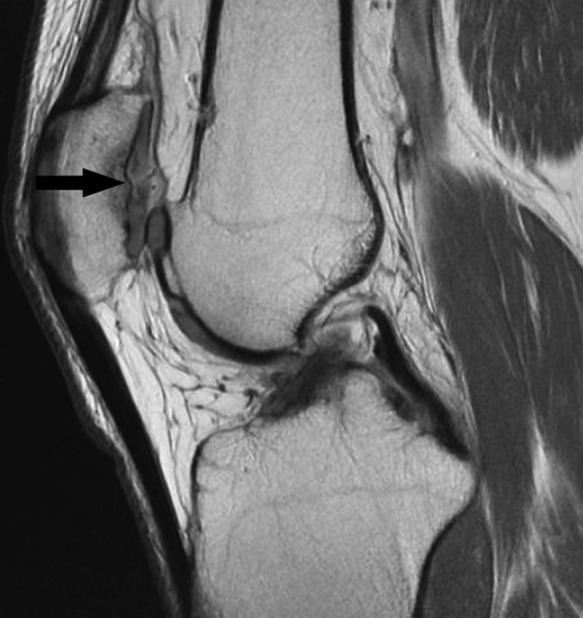
Sagittal proton density–weighted image shows a full-thickness chondral fissure over the patella apex communicating with perpendicular thin similar high signal (arrow) paralleling the tidemark (adjacent low signal), reflecting chondral delamination.
Bone
Subchondral fractures may occur in the setting of meniscus tears, nearly always in the setting of posterior root tears of either meniscus or after partial meniscectomy, most likely due to increased loads produced in these settings. These fractures are diagnosed when thin linear or curvilinear subchondral low signal is present, surrounded by a marrow edema pattern that is disproportionate to the size of the fracture (Figure 32). When a marked subchondral marrow edema pattern is present in a femoral or tibial condyle, a search should always be made for an underlying subchondral fracture. The fractures can progress to osteonecrosis and subchondral collapse, particularly if protected weightbearing is not instituted.
Figure 32.
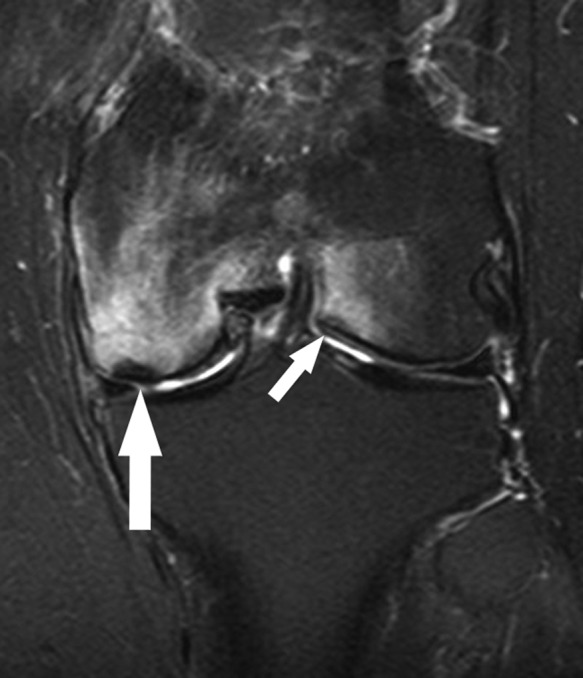
Six weeks after partial medial meniscectomy, coronal fat-saturated T2-weighted image shows a subchondral fracture of the medial femoral condyle (large arrow) with marked surrounding marrow edema pattern as well as a smaller subchondral fracture of the central aspect of the lateral femoral condyle (small arrow) with less extensive adjacent marrow edema pattern. This case is unusual given subchondral fractures in both compartments; usually, the fracture occurs in the compartment where there has been prior partial meniscectomy or where there is a radial split or complex tear of the posterior horn root.
Avascular necrosis/osteonecrosis can occur in the knee as in any other portion of the skeleton. Findings are no different than in other locations: the involved bone is circumscribed by a double-layered serpentine margin that is internally hyperintense and externally hypointense on fluid-sensitive sequences (Figure 33).
Figure 33.
(A) Coronal fat-saturated T2-weighted image shows multiple geographic areas with serpentine margins, predominantly of high signal with 2 discrete areas of high signal marginated by low signal (arrows), reflecting the interface between granulation tissue and necrotic bone (the so-called “double-line sign” of osteonecrosis). (B) Sagittal fat-saturated T2-weighted image again shows a geographic area marginated by predominantly high signal extending to the intact subchondral plate of the medial femoral condyle with 2 areas demonstrating the classic “double-line sign” (arrows). (C) A sagittal T1-weighted image in the same location as B shows the large area of osteonecrosis with predominantly intermediate signal margins with 2 discrete areas of low signal (arrows), reflecting necrotic bone. This patient had taken corticosteroids and bevacizumab (Avastin; Genentech, South San Francisco, California) as therapy for pilocytic astrocytoma. The patient also had osteonecrosis of both humeral and femoral heads.
Tibiofibular Joint
The anterior and posterior tibiofibular ligaments, thin ligaments that extend superomedially from the fibula to the tibia, can be identified on MRI. Because of the ligaments’ relatively short oblique course, accurate assessment of their integrity is often challenging on MRI. In cases of subluxation or prior dislocation, abnormal intermediate signal, frank disruption, or absence of 1 or both of the ligaments can be seen, usually with surrounding marrow and soft tissue edema in the acute or subacute stage (Figure 34). These findings can be seen in chronic tibiofibular instability as well, usually without the adjacent edema.
Figure 34.
Axial fat-saturated T2-weighted images in this case of anterolateral fibula dislocation. (A) Tear of the anterior tibiofibular ligament at the tibia with small fluid-filled gap; the thin hypointense band coursing to the tibia just lateral to the arrow is a portion of the biceps tendon expansion to the tibia. (B) Disruption of the posterior tibiofibular ligament at the fibula, denoted by the arrow. Note the surrounding soft tissue edema, including high-grade tear of the fibular origin of the soleus muscle. (C) For comparison, normal anterior and posterior tibiofibular ligaments are shown (arrows).
Synovial Pathology
Imaging of synovial disorders is beyond the scope of this article. Synovitis can be visualized on MRI without the use of intravenous contrast. Synovitis is usually seen as small punctate and/or linear low signal foci within the joint fluid, best visualized on fluid-sensitive sequences (Figure 35). It is most easily seen in the suprapatellar pouch because of its relative capaciousness, although it can be visualized on MRI in all locations. In the setting of chondral loss, synovitis is usually attributed to the associated upregulation of inflammatory mediators. However, in the absence of chondral loss, other etiologies should be considered; differential considerations in this setting include a systemic inflammatory arthropathy, a crystalline arthropathy (eg, gout), and infectious arthritis. Importantly septic arthritis, unlike most arthritides, typically manifests as prominent pericapsular edema involving the surrounding soft tissues and musculature as well as a prominent focal or multifocal subchondral marrow edema pattern (Figure 36). If focal or diffuse conglomerate/masslike synovial proliferation is present, typical considerations include pigmented villonodular synovitis (PVNS) or synovial chondromatosis. Nodular deposits of PVNS are typically of intermediate signal intensity on proton density–weighted images and occasionally demonstrate low signal intensity foci due to the deposition of hemosiderin, which distinguishes these lesions from chondral loose bodies.
Figure 35.
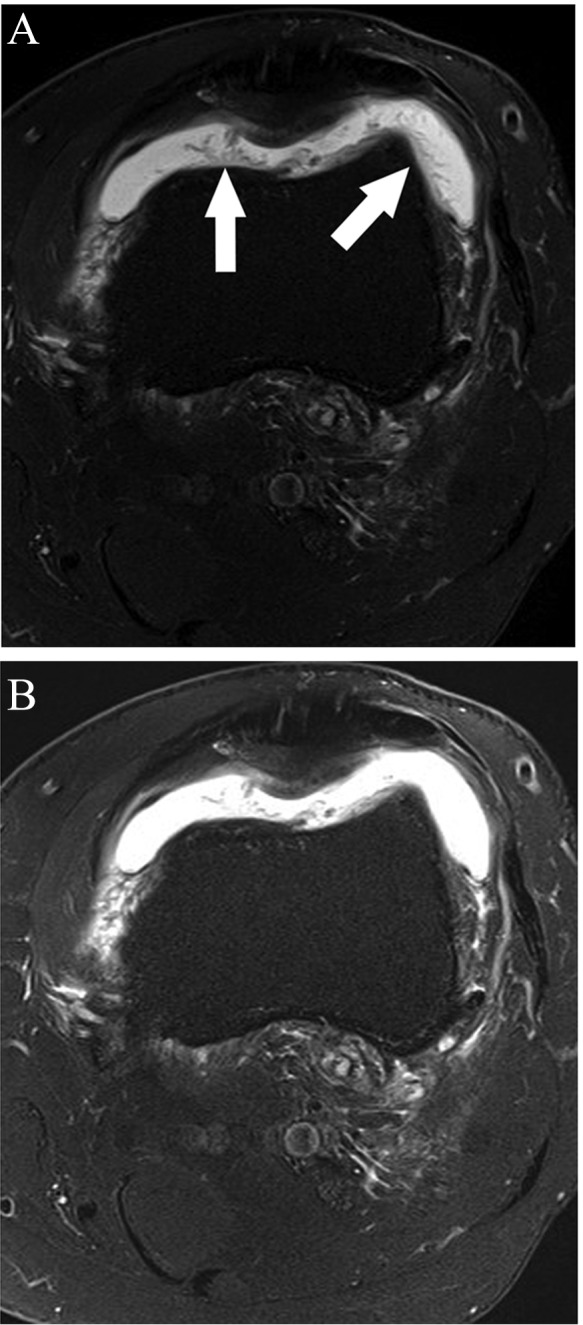
(A) Axial fat-saturated T2-weighted sequence shows marked frondlike synovial thickening, denoted by arrows. Note that decreasing contrast (“windowing” the images) on fluid-sensitive sequences allows for better detection of synovitis (B, for comparison, is the same image prior to decreasing contrast).
Figure 36.
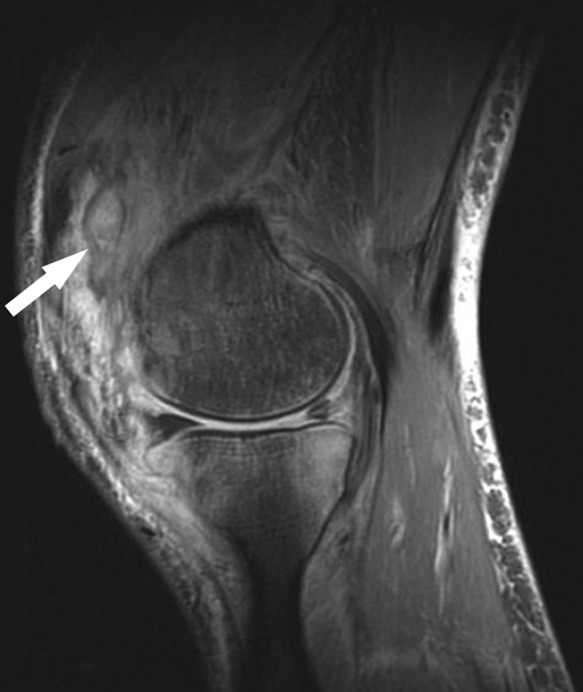
Sagittal fat-saturated T2-weighted sequence shows extensive muscle and subcutaneous edema with synovitis (arrow), reflecting septic arthritis. Confluent marrow edema pattern in the posterior aspect of the medial tibial plateau is suspicious for osteomyelitis. This was 6 weeks after partial medial meniscectomy.
Acknowledgments
The author would like to thank Hollis G. Potter, MD, Hospital for Special Surgery, for her analysis and insightful comments in the production of this article.
References
- 1.Ahn JH, Lee YS, Chang JY, et al. Arthroscopic all inside repair of the lateral meniscus root tear. Knee. 2009;16:77-80 [DOI] [PubMed] [Google Scholar]
- 2.Akisue T, Kurosaka M, Yoshiya S, et al. Evaluation of healing of the injured posterior cruciate ligament: analysis of instability and magnetic resonance imaging. Arthroscopy. 2001;17(3):264-269 [DOI] [PubMed] [Google Scholar]
- 3.Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Joint Surg Am. 2008;90:1922-1931 [DOI] [PubMed] [Google Scholar]
- 4.Anderson JJ, Connor JF, Helms CA. New observations on meniscal cysts. Skeletal Radiol. 2010;39:1187-1191 [DOI] [PubMed] [Google Scholar]
- 5.Brody JM, Lin HM, Hulslyn MJ, Tung GA. Lateral meniscus root tear and meniscus extrusion with anterior cruciate ligament tear. Radiology. 2006;239(3):805-810 [DOI] [PubMed] [Google Scholar]
- 6.Campbell SE, Sanders TG, Morrison WB. MR imaging of meniscal cysts: incidence, location, and clinical significance. AJR. 2001;177: 409-413 [DOI] [PubMed] [Google Scholar]
- 7.Cha JH, Chung HW, Kwon JW, et al. Longitudinal split of the posterior cruciate ligament: description of a new MR finding and evaluation of its potential clinical significance. Clin Radiol. 2011;66:269-274 [DOI] [PubMed] [Google Scholar]
- 8.Chan WP, Peterfy C, Fritz RC, Genant HK. MR diagnosis of complete tears of the anterior cruciate ligament of the knee: importance of anterior subluxation of the tibia. AJR. 1994;162:355-360 [DOI] [PubMed] [Google Scholar]
- 9.Chen FS, Rokito AS, Pitman AI. Acute and chronic posterolateral rotatory instability of the knee. J Am Acad Orthop Surg. 2000;8(2):97-110 [DOI] [PubMed] [Google Scholar]
- 10.Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468:289-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa CR, Morrison WB, Carrino JA. Medial meniscus extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? AJR. 2004;183:17-23 [DOI] [PubMed] [Google Scholar]
- 12.DeFranco MJ, Bach BR. Current concepts review: comprehensive review of partial anterior cruciate ligament tears. J Bone Joint Surg Am. 2009;91:198-208 [DOI] [PubMed] [Google Scholar]
- 13.De Smet AA, Blankenbaker DG, Kijowski R, et al. MR diagnosis of posterior root tears of the lateral meniscus using arthroscopy as the reference standard. AJR. 2009;192:480-486 [DOI] [PubMed] [Google Scholar]
- 14.De Smet AA, Tuite MJ. Use of the “two-slice-touch” rule for the MRI diagnosis of meniscal tears. AJR. 2006;187:911-914 [DOI] [PubMed] [Google Scholar]
- 15.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee: a biomechanical study. J Bone Joint Surg Am. 1987;69-A(2):233-242 [PubMed] [Google Scholar]
- 16.Hede A, Larsen E, Sandberg H. Partial versus total meniscectomy: a prospective, randomized study with long-term follow-up. J Bone Joint Surg Br. 1992;74-B:118-121 [DOI] [PubMed] [Google Scholar]
- 17.Horton KL, Jacobson JA, Lin J, Hayes CW. MR imaging of anterior cruciate ligament reconstruction graft. AJR. 2000;175:1091-1097 [DOI] [PubMed] [Google Scholar]
- 18.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795-801 [DOI] [PubMed] [Google Scholar]
- 19.Hwang BY, Kim SJ, Lee SW, et al. Risk factors for medial meniscus posterior root tear. Am J Sports Med. 2012;40:1606-1610 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan PA, Gehl RH, Dussault RG, et al. Bone contusions of the posterior lip of the medial tibial plateau (contrecoup injury) and associated internal derangements of the knee at MR imaging. Radiology. 1999;211:747-753 [DOI] [PubMed] [Google Scholar]
- 21.Kenny C. Radial displacement of the medial meniscus and Fairbank’s signs. Clin Orthop Relat Res. 1997;339:163-173 [DOI] [PubMed] [Google Scholar]
- 22.Koenig JH, Ranawat AS, Umans HR, DiFelice GS. Mensical root tears: diagnosis and treatment. Arthroscopy. 2009;25(9):1025-1032 [DOI] [PubMed] [Google Scholar]
- 23.LaPrade RF, Bollom TS, Wentorf FA, et al. Mechanical properties of the posterolateral structures of the knee. Am J Sports Med. 2005;33(9):1386-1391 [DOI] [PubMed] [Google Scholar]
- 24.LaPrade RF, Gilbert TJ, Bollom TS, et al. The magnetic resonance imaging appearance of individual structures of the posterolateral knee. Am J Sports Med. 2000;28(2):191-199 [DOI] [PubMed] [Google Scholar]
- 25.LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860 [DOI] [PubMed] [Google Scholar]
- 26.LaPrade RF, Wentorf FA, Crum JA. Assessment of healing of grade III posterolateral corner injuries: an in vivo model. J Orthop Res. 2004;22:970-975 [DOI] [PubMed] [Google Scholar]
- 27.Laundre BJ, Collins MS, Bond JR, et al. MRI accuracy for tears of the posterior horn of the lateral meniscus in patients with acute anterior cruciate ligament injury and the clinical relevance of missed tears. AJR. 2009;193:515-523 [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR, Mooar LA, Moorman CT, McGinniss GH. Partial tears of the anterior cruciate ligament: progression to complete ligament deficiency. J Bone Joint Surg Br. 1989;71-B:825-833 [DOI] [PubMed] [Google Scholar]
- 29.Pacheco RJ, Ayre CA, Bollen SR. Posterolateral corner injuries of the knee: a serious injury commonly missed. J Bone Joint Surg Br. 2011;93-B:194-197 [DOI] [PubMed] [Google Scholar]
- 30.Park LS, Jacobson JA, Jamadar DA, et al. Posterior horn lateral meniscal tears simulating meniscofemoral ligament attachment in the setting of ACL tear: MRI findings. Skeletal Radiol. 2007;36:399-403 [DOI] [PubMed] [Google Scholar]
- 31.Phillipot R, Chouteau J, Wegrzyn J, et al. Medial patellofemoral ligament anatomy: implications for its surgical reconstruction. Knee Surg Sports Traumatol Arthrosc. 2009;17:475-479 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez W, Vinson EN, Helms CA, et al. MRI appearance of posterior cruciate ligament tears. AJR. 2008;191:W155-W159 [DOI] [PubMed] [Google Scholar]
- 33.Sanders TG, Linares RC, Lawhorn KW, et al. Oblique meniscomeniscal ligament: another potential pitfall for a meniscal tear—anatomic description and appearance at MR imaging in three cases. Radiology. 1999;213:213-216 [DOI] [PubMed] [Google Scholar]
- 34.Schillhammer CK, Werner FW, Scuderi MG, Cannizzaro JP. Repair of lateral meniscus posterior horn detachment lesions: a biomechanical evaluation. Am J Sports Med. 2012;40(11):2064-2609 [DOI] [PubMed] [Google Scholar]
- 35.Shelbourne KD, Davis TJ, Patel DV. The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries: a prospective study. Am J Sports Med. 1999;27(3):276-283 [DOI] [PubMed] [Google Scholar]
- 36.Shelbourne KD, Jennings RW, Vahey TN. Magnetic resonance imaging of posterior cruciate ligament injuries: assessment of healing. Am J Knee Surg. 1999;12(4):209-213 [PubMed] [Google Scholar]
- 37.Smirk C, Morris H. The anatomy and reconstruction of the medial patellofemoral ligament. Knee. 2003;10:221-227 [DOI] [PubMed] [Google Scholar]
- 38.Tuxoe JI, Teir M, Winge S, Nielsen PL. The medial patellofemoral ligament: a dissection study. Knee Surg Sports Traumatol Arthrosc. 2002;10:138-140 [DOI] [PubMed] [Google Scholar]
- 39.Vahey TN, Hunt JE, Shelbourne KD. Anterior translocation of the tibia at MR imaging: a secondary sign of anterior cruciate ligament tear. Radiology. 1993;187:817-819 [DOI] [PubMed] [Google Scholar]
- 40.Van Dyck P, De Smet E, Veryser J. Partial tear of the anterior cruciate ligament of the knee: injury patterns on MR imaging. Knee Surg Sports Traumatol Arthrosc. 2012;20:256-261 [DOI] [PubMed] [Google Scholar]
- 41.Van Dyck P, Gielen JL, Vanhoenacker FM, et al. Stable or unstable tear of the anterior cruciate ligament of the knee: an MR diagnosis? Skeletal Radiol. 2012;41:273-280 [DOI] [PubMed] [Google Scholar]
- 42.Van Dyck P, Vanhoenacker FM, Gielen JL, et al. Three tesla magnetic resonance imaging of the anterior cruciate ligament of the knee: can we differentiate complete from partial tears? Skeletal Radiol. 2011;40:701-707 [DOI] [PubMed] [Google Scholar]
- 43.Veltri DM, Deng X, Torzilli PA, et al. The role of the popliteofibular ligament in the stability of the human knee. Am J Sports Med. 1996;24(1):19-27 [DOI] [PubMed] [Google Scholar]
- 44.Yoon KH, Lee JH, Bae DK, et al. Comparison of clinical results of anatomic posterolateral corner reconstruction for posterolateral rotatory instability of the knee with or without popliteal tendon reconstruction. Am J Sports Med. 2011;39(11):2421-2428 [DOI] [PubMed] [Google Scholar]