Abstract
Entomopathogenic nematodes survive in the soil as stress-resistant infective juveniles that seek out and infect insect hosts. Upon sensing internal host cues, the infective juveniles regurgitate bacterial pathogens from their gut that ultimately kill the host. Inside the host, the nematode develops into a reproductive adult and multiplies until unknown cues trigger the accumulation of infective juveniles. Here, we show that the entomopathogenic nematode Heterorhabditis bacteriophora uses a small-molecule pheromone to control infective juvenile development. The pheromone is structurally related to the dauer pheromone ascarosides that the free-living nematode Caenorhabditis elegans uses to control its development. However, none of the C. elegans ascarosides are effective in H. bacteriophora, suggesting that there is a high degree of species specificity. Our report is the first to show that ascarosides are important regulators of development in a parasitic nematode species. An understanding of chemical signaling in parasitic nematodes may enable the development of chemical tools to control these species.
The entomopathogenic nematode Heterorhabditis bacteriophora is a lethal parasite of insects that engages in a symbiotic relationship with a bacterial partner, Photorhabdus luminescens, which it uses to kill insect hosts.1-3 Small-molecule cues are critical mediators of the interactions between nematode, bacterium, and insect in this tripartite system. In the soil, the nematode survives as a developmentally arrested infective juvenile (IJ) that seeks out an insect host based on chemical cues that the insect produces.4 Once the nematode enters the insect host, it responds to chemical cues in the insect’s hemolymph by regurgitating the symbiotic bacteria that it stores in its gut.2 The bacterial pathogens multiply inside the insect, disabling the insect’s immune system and producing antibiotics that prevent other microorganisms from proliferating on the insect’s carcass.5 In addition, the bacteria also produce small-molecule signals that induce the IJs to develop into adults and transition into the reproductive phase of their life cycle.6-9 Ultimately, once the insect has been consumed by the bacteria and nematodes feeding off of it, IJs accumulate inside the insect and then disperse to seek out new insect prey.
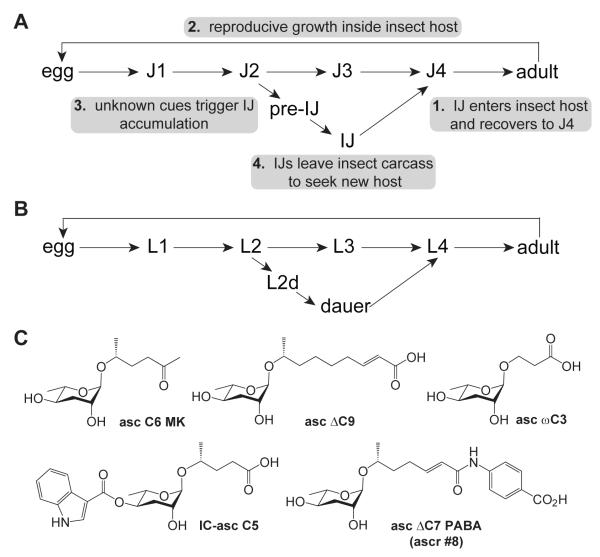
Initially, after IJs infect an insect host and recover to the adult stage, the adults lay eggs that develop through four larval stages (J1-J4) to the next generation of adults (Figure 1A). After one or two rounds of egg laying, however, the adults begin to retain eggs inside their body cavity.1 After these retained eggs hatch, the resulting juveniles digest maternal tissues and develop into IJs. This process, known as endotokia matricida, leads to maternal death, followed by emergence of the IJs. Low food availability within the maternal uterus is thought to trigger egg retention and IJ formation.10 Endotokia matricida may facilitate transmission of P. luminescens to the gut of the IJs before they leave the insect host.1 Although IJs can form via endotokia matricida at both low and high nematode densities, at low nematode densities the IJs will recover to the J4 stage, but at high nematode densities the IJs will accumulate.1
Figure 1.
The life cycles of H. bacteriophora and C. elegans. (A) Inside an insect, H. bacteriophora undergoes reproductive growth, progressing through four larval stages (J1–J4) to the adult stage. IJs form in large numbers via endotokia matricida and break out of the insect carcass to find a new host. (B) During reproductive growth, C. elegans progresses through four larval stages (L1–L4) to the adult stage. High population density (i.e., dauer pheromone ascarosides) promotes dauer development. (C) C. elegans dauer pheromone ascarosides. More descriptive names have been provided than in the original publications of these molecules: asc C6 MK (methylketone) (originally called asc C612, 17), asc μC9 (originally called asc C912, 17), asc ωC3 (originally called asc C313, 18), IC (indolecarboxyl)-asc C514, and asc μC7 PABA (para-aminobenzoic acid) (also called ascr #815).
The IJ stage of entomopathogenic nematodes is similar to the dauer stage of Caenorhabditis elegans (Figure 1B), in that it is developmentally arrested, non-feeding, and specialized for dispersal. The IJ stage of H. bacteriophora enables the worm to survive in the environment as it seeks out a new insect host. Similarly, the dauer stage of C. elegans enables the worm to survive in the environment as it seeks out a bacteria-rich food source, such as a rotting piece of fruit.11 In C. elegans, dauer development is controlled by the dauer pheromone, which the nematode secretes into its environment during reproductive growth and uses to sense its population density. The active components of the dauer pheromone, which include several derivatives of the 3,6-dideoxysugar ascarylose with different fatty acid-like side chains (Figure 1C), promote dauer formation and inhibit dauer recovery.12-15 The dauer pheromone targets G protein-coupled receptors on exposed chemosensory neurons in the head of the worm and suppresses signaling by the insulin / insulin-like growth factor-1 (IGF-1) and TGFβ neuroendocrine pathways.16-18
Unlike the free-living C. elegans, H. bacteriophora is an obligate parasite in nature and has many parasitic adaptations, including a buccal tooth to slice into hosts.3 H. bacteriophora is closely related to the strongylids, vertebrate parasites which include human hookworms.19 The IJ larval stage is similar to the infective L3 (iL3) larval stage of animal-parasitic nematodes in that it is required for successful infection and in that it resumes reproductive development in response to host cues.20-22 Furthermore, there is conservation in the neurons and signaling pathways that control IJ recovery in H. bacteriophora23 and iL3 recovery in animal-parasitic nematodes22, 24-27. Importantly, H. bacteriophora can be cultivated in the laboratory outside of its host on a lawn of bacterial symbionts, making it much easier to manipulate than an animal-parasitic nematode. The genome sequence of H. bacteriophora, as well as that of P. luminescens, is publicly available, and RNA interference, which has been an important technique for examining gene function in C. elegans, can also be used to study signaling pathways in H. bacteriophora.3, 28
Here, we show that H. bacteriophora secretes a pheromone that prevents IJ recovery to the J4 stage. Using activity-guided fractionation and NMR-based structure elucidation, we identify the chemical structure of the pheromone as a structurally novel ascaroside. Other ascarosides, including the dauer pheromone ascarosides from C. elegans, have much less activity in H. bacteriophora, suggesting that there is a high degree of structural specificity. Once the nematodes reach a high density inside an insect host, the IJ pheromone may facilitate the accumulation of IJs by preventing IJ recovery.
Results and Discussion
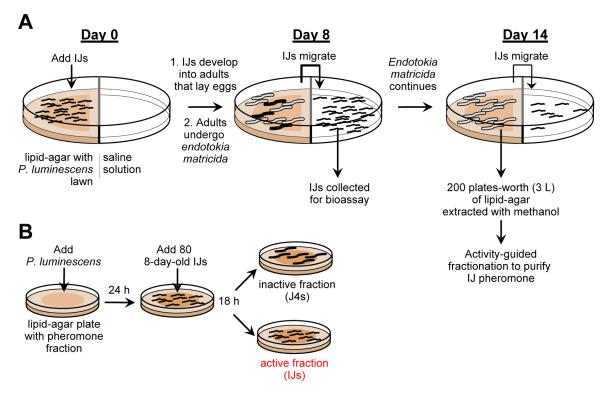
When adults undergo endotokia matricida at low nematode densities, the IJs that are generated ultimately recover to the J4 stage, but when adults undergo endotokia matricida at high nematode densities, the IJs that are generated do not recover, allowing IJs to accumulate in large numbers inside the insect host.1 This accumulation of IJs could potentially be triggered by low food availability, a pheromone, or some other type of signal. To address this question, H. bacteriophora was cultivated at high densities by placing IJs on the agar side of a split petri plate, with lipid-agar and a lawn of P. luminescens on one side and saline solution on the other side (Figure 2A). The IJs recovered to the adult stage, multiplied, and began to undergo endotokia matricida to form new IJs in mass on day 7–8. These IJs migrated to the saline solution side of the plate where they could be collected. After all of the adults had undergone endotokia matricida (day 14), the lipid-agar was collected and extracted with methanol, and the crude extract was tested in an IJ recovery assay for its ability to prevent the recovery of the IJs to the J4 stage (Figure 2B). In the IJ recovery assay, the crude extract was incorporated into a small lipid-agar plate, a small amount of P. luminescens was added to the plate, and approximately 80 IJs were spotted on the plate (Figure 2B). The number of IJs that recovered was assessed after 18 h. The crude extract was able to prevent IJ recovery when diluted to the same concentration as found in the original lipid-agar plates used to generate the extract, suggesting that H. bacteriophora produces a pheromone that prevents IJ recovery (Supporting Figure 1). Older IJs appeared to have a greater propensity to recover because they were less sensitive to pheromone. Thus, only ‘young’ IJs, collected from the split plates on day 7 or 8, were used in the assay.
Figure 2.
Preparation of crude IJ pheromone and activity-guided fractionation using the IJ recovery assay. (A) IJs were placed on a P. luminescens lawn on the lipid-agar side of a split petri plate. The IJs developed into adults, which laid eggs for several days but then underwent endotokia matricida, generating IJs that dispersed and got trapped in the saline solution. The IJs were collected on day 7–8 for use in the IJ recovery assay, and lipid-agar was harvested on day 14 for extraction to generate crude IJ pheromone. (B) The IJ recovery assay was used in activity-guided fractionation of the crude IJ pheromone. IJs were placed on a thin lawn of P. luminescens on small lipid-agar petri plates, and recovery was observed in the presence and absence of pheromone.
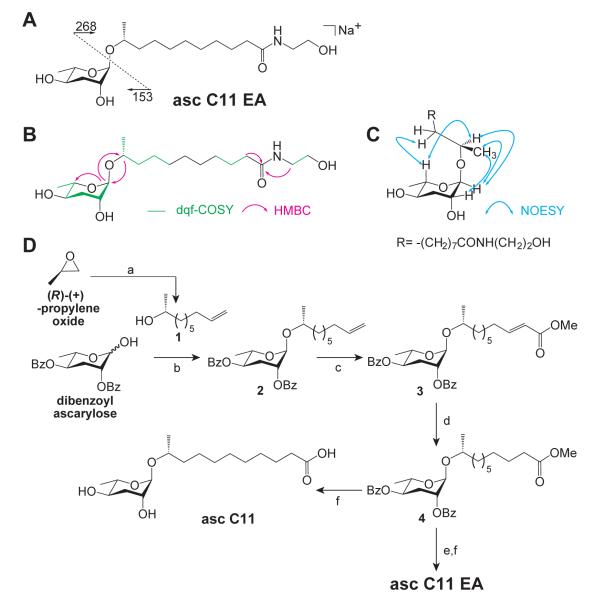
The crude IJ pheromone was fractionated using solvent partition, followed by C18chromatography, and then silica gel chromatography. At each fractionation stage, the activity was followed using the IJ recovery assay. The most active fractions after silica gel chromatography were pooled and further fractionated by reversed-phase high pressure liquid chromatography (HPLC). The most active HPLC fraction contained a single, active compound, and the structure of this compound was elucidated using multidimensional NMR, including dqf-COSY, HSQC, HMBC, and ROESY experiments (Supporting Figure 2A–E), high-resolution mass spectrometry (HRMS), and MS-MS fragmentation (Figure 3A). dqf-COSY experiments established the presence of several fragments, including an ascarylose sugar, a carbon side chain with an ω-1 alcohol, and an ethanolamine fragment (Figure 3A,B). HSQC and HMBC correlations established the connection between the sugar and the side chain, as well as the connection of the side chain to the ethanolamine via a carbonyl (Figure 3B). Coupling constants and ROESY correlations were used to assign tentatively the relative stereochemistry of the sugar, as well as the β configuration at the anomeric carbon and the R configuration of the alcohol at the ω-1 position of the side chain (Figure 3C). HRMS indicated a formula of C19H37NO6, which along with MS-MS fragmentation, suggested that the fatty acid-derived portion of the side chain was 11 carbons in length (Figure 3A). To be consistent with our previous nomenclature of the C. elegans dauer pheromone ascarosides, we refer to this molecule as ascaroside C11 ethanolamine (asc C11 EA).
Figure 3.
Structure elucidation of asc C11 EA. (A) Chemical structure of asc C11 EA and MS-MS fragmentation. (B) dqf-COSY and key HMBC correlations. (C) Key ROESY correlations. (D) Chemical synthesis of asc C11 EA and asc C11. Reagents: (a) 6-heptenyl-MgBr, CuBr, 43%; (b) 4 Å MS, BF. 3OEt2, CH2Cl2, 0° C, 70%; (c) methyl acrylate (5 eq.), Grubbs’ II, CH2Cl2, reflux, 91%; (d) 1 atm H2, 10% Pd/C, EtOAc, 25° C, 97%; (e) ethanolamine (excess), pyridine, reflux; (f) 1 M LiOH, tBuOH, RT, 81% (2 steps).
In order to verify the chemical structure of the active molecule, including its relative stereochemistry, and to enable further biological studies, the candidate structure was chemically synthesized (Figure 3D). Cuprate addition to (R)-(+)-propylene oxide provided (R)-9-decen-2-ol (1), which was glycosylated with dibenzoyl ascarylose29 to give 2. Cross metathesis of the resulting terminal alkene with methyl acrylate yielded 3, and catalytic hydrogenation afforded a saturated methyl ester (4). This saturated methyl ester was then subjected to aminolysis (exchange of methyl ester for ethanolamide and monodebenzoylation) and alkaline hydrolysis (removal of second benzoate) to provide asc C11 EA (Figure 3D; see Supporting Methods for further details). The NMR data of the synthetic molecule matched that of the natural one (Supporting Figure 3A–E and Supporting Table 1). In order to verify that synthetic and natural asc C11 EA had the same absolute stereochemistry, the two compounds were derivatized with 4-nitrobenzoyl chloride and their stereochemistry was compared by circular dichroism (Supporting Methods).
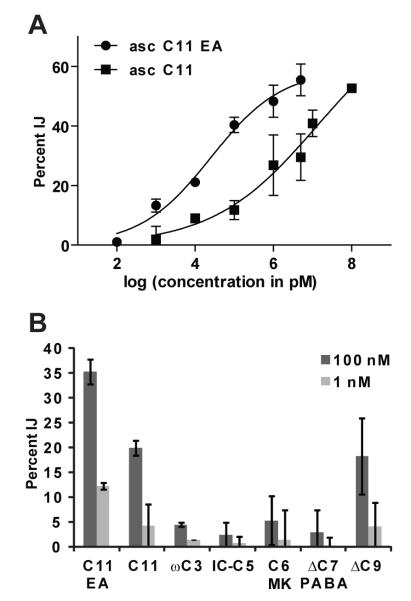
The synthetic asc C11 EA was titrated in the IJ recovery assay, which indicated an EC50 of 25 nM (Figure 4A). Based on the isolated yield of about 1 mg from 3 L of lipid agar, the concentration of the asc C11 EA in the lipid-agar side of the split plates was estimated at approximately 1 μM. By comparing the activity of the crude pheromone extract (the original organic extract from the lipid-agar split plates) in the IJ recovery assay to that of the synthetic asc C11 EA, it was shown that asc C11 EA accounts for the majority of the activity seen in the crude pheromone (Supporting Figure 1). In order to test the importance of the ethanolamine moiety to the pheromone’s activity, asc C11, a derivative that is similar to asc C11 EA but lacks the ethanolamine moiety, was chemically synthesized (Figure 3D; see Supporting Methods for further details) and titrated in the IJ recovery assay. This derivative was about 500-fold less potent at inhibiting IJ recovery than asc C11 EA with an EC50 of 13 μM, suggesting that the ethanolamine is critical to activity (Figure 4A).
Figure 4.
Biological activity of synthetic asc C11 EA and ascaroside derivatives. (A) Comparison of the activity of asc C11 EA and a derivative lacking the ethanolamine, asc C11, in the H. bacteriophora IJ recovery assay. Data represent the average of two independent assays (± one standard deviation). (B) Comparison of the activity of asc C11 EA and C. elegans dauer pheromone ascarosides in the H. bacteriophora IJ recovery assay. Data represent the average of two independent assays (± one standard deviation).
Due to the similarity in structure of C. elegans dauer pheromone ascarosides and asc C11 EA, the cross-species specificity of the pheromones was tested. Specifically, the C. elegans dauer pheromone ascarosides were tested in the H. bacteriophora IJ recovery assay, alongside asc C11 EA and asc C11. Asc μC912, 17 showed weak activity at inhibiting IJ recovery in H. bacteriophora and was roughly equivalent to asc C11 in terms of potency (Figure 4B). Asc C6 MK (methylketone)12, 17, asc ωC313, 18, IC (indolecarboxyl)-asc C514 and asc μC7 PABA (para-aminobenzoic acid) (also called ascr #815) showed virtually no activity at inhibiting IJ recovery in H. bacteriophora. Longer side chain lengths thus appear to be more potent at inhibiting IJ recovery in H. bacteriophora.
Given that IJs have only been observed to form inside mothers, it is not known whether H. bacteriophora actively secretes a pheromone or whether asc C11 EA is actually an internal signal (and thus technically a hormone). Because asc C11 EA was isolated from the lipid-agar side of the split plate where the H. bacteriophora adults had undergone endotokia matricida, asc C11 EA may have been actively secreted into the agar or it could have been simply released into the agar after death and disintegration of the adults. In order to address this question, 100 μL of settled adults were soaked in 1 mL of buffer for 3 h, at which time the adults were observed under a microscope to establish that they were still intact and moving. The concentration of asc C11 EA in the buffer was then estimated by extracting the buffer and subjecting the extract to liquid chromatography-mass spectrometry. Asc C11 EA accumulated in the buffer at a rate of about 1.7 nM h−1, suggesting that the molecule is actively secreted or excreted by intact, live adults at a relatively high rate. This data does not exclude, however, the possibility that asc C11 EA plays an internal signaling role as well.
In summary, the entomopathogenic nematode H. bacteriophora secretes a novel ascaroside, asc C11 EA, which prevents IJ recovery. As has been shown previously, when lower densities of H. bacteriophora adults undergo endotokia matricida, the IJs that form ultimately recover once they emerge from the adult.1 Thus, asc C11 EA, which likely increases in concentration at higher nematode densities, may prevent this recovery, allowing IJs to accumulate on an agar plate or inside an insect host late in the infection process. The ascaroside may also function inside H. bacteriophora adults to induce IJ formation, although this remains to be established. In future work, it will be interesting to determine whether the IJ pheromone influences additional aspects of the parasitic life cycle of H. bacteriophora, including IJ formation, pathogen regurgitation, and host infection rates. A further understanding of ascarosides in parasitic nematode species may lead to novel means to control the infection process.
Methods
General procedures
For natural and synthetic asc C11 EA, NMR spectra were recorded on a Varian INOVA 600 MHz spectrometer (600 MHz for 1H NMR, 151 MHz for 13C), and HRMS was performed on a Micromass Q-TOF Ultima spectrometer.
Strains and general culture methods
P. luminescens subspecies laumondii was grown at 28 °C in luria-bertani (LB) medium or agar plates containing 0.1% sodium pyruvate. H. bacteriophora TT01 was propagated at 28 °C on split plates containing lipid-agar (LA; 2.5% nutrient broth (NB), 1.5% agar, 1% corn oil) on one side of a split petri plate and saline solution (0.85% NaCl) on the other. IJs were added to lawns of P. luminescens that had been grown overnight at 28 °C on LA, and after 7–8 d IJs were collected from the saline solution.1
Preparation of crude IJ pheromone
Crude IJ pheromone was prepared by propagating H. bacteriophora on approximately 3 L worth of LA split plates. After 14 d the LA side of the split plates were collected and extracted with methanol. The extract was filtered over Celite, concentrated to dryness, and then partitioned in 1:1 water/ethyl acetate.
IJ Recovery Assay
The samples in vehicle (ethanol) were added to 100 μL of water at 30 times the assay concentration. The assay plates were made by mixing this 100 μL with 3 mL of 0.5X NB LA (1 g NB, 3.75 g agar, and 3 ml Mazola oil in 250 mL of water) in 3.5 cm plates. Approximately 20 μL of a P. luminescens culture (0.1 OD) in LB was added to the center of each assay plate and allowed to dry before incubating at 28 °C for ~ 24 h. Approximately 80 7- or 8-day-old IJs were added to each assay plate, and the plates were incubated at 28 °C for ~ 18 h. The number of IJs and recovered worms was then counted, and the percent IJ value was calculated by determining the percentage of IJs on each plate and subtracting the percentage of IJs on a control plate containing vehicle. The EC50 value was defined as the concentration at which the sample reached half of its maximal activity and was determined using Prism software. The titration curve of each compound was fit with a sigmoidal curve with the lower limit set at 0 and the upper limit undefined.
Purification and Characterization of IJ pheromone
The crude IJ pheromone was fractionated by C18 column chromatography with a stepwise gradient of aqueous methanol (0%–100%). The active fractions were combined and further fractionated on a silica gel column eluting with a gradient (100% ethyl acetate to 100% methanol). The most active group of fractions was further purified by reverse-phase HPLC on a Supelco Discovery HS C18 column using an aqueous acetonitrile gradient (10%–100%). Asc C11 EA was isolated as a colorless oil. See Supplemental Figure 2 and Supplemental Table 1 for NMR data. HR-ESIMS (m/z): [M+Na]+ calcd. for C19H37NO6, 398.2513; found 398.2527.
Supplementary Material
Acknowledgment
We acknowledge financial support from the NIH (GM087533 to R.A.B.). We thank S. Hagen (University of Florida) for providing access to a CD spectrometer. We also thank A. Hassan and C. David (Louisiana State University) for assistance with HRMS.
Footnotes
Supporting Information Supplementary methods, figures, and tables. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ciche TA, Kim KS, Kaufmann-Daszczuk B, Nguyen KC, Hall DH. Cell Invasion and Matricide during Photorhabdus luminescens Transmission by Heterorhabditis bacteriophora Nematodes. Appl Environ Microbiol. 2008;74:2275–2287. doi: 10.1128/AEM.02646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciche TA, Ensign JC. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol. 2003;69:1890–1897. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciche T. The biology and genome of Heterorhabditis bacteriophora. WormBook. 2007:1–9. doi: 10.1895/wormbook.1.135.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallem EA, Dillman AR, Hong AV, Zhang Y, Yano JM, DeMarco SF, Sternberg PW. A sensory code for host seeking in parasitic nematodes. Curr Biol. 2011;21:377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bode HB. Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol. 2009;13:224–230. doi: 10.1016/j.cbpa.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Aumann J, Ehlers RU. Physico-chemical properties and mode of action of a signal from the symbiotic bacterium Photorhabdus luminescens inducing dauer juvenile recovery in the entomopathogenic nematode Heterorhabditis bacteriophora. Nematology. 2001;3:849–853. [Google Scholar]
- 7.Ciche TA, Blackburn M, Carney JR, Ensign JC. Photobactin: a catechol siderophore produced by Photorhabdus luminescens, an entomopathogen mutually associated with Heterorhabditis bacteriophora NC1 nematodes. Appl Environ Microbiol. 2003;69:4706–4713. doi: 10.1128/AEM.69.8.4706-4713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciche TA, Bintrim SB, Horswill AR, Ensign JC. A Phosphopantetheinyl transferase homolog is essential for Photorhabdus luminescens to support growth and reproduction of the entomopathogenic nematode Heterorhabditis bacteriophora. J Bacteriol. 2001;183:3117–3126. doi: 10.1128/JB.183.10.3117-3126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce SA, Brachmann AO, Glazer I, Lango L, Schwar G, Clarke DJ, Bode HB. Bacterial biosynthesis of a multipotent stilbene. Angew Chem Int Ed Engl. 2008;47:1942–1945. doi: 10.1002/anie.200705148. [DOI] [PubMed] [Google Scholar]
- 10.Johnigk S-A, Ehlers R-U. Endotokia matricida in hermaphrodites of Heterorhabditis spp. and the effect of the food supply. Nematology. 1999;1:717–726. [Google Scholar]
- 11.Felix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–969. doi: 10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 12.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 13.Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in C. elegans that acts synergistically with other components. Proc Natl Acad Sci U S A. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butcher RA, Ragains JR, Clardy J. An indole-containing dauer pheromone component with unusual dauer inhibitory activity at higher concentrations. Org Lett. 2009;11:3100–3103. doi: 10.1021/ol901011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer RJ, Streit A. Comparative genetics and genomics of nematodes: genome structure, development, and lifestyle. Annu Rev Genet. 2011;45:1–20. doi: 10.1146/annurev-genet-110410-132417. [DOI] [PubMed] [Google Scholar]
- 20.Nikolaou S, Gasser RB. Prospects for exploring molecular developmental processes in Haemonchus contortus. Int J Parasitol. 2006;36:859–868. doi: 10.1016/j.ijpara.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Hotez P, Hawdon J, Schad GA. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans Daf-c paradigm. Parasitol Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 22.Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci U S A. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol. 2007;17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol. 2004;34:909–914. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int J Parasitol. 2003;33:787–793. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 26.Ashton FT, Zhu X, Boston R, Lok JB, Schad GA. Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa A, Streit A, Antebi A, Sommer RJ. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr Biol. 2009;19:67–71. doi: 10.1016/j.cub.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciche TA, Sternberg PW. Postembryonic RNAi in Heterorhabditis bacteriophora: a nematode insect parasite and host for insect pathogenic symbionts. BMC Dev Biol. 2007;7:101. doi: 10.1186/1471-213X-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.