Abstract
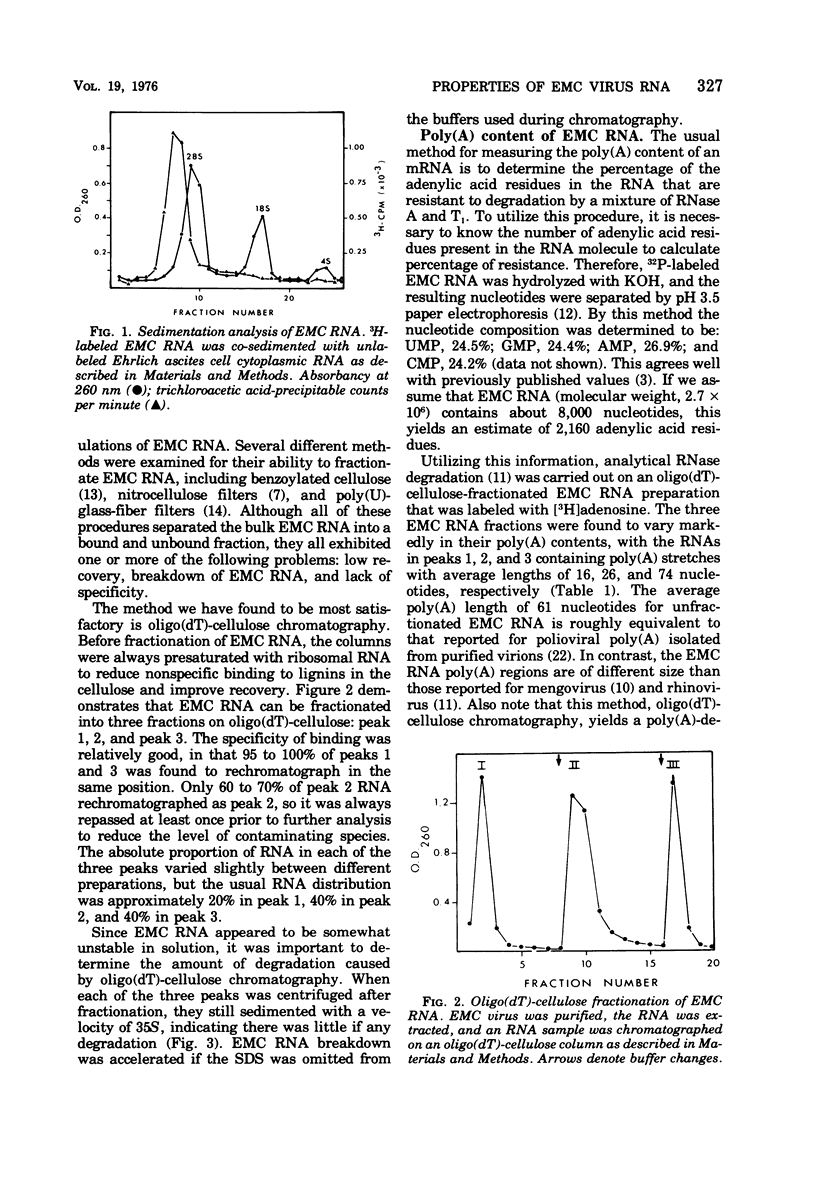
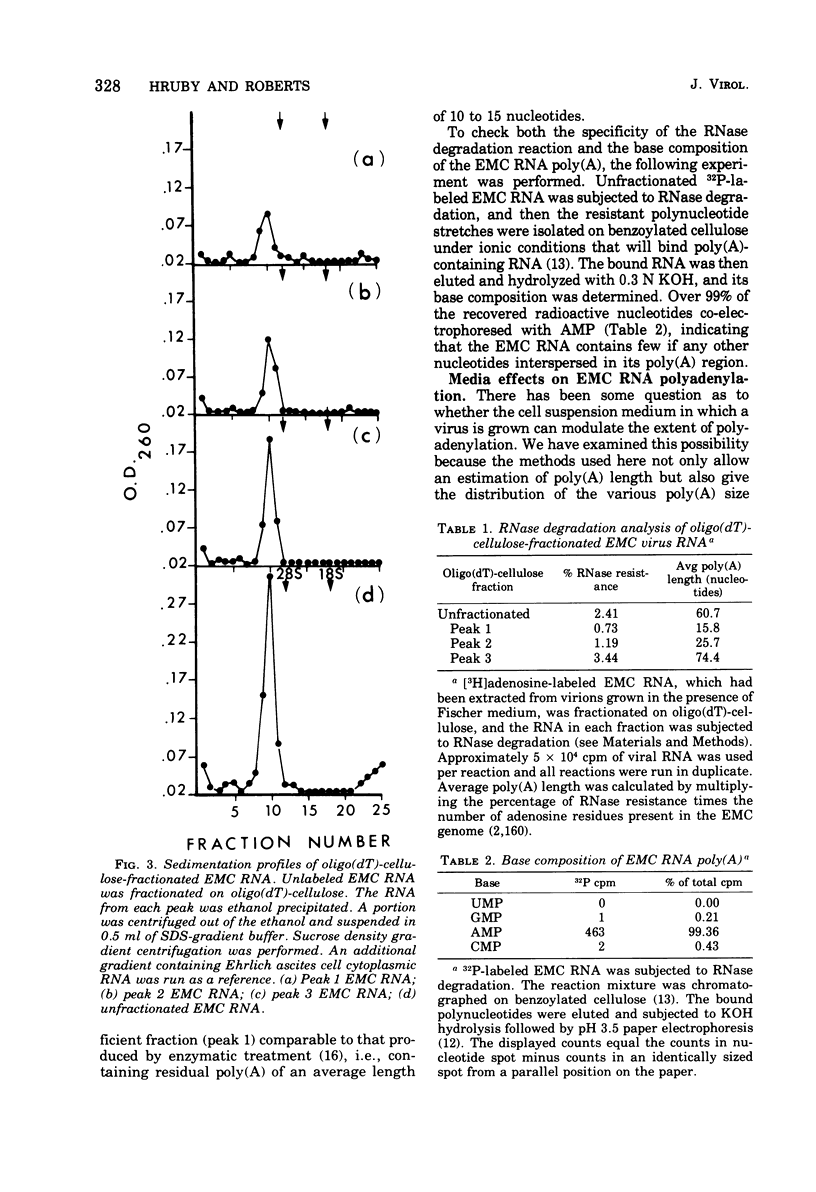
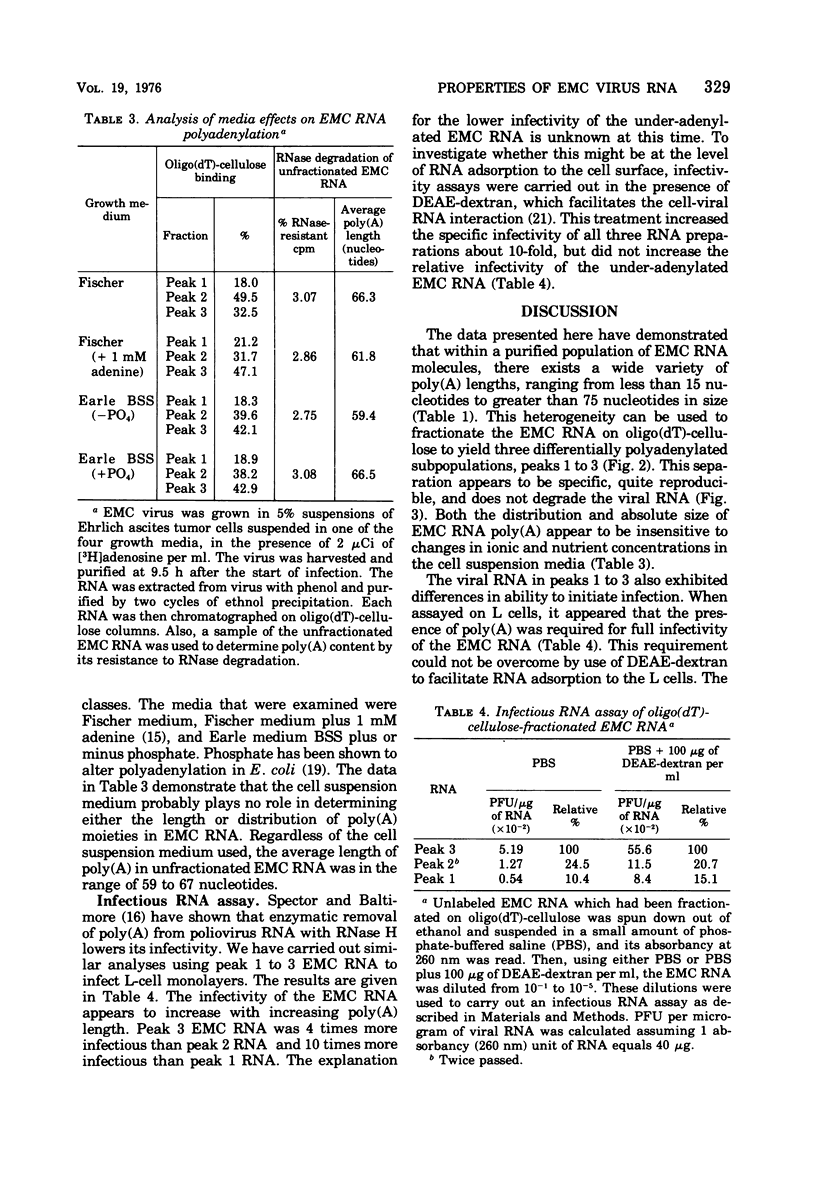
Encephalomyocarditis (EMC) viral RNA was isolated from purified virus grown in Ehrlich ascites tumor cells. The viral RNA was found to contain polyadenylic acid [poly(A)] regions that were very heterogeneous in length. Chromatography of the EMC viral RNA on oligo(dT)-cellulose columns separated the RNA into three distinct fractions (peaks 1 to 3). Approximately 20% of the EMC viral RNA appeared as peak 1, 40% as peak 2, and 40% as peak 3. The RNA in each fraction appeared to be intact as shown by co-sedimentation with 35S unfractionated EMC viral RNA in SDS-sucrose density gradients. Approximately 95 to 100% of peaks 1 and 3, and 60 to 70% of peak 2, reappeared at the same elution position after rechromatography on oligo(dT)-cellulose. The RNA in peak 1 contained poly(A) with an average length of 16 nucleotides, peak 2 contained poly(A) with an average of 26 nucleotides, and peak 3 contained an average of 74 nucleotides in its poly(A) region. The distribution in the three fractions, as well as the average length of the poly(A) moieties, was relatively unaffected by changes in the cell suspension medium used during infection. Finally, each of the three viral RNA fractions was assayed for biological activity using an infectious RNA assay on L-cell monolayers. Infectivity of the viral RNA was found to increase with poly(A) length, with peak 3 viral RNA being approximately 10 times more infectious than peak 1 viral RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burness A. T. Ribonucleic acid content of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):373–380. doi: 10.1099/0022-1317-6-3-373. [DOI] [PubMed] [Google Scholar]
- Caldwell I. C., Chan M. F. Tumor cell metabolism in vitro: a study of incubation media. Can J Biochem. 1970 Apr;48(4):517–519. doi: 10.1139/o70-083. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Takemoto K., Robert M., Gallo R. C. Polyadenylic acid in Visna virus RNA. Science. 1973 Mar 30;179(4080):1328–1330. doi: 10.1126/science.179.4080.1328. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Cohen N., Work T. S. Factors controlling amino acid incorporation by ribosomes from krebs II mouse ascites-tumour cells. Biochem J. 1966 Mar;98(3):826–835. doi: 10.1042/bj0980826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy C. C., Schmukler M., Frank J. J., Karpetsky T. P., Jewett P. B., Hieter P. A., LeGendre S. M., Dorr R. G. Possible role for poly(A) as an inhibitor of endonuclease activity in eukaryotic cells. Nature. 1975 Jul 24;256(5515):340–342. doi: 10.1038/256340a0. [DOI] [PubMed] [Google Scholar]
- MARTIN E. M., MALEC J., SVED S., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 1. Encephalomyocarditis virus in Krebs II mouse-ascites-tumour cells. Biochem J. 1961 Sep;80:585–597. doi: 10.1042/bj0800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Purification of mengovirus and identification of an A-rich segment in its ribonucleic acid. J Gen Virol. 1972 Dec;17(3):349–353. doi: 10.1099/0022-1317-17-3-349. [DOI] [PubMed] [Google Scholar]
- Nair C. N., Owens M. J. Preliminary observations pertaining to polyadenylation of rhinovirus RNA. J Virol. 1974 Feb;13(2):535–537. doi: 10.1128/jvi.13.2.535-537.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K. Studies on RNA synthesis in Ehrlich ascites cells extraction and properties of labeled RNA. Biochim Biophys Acta. 1965 Nov 8;108(3):474–488. doi: 10.1016/0005-2787(65)90039-0. [DOI] [PubMed] [Google Scholar]
- Roberts W. K. Use of benzoylated cellulose columns for the isolation of poly(adenylic acid) containing RNA and other polynucleotides with little secondary structure. Biochemistry. 1974 Aug 27;13(18):3677–3682. doi: 10.1021/bi00715a009. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R., Sachs L. Induction of polyadenylate polymerase and differentiation in neuroblastoma cells. Eur J Biochem. 1975 Jun 16;55(1):9–14. doi: 10.1111/j.1432-1033.1975.tb02132.x. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. III. In vitro addition of polyadenylic acid to poliovirus RNAs. J Virol. 1975 Jun;15(6):1432–1439. doi: 10.1128/jvi.15.6.1432-1439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Villa-Komaroff L., Baltimore D. Studies on the function of polyadenylic acid on poliovirus RNA. Cell. 1975 Sep;6(1):41–44. doi: 10.1016/0092-8674(75)90071-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan P. R., Ramanarayanan M., Rabbani E. Presence of polyriboadenylate sequences in pulse-labeled RNA of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2910–2914. doi: 10.1073/pnas.72.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Shatkin A. J., Banerjee A. K. Absence of polyadenylic acid from reovirus messenger ribonucleic acid. J Biol Chem. 1973 Dec 10;248(23):7993–7998. [PubMed] [Google Scholar]
- Tovell D. R., Colter J. S. The interaction of tritium-labelled mengo virus RNA and L cells: the effects of DMSO and DEAE-dextran. Virology. 1969 Apr;37(4):624–631. doi: 10.1016/0042-6822(69)90280-3. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]



