Abstract
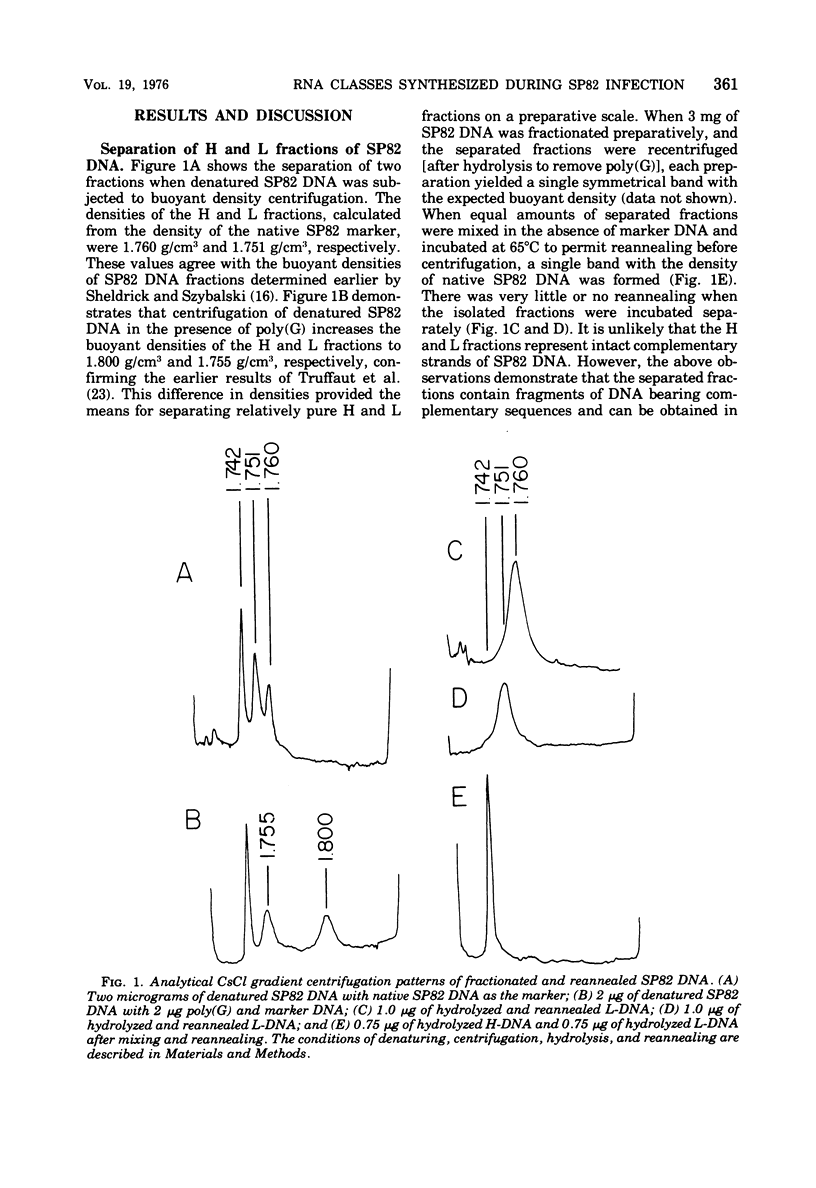
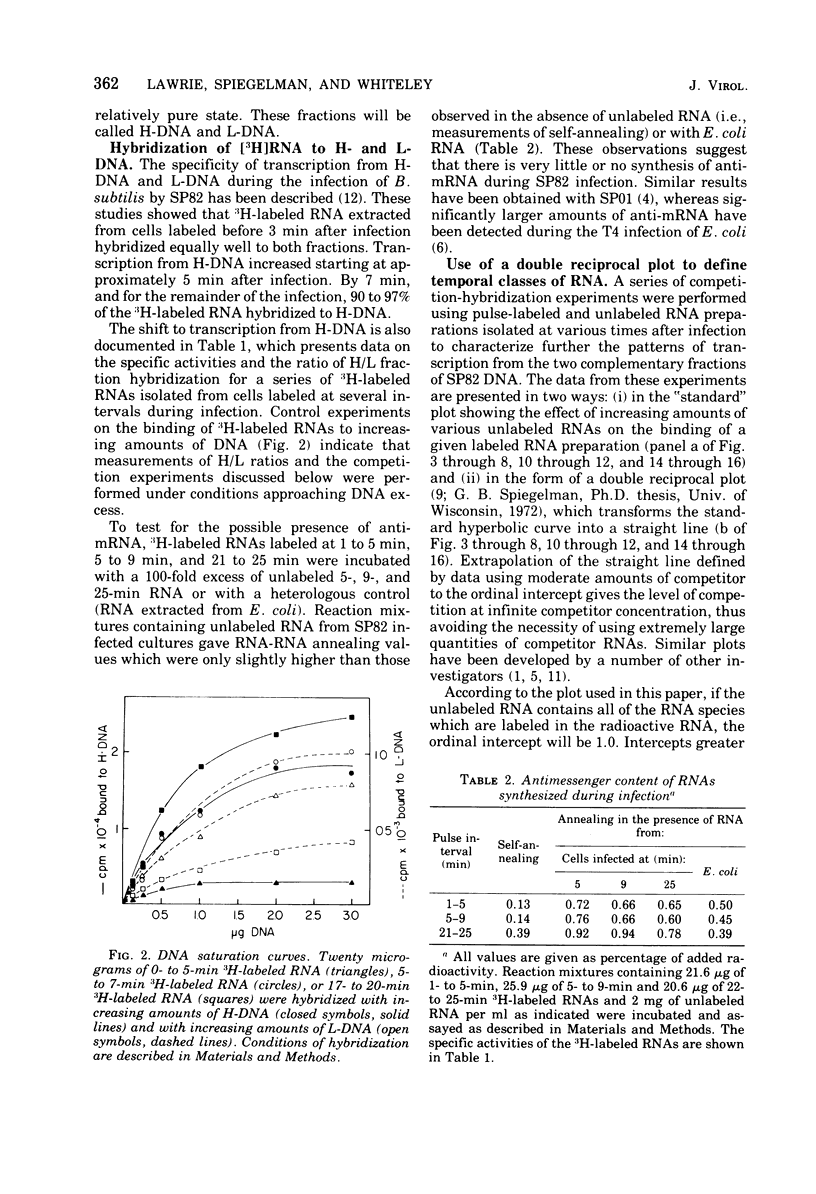
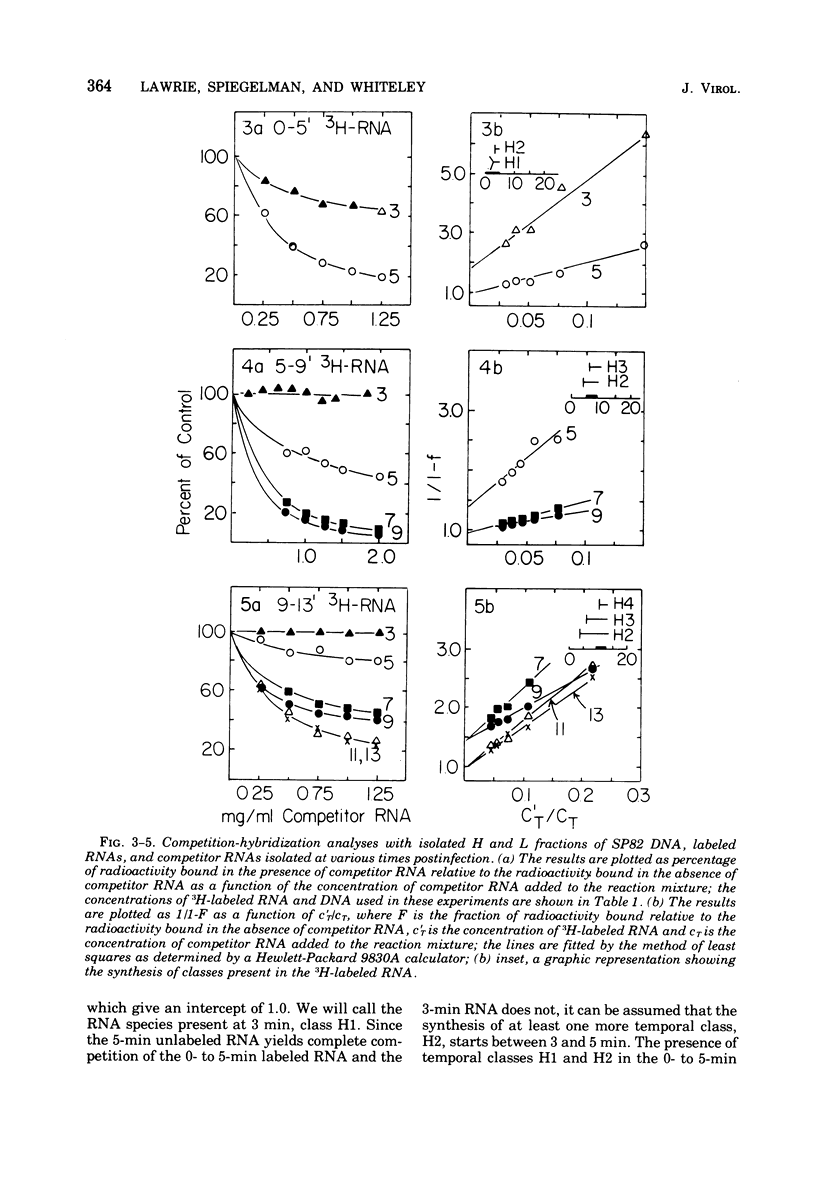
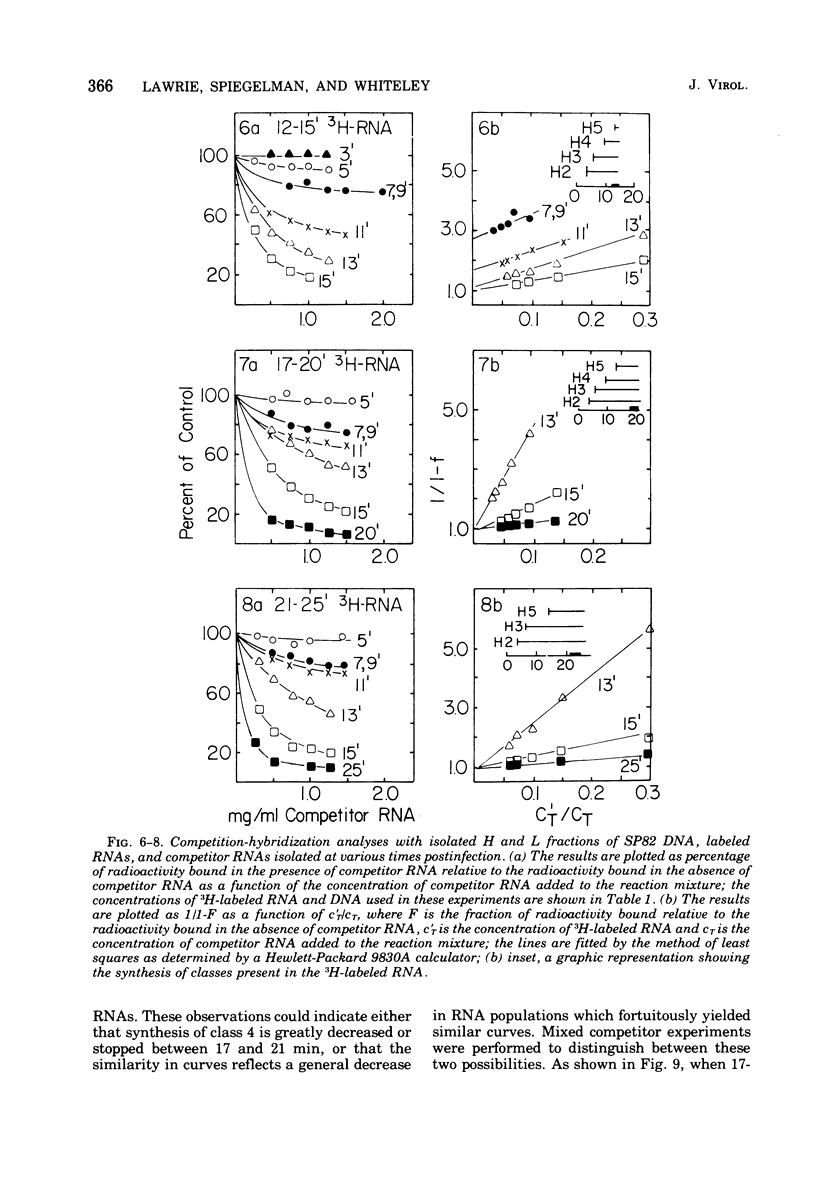
The DNA of the Bacillus subtilis bacteriophage SP82 has been separated into heavy (H) and light (L) fractions by centrifugation in buoyant density gradients in the presence of polyguanylic acid. Competition-hybridization experiments were performed with these separated fractions using RNAs isolated from cells labeled at intervals which account for 80% of the lytic cycle and unlabeled competitor RNAs isolated from phage-infected cells at 2-min intervals throughout infection. The analysis of temporal RNA classes were facilitated by use of a double reciprocal plot of the data. Five temporal classes binding to the H fraction and three binding to the L fraction were detected; the possible existence of an additional class transcribed from the H fraction is discussed. RNA synthesized in the presence of chloramphenicol contains two of the three classes produced from L-DNA and two of the five classes transcribed from H-DNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. O., Robertson F. W., Burns J. A., Melli M. Methods for the analysis of deoxyribonucleic acid-ribonucleic acid hybridization data. Biochem J. 1969 Nov;115(3):361–370. doi: 10.1042/bj1150361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P. R., Bandyopadhyay P., Huang H. H., Maitra U. Fidelity of in vitro transcription of T3 deoxyribonucleic acid by bacteriophage T3-induced ribonucleic acid polymerase and by Escherichia coli ribonucleic acid polymerase. J Biol Chem. 1974 Nov 10;249(21):6901–6909. [PubMed] [Google Scholar]
- Esche H., Spatz H. C. Asymmetric transcription of SPP1 in vivo. Mol Gen Genet. 1973 Jul 31;124(1):57–63. doi: 10.1007/BF00267164. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriphage SPO1 development. II. Some modulations and prerequisites of the transcription program. Virology. 1971 Apr;44(1):200–210. doi: 10.1016/0042-6822(71)90165-6. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W. Fractionation of the complementary strands of coliphage T4 DNA based on the asymmetric distribution of the poly U and poly U,G binding sites. Virology. 1968 Apr;34(4):608–616. doi: 10.1016/0042-6822(68)90082-2. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Hansen J. N., Spiegelman G., Halvorson H. O. Bacterial spore outgrowth: its regulation. Science. 1970 Jun 12;168(3937):1291–1298. doi: 10.1126/science.168.3937.1291. [DOI] [PubMed] [Google Scholar]
- Jayaraman R. Transcription of bacteriophage T4 DNA by Escherichia coli RNA polymerase in vitro: identification of some immediate-early and delayed-early genes. J Mol Biol. 1972 Sep 28;70(2):253–263. doi: 10.1016/0022-2836(72)90537-2. [DOI] [PubMed] [Google Scholar]
- Lavallé R., De Hauwer G. Messenger RNA synthesis during amino acid starvation in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):269–288. doi: 10.1016/0022-2836(68)90267-2. [DOI] [PubMed] [Google Scholar]
- Lawrie J. M. DNA strand specificity of transcripts produced in vivo and in vitro by RNA polymerase from SP82-infected Bacillus subtilis. J Virol. 1975 May;15(5):1286–1288. doi: 10.1128/jvi.15.5.1286-1288.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., De Sain C. V., Anderson D. L. Transcription during the development of bacteriophage phi29: definition of "early" and "late" phi29 ribonucleic acid. J Virol. 1973 Jan;11(1):9–16. doi: 10.1128/jvi.11.1.9-16.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., De Sain C. V., Hawley L. A., Anderson D. L. Transcription during the development of bacteriophage phi 29: production of host- and phi 29-specific ribonucleic acid. J Virol. 1972 Dec;10(6):1170–1178. doi: 10.1128/jvi.10.6.1170-1178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. In vivo and in vitro transcription by ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1483–1489. [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. Purification of ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1476–1482. [PubMed] [Google Scholar]
- Stevens A. Deoxyribonucleic acid dependent ribonucleic acid polymerases from two T4 phage-infected systems. Biochemistry. 1974 Jan 29;13(3):493–503. doi: 10.1021/bi00700a015. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Click B., Tole M. F. DNA replication and late protein synthesis during SP82 infection of Bacillus subtilis. Virology. 1972 Dec;50(3):653–663. doi: 10.1016/0042-6822(72)90419-9. [DOI] [PubMed] [Google Scholar]
- Truffaut N., Revet B., Soulie M. O. Etude comparative des DNA de phages 2C, SP8*, SP82, phi e, SP01 et SP50. Eur J Biochem. 1970 Aug;15(2):391–400. doi: 10.1111/j.1432-1033.1970.tb01020.x. [DOI] [PubMed] [Google Scholar]



