Abstract
Background
Children under five bear the largest cholera burden. We therefore sought to identify modifiable risk factors among Bangladeshi children.
Methodology/Principal Findings
We used multivariate Poisson regression to assess risk factors for severe cholera among diarrheal patients presenting at hospitals in Matlab (rural) and Dhaka (urban), Bangladesh. Risk increased with age. Compared to those under one, rural and urban four-year-olds had adjusted risk ratios (aRR) of 4.17 (95% confidence interval (CI) 2.43–7.15) and 6.32 (95% CI: 4.63–8.63), respectively. Breastfeeding halved the risk in both rural (aRR = 0.49, 95% CI: 0.35–0.67) and urban (aRR = 0.51, 95% CI: 0.41–0.62) settings. Rural children’s risk decreased with maternal education (P-trend: <0.001) and increased among those with a family member with diarrhea in the past week (aRR = 1.61, 95% CI: 1.22–2.14) and those with prior vitamin A supplementation (aRR = 1.65, 95% CI: 1.12–2.43). Urban children whose mothers daily (aRR = 0.41, 95% CI: 0.21–0.79) or occasionally (aRR = 0.55, 95% CI: 0.36–0.84) read a newspaper experienced reduced risk. Urban children from households with incomes between 34–84 USD/month had a 30% increased risk compared to those from households with incomes >84 USD/month.
Conclusion/Significance
Increasing age, lower socioeconomic status, and lack of breastfeeding are key correlates of increased risk for cholera hospitalization among those under five in rural and urban Bangladesh. In addition, having a family member with diarrhea in the past week was associated with increased risk among rural children. Continued attention should be directed to the promotion of breastfeeding. Further research is needed to elucidate the relationship between maternal education and cholera risk. Renewed research regarding the use of chemoprophylaxis among family members of cholera cases may be warranted in rural endemic settings.
Introduction
Cholera is a potentially life-threatening, primarily waterborne, diarrheal disease caused by infection with Vibrio cholerae bacteria. A 2012 review of cholera’s global burden estimated that 1.4 billion people are at risk for cholera, with 2.8 million cases and 91,000 related deaths in endemic regions annually [1]. This burden is disproportionately borne by the young, with children under five having the highest incidence of cholera and contributing almost half of the mortality [1]. More than 40 years ago it was reported that the cholera case fatality rate among children one to five years old was more than 10 times that of adults [2], but description of this disparity has not resulted in large-scale studies of cholera risk factors unique to young children. Most prior studies have been small [3], focused on specific risk factors such as breastfeeding [4], [5], or assessed risk factors for diarrhea in general [6]. More recently, other studies have examined risks for duration of diarrheal illness [7] and diarrheal disease associated death [8], [9]. Research that specifically explores cholera risk factors in children under five may provide an important new perspective.
Although an oral bivalent cholera vaccine campaign was initiated in response to the epidemic that started in Haiti in 2010, and a large-scale feasibility trial of this same vaccine began in Bangladesh in 2011 (clinicaltrials.gov id: NCT01339845), there is currently no evidence that this vaccine will be able to stop an epidemic or significantly reduce cholera burden in endemic settings. Furthermore, a 2011 Cochrane review reported that the protective efficacy of five types of killed whole cell cholera vaccine in children under five was significantly lower than among older children and adults [10].
Due to uncertainty regarding the efficacy of cholera vaccines in young children, we sought to identify risk factors amenable to non-immunologic intervention by developing predictive models for severe cholera in children under five. Due to substantial environmental and socioeconomic regional differences, we examined children in rural (Matlab) and urban (Dhaka) Bangladesh separately.
Methods
Ethics Statement
Hospital surveillance activities were approved by the Ethical Review Committee (ERC) and the Research Review Committee (RRC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). Informed oral consent was obtained from all participants and was documented in the DDSS database by ICDDR,B staff. For minors, informed oral consent was obtained from parents, guardians, caretakers, or next of kin. Anonymized medical records were used in all data analyses. This research was exempted from human subjects review by the University of Washington Institutional Review Board. The ERC and RRC approved the use of oral consent because of the high proportion of illiterate patients.
Study Design & Setting
We performed a hospital-based surveillance study using the Diarrhoeal Diseases Surveillance System (DDSS) databases from Matlab and Dhaka ICDDR,B hospitals. The DDSS records clinical, demographic, socioeconomic, and enteric pathogen data from diarrheal patients. All DDSS patients had stool cultured for enteric pathogens following standard bacteriological methods [11]–[13]. In addition to V. cholerae, stools were systematically tested for rotavirus, Shigella, Salmonella, amoeba and Giardia species.
Matlab Upazila (sub-district) is a predominantly rural area of Bangladesh, with villagers comprising more than 97% of the population. In Matlab, a Health and Demographic Surveillance System (HDSS) covering approximately 200,000 residents was established in 1966. All diarrheal patients living in the HDSS catchment area were enrolled in the DDSS.
Dhaka is a densely populated city, with more than 12 million residents in 2008. Every fiftieth diarrheal patient visiting the Dhaka hospital has been enrolled in the DDSS since 1996 [12]. In both settings, ICDDR,B hospitals provide excellent diarrhea treatment at no cost to the patient.
For this analysis, Matlab patients who lived in villages were considered rural dwellers. Dhaka patients who lived in high-density residential or mixed-use areas, or slums, were considered urban dwellers.
Study Population
We limited the analysis to patients under five years old entering ICDDR,B hospitals between January 1, 2000 and December 31, 2008. Patients missing age data, Dhaka patients residing in villages, and Matlab patients who reported residing in slums or high-density residential or mixed use areas were excluded. Due to the inability to attribute severe diarrhea to a particular pathogen in patients who were co-infected with V. cholerae and another pathogen, we excluded these patients. There were no other exclusion criteria. Multiple hospital admissions of the same child could not be identified because anonymized data were used.
Cholera Definition
Laboratory-confirmed culture of V. cholerae (negative, positive) was the outcome of interest. V. cholerae status was positive when V. cholerae O1 Classical Inaba, V. cholerae O1 Classical Ogawa, V. cholerae O1 El Tor Inaba, V. cholerae O1 El Tor Ogawa, or V. cholerae O139 (Bengal) was recorded as one of the first three isolated pathogens in the DDSS database.
Data Analysis
Characteristics of cholera and non-cholera patients, stratified by rural or urban residence, were compared using chi-squared tests for categorical variables. The Mann–Whitney U test was used for the number of household members, a continuous variable with a right-skewed distribution. Sociodemographic characteristics were self-reported and included age, sex, the number of household members, maternal education, maternal newspaper readership, monthly household income, residence in a slum, home ownership, and the presence of concrete floors in the home.
Self-reported water and sanitation characteristics included household toilet facilities, distance from the kitchen to drinking water, sources of drinking and washing/bathing water, and drinking water treatment.
Other variables of interest included a family member with diarrhea in the past week, breastfeeding status, history of vitamin A supplementation, and distance from home to the hospital, all of which were self-reported. Severe acute malnutrition (mid-upper arm circumference <11.5 cm) was assessed by ICDDR,B medical personnel.
Clinical characteristics included the general condition on admission (assessed by medical personnel), diarrhea duration prior to arrival (self-reported), degree of clinical dehydration (assessed by medical personnel), watery stool, stool contents, and the number of stools and bouts of vomiting in the 24 hours prior to admission (all self-reported). We also assessed the distribution of enteric pathogens detected by ICDDR,B laboratories.
Risk Factors Examined
Sociodemographic risk factors examined were age, sex, maternal education (none, 1–5, 6–9, 10–12, >12 years), maternal newspaper readership (never, <7 days/week, daily), monthly household income (84+, 50–84, 34–50, <34 USD (converted from Taka at 59.4 Taka/USD, the mid-market rate on July 1, 2004, the mid-point of our study [14])), residence in a slum (yes, no), home ownership (own, rent), and concrete floors in the home (yes, no).
Water and sanitation risk factors included household toilet facilities (improved or unimproved, as defined by the WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation [15]), distance from the kitchen to drinking water (10-meter increments), water source (ranked in decreasing order of safety as tap, tube well, or surface; if different sources were used for drinking and washing/bathing water, the least safe source was analyzed), and drinking water treatment (none, boiling, other). Water sources used in food preparation were not available. Surface water was defined as water from a pond, river, or ditch. Other water treatment included filtering, sieving, and treatment with tablets.
A history of a family member with diarrhea in the past week (yes, no), current breastfeeding (yes, no), severe acute malnutrition (yes, no), prior vitamin A supplementation (never, ever), and distance from home to the hospital (5 km increments) were also assessed.
Statistical Methods
Risk ratios (RR) and 95% confidence intervals (95% CI) for cholera risk factors were assessed using Poisson regression, with robust variance estimates to compensate for variance overestimation [16]. Candidate risk factors with more than 5% missing data were excluded from analysis. A linear trend test was performed for ordinal variables with ≥4 strata.
Univariate risk factors associated with cholera with a p-value <0.10 and an RR of <0.9 or >1.1 were candidates for the multivariate model. We excluded risk factors with an RR between 0.9 and 1.1 because of the likelihood that weak (epidemiologically unimportant) associations would be found statistically significant solely due to our large sample size. Collinearity among multivariate candidates was assessed using variance inflation factors (VIF), with a VIF of ≥10 indicating collinearity. If collinear candidates were found, only the predictor judged to be more biologically plausible was considered for the multivariate model.
A multivariate regression model was built by adding and testing candidate predictors individually, in order of effect size. Continuous predictors were retained if the Wald test was significant (p-value <0.05). Retention of categorical predictors was also dependent on a significant Wald test for at least one stratum. Since regression with robust standard errors does not provide log likelihoods, we could not perform likelihood ratio tests to compare models. After inclusion in the model, risk factors were not reevaluated in subsequent model building steps.
Sex, the number of household members (continuous), and cholera seasonality were a priori confounders and were included in the multivariate model as adjustment variables. Seasonality was constructed by creating a restricted cubic spline of the day of the year on the date of visit (1–366) with seven knots [17].
Analyses used two-sided significance levels and were performed with Stata/IC 11.2 (StataCorp LP, College Station, TX).
Results
Study Population
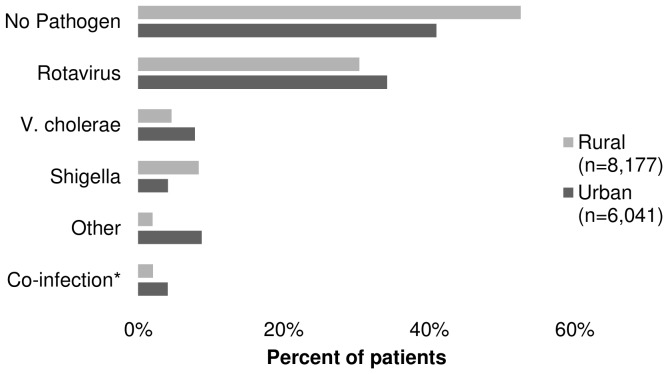
Of the 13,839 Matlab patient records in the DDSS, four (<0.01%) had missing age data, 304 (2.2%) were non-rural dwellers, 5,193 (37.5%) were older than five, and 161 (1.2%) had laboratory-confirmed co-infections with V. cholerae and another enteric pathogen. Among the remaining 8,177 rural children, there were 378 (4.6%) laboratory-confirmed V. cholerae cases. Of the 19,332 Dhaka patient records, 41 (0.2%) had missing age data, 6,938 (36%) were non-urban dwellers, 6,176 (31.9%) were older than five, and 136 (0.7%) had laboratory-confirmed infections with V. cholerae and another enteric pathogen. Among the remaining 6,041 urban children, there were 473 (7.8%) cases of cholera. Dhaka had a higher proportion of cholera cases (P<0.001). A known pathogen was detected in 47% of rural patients and 59% of urban patients (Figure 1).
Figure 1. Frequency of detected enteric pathogens among children under five in ICDDR,B hospitals, Bangladesh, 2000–2008.
* Co-infection is defined as a positive assay for two or more non-cholera pathogens. Patients with V. cholerae co-infection were excluded from analyses.
Comparison of Cholera and Non-cholera Diarrhea Cases
In the rural setting, cholera patients were older (median age 30 vs. 14 months), had fewer household members, and were more likely to have uneducated mothers who never read newspapers than other diarrhea patients (Table 1). They also came from families with lower household incomes and their homes were less likely to have concrete floors, improved toilet facilities (6.3% vs. 10.2%), and a drinking water source within 10 meters of their kitchen (41.5% vs. 55.9%). They were more likely to use surface water for drinking or washing, to have had a family member with diarrhea in the past week (11.6% vs. 6.2%), and less likely to be breastfed (48.8% vs. 88.4%) and have severe acute malnutrition. They were also more likely to have had prior vitamin A supplementation (82.3% vs. 49.9%) and to live further from the hospital than other diarrhea patients.
Table 1. Sociodemographic, water and sanitation, and other potential correlates of diarrhea among diarrheal patients <5-years-old in ICDDR,B hospitals, Bangladesh, 2000–2008.
| Rural | Urban | |||||||||||||||||
| Cholera (n = 378) | Other (n = 7,799) | Cholera (n = 473) | Other (n = 5,568) | |||||||||||||||
| N | % | N | % | P a | N | % | N | % | P a | |||||||||
| Sociodemographic | ||||||||||||||||||
| Age (yr) | <0.001 | <0.001 | ||||||||||||||||
| 0 | 65 | 17.2 | 4056 | 52.0 | 103 | 21.8 | 3397 | 61.0 | ||||||||||
| 1 | 75 | 19.8 | 2628 | 33.7 | 112 | 23.7 | 1496 | 26.9 | ||||||||||
| 2 | 108 | 28.6 | 651 | 8.3 | 102 | 21.6 | 358 | 6.4 | ||||||||||
| 3 | 74 | 19.6 | 286 | 3.7 | 88 | 18.6 | 198 | 3.6 | ||||||||||
| 4 | 56 | 14.8 | 178 | 2.3 | 68 | 14.4 | 119 | 2.1 | ||||||||||
| Female sex | 154 | 40.7 | 3043 | 39.0 | 0.503 | 215 | 45.5 | 2252 | 40.4 | 0.033 | ||||||||
| No. household members b | 5 | 2.1 | 5 | 2.4 | 0.010 | 4 | 1.9 | 4 | 2.3 | 0.751 | ||||||||
| Maternal education (yr) | <0.001 | <0.001 | ||||||||||||||||
| None | 142 | 37.6 | 1659 | 21.3 | 216 | 45.7 | 1842 | 33.1 | ||||||||||
| 1–5 | 99 | 26.2 | 1983 | 25.4 | 105 | 22.2 | 1138 | 20.4 | ||||||||||
| 6–9 | 110 | 29.1 | 2974 | 38.1 | 106 | 22.4 | 1410 | 25.3 | ||||||||||
| 10–12 | 25 | 6.6 | 922 | 11.8 | 27 | 5.7 | 721 | 12.9 | ||||||||||
| >12 | 2 | 0.5 | 261 | 3.3 | 19 | 4.0 | 457 | 8.2 | ||||||||||
| Maternal newspaper readership | 0.004 | <0.001 | ||||||||||||||||
| Never | 347 | 92.0 | 6756 | 86.8 | 440 | 93.4 | 4662 | 84.1 | ||||||||||
| <7 days/week | 29 | 7.7 | 855 | 11.0 | 22 | 4.7 | 568 | 10.2 | ||||||||||
| Daily | 1 | 0.3 | 175 | 2.2 | 9 | 1.9 | 315 | 5.7 | ||||||||||
| Monthly household income (USD) | <0.001 | <0.001 | ||||||||||||||||
| 84+ | 142 | 37.6 | 3850 | 49.4 | 188 | 39.7 | 2889 | 51.9 | ||||||||||
| 50–84 | 169 | 44.7 | 3091 | 39.6 | 210 | 44.4 | 1965 | 35.3 | ||||||||||
| 34–50 | 57 | 15.1 | 774 | 9.9 | 62 | 13.1 | 543 | 9.8 | ||||||||||
| <33 | 10 | 2.6 | 84 | 1.1 | 13 | 2.7 | 171 | 3.1 | ||||||||||
| Residence in a slum | – | – | – | – | 101 | 21.4 | 827 | 14.9 | <0.001 | |||||||||
| Homeowner | 367 | 97.1 | 7558 | 96.9 | 0.854 | 49 | 10.4 | 823 | 14.8 | 0.009 | ||||||||
| Concrete floor in home | 24 | 6.3 | 911 | 11.7 | 0.001 | 342 | 72.3 | 4405 | 79.1 | 0.001 | ||||||||
| Water & Sanitation | ||||||||||||||||||
| Improved toilet facilities | 24 | 6.3 | 792 | 10.2 | 0.016 | 285 | 60.3 | 3977 | 71.4 | <0.001 | ||||||||
| Distance to drinking water (10 m increments) | <0.001 | <0.001 | ||||||||||||||||
| 0 | 0 | 0 | 20 | 0.3 | 79 | 16.7 | 1661 | 29.8 | ||||||||||
| <1 | 157 | 41.5 | 4332 | 55.6 | 282 | 59.6 | 2860 | 51.4 | ||||||||||
| 1–2 | 58 | 15.3 | 1229 | 15.8 | 79 | 16.7 | 689 | 12.4 | ||||||||||
| 2–5 | 71 | 18.8 | 1146 | 14.7 | 25 | 5.3 | 295 | 5.3 | ||||||||||
| 5+ | 92 | 24.3 | 1071 | 13.7 | 8 | 1.7 | 62 | 1.1 | ||||||||||
| Water source | 0.001 | 0.035 | ||||||||||||||||
| Tap | 0 | 0 | 46 | 0.6 | 392 | 82.9 | 4843 | 87.0 | ||||||||||
| Tube well | 37 | 9.8 | 1280 | 16.4 | 77 | 16.3 | 678 | 12.2 | ||||||||||
| Surfacec | 341 | 90.2 | 6467 | 83.0 | 4 | 0.8 | 44 | 0.8 | ||||||||||
| Drinking water treatment | 0.099 | <0.001 | ||||||||||||||||
| None | 346 | 91.5 | 7334 | 94 | 291 | 61.5 | 2658 | 47.7 | ||||||||||
| Boiling | 7 | 1.9 | 127 | 1.6 | 179 | 37.8 | 2851 | 51.2 | ||||||||||
| Other | 25 | 6.6 | 337 | 4.3 | 3 | 0.6 | 58 | 1.0 | 0.069 | |||||||||
| Other Potential Correlates | ||||||||||||||||||
| Family member with diarrhea in past week | 44 | 11.6 | 484 | 6.2 | <0.001 | 76 | 16.1 | 560 | 10.1 | <0.001 | ||||||||
| Currently breastfed | 182 | 48.4 | 6878 | 88.4 | <0.001 | 235 | 50.0 | 4574 | 82.3 | <0.001 | ||||||||
| Severe acute malnutrition d | 19 | 5.0 | 624 | 8.0 | 0.035 | 63 | 14.1 | 986 | 18.1 | 0.032 | ||||||||
| Prior vitamin A supplementation | 311 | 82.3 | 3891 | 49.9 | <0.001 | 252 | 53.3 | 2659 | 47.8 | 0.021 | ||||||||
| Distance to hospital (km) | 0.004 | <0.001 | ||||||||||||||||
| ≤3 | 61 | 16.1 | 1782 | 22.8 | 19 | 4.0 | 451 | 8.1 | ||||||||||
| >3 & ≤5 | 122 | 32.3 | 2562 | 32.9 | 18 | 3.8 | 373 | 6.7 | ||||||||||
| >5 & ≤7 | 55 | 14.6 | 1112 | 14.3 | 39 | 8.2 | 628 | 11.3 | ||||||||||
| >7 | 140 | 37.0 | 2343 | 30.0 | 397 | 83.9 | 4115 | 73.9 | ||||||||||
Two-sided chi-squared test for categorical variables and Mann-Whitney U test for continuous variables; cholera vs. other diarrhea.
Median and standard deviation provided for continuous variables.
Surface water includes ponds, rivers, and ditches.
Severe acute malnutrition is defined as mid-upper arm circumference (MUAC) <11.5 cm.
Similar relationships were observed for cholera vs. other diarrhea patients in the urban setting, with the following exceptions. Urban cholera patients were more likely to be female (45.5% vs. 40.4%) and did not differ significantly in the number of household members compared to non-cholera patients. Cholera patients were also more likely to reside in a slum (21.4% vs. 14.9%) and less likely to come from a family that owned a home, to use tap water, or to treat their drinking water.
In both settings, cholera patients were less likely to present in normal physical condition, and more likely to present within one day of diarrhea onset than other diarrhea patients (Table 2). They were also more likely to have severe dehydration, watery stool, non-bloody, non-mucusy stool contents, and more than 10 bowel movements and vomiting in the prior 24 hours.
Table 2. Clinical characteristics of diarrheal patients <5-years-old in ICDDR,B hospitals, Bangladesh, 2000–2008.
| Rural | Urban | |||||||||||||
| Cholera (n = 378) | Other (n = 7,799) | Cholera (n = 473) | Other (n = 5,568) | |||||||||||
| N | % | N | % | P a | N | % | N | % | P a | |||||
| Clinical Characteristics | ||||||||||||||
| General Condition | <0.001 | <0.001 | ||||||||||||
| Normal | 222 | 58.7 | 7026 | 90.2 | 85 | 18.0 | 3552 | 63.9 | ||||||
| Restless | 72 | 19.0 | 542 | 7.0 | 6 | 1.3 | 48 | 0.9 | ||||||
| Lethargic but irritable | 73 | 19.3 | 207 | 2.7 | 237 | 50.1 | 1828 | 32.9 | ||||||
| Drowsy/cold & sweating | 11 | 2.9 | 12 | 0.2 | 145 | 30.7 | 132 | 2.4 | ||||||
| Duration of diarrhea prior to arrival (days) | <0.001 | <0.001 | ||||||||||||
| <1 | 186 | 49.2 | 2430 | 31.2 | 223 | 47.1 | 1237 | 22.2 | ||||||
| 1–6 | 182 | 48.1 | 5045 | 64.7 | 229 | 48.4 | 3818 | 68.6 | ||||||
| 7–14 | 9 | 2.4 | 280 | 3.6 | 18 | 3.8 | 434 | 7.8 | ||||||
| 15+ | 1 | 0.3 | 44 | 0.6 | 3 | 0.6 | 78 | 1.4 | ||||||
| Clinical Dehydration | <0.001 | <0.001 | ||||||||||||
| None | 154 | 40.7 | 6705 | 86.0 | 84 | 17.8 | 3549 | 63.8 | ||||||
| Some | 164 | 43.4 | 1052 | 13.5 | 229 | 48.4 | 1865 | 33.5 | ||||||
| Severe | 60 | 15.9 | 39 | 0.5 | 160 | 33.8 | 146 | 2.6 | ||||||
| Watery stool | 320 | 84.7 | 5641 | 72.3 | <0.001 | 466 | 98.5 | 5239 | 94.1 | <0.001 | ||||
| Stool contents | <0.001 | <0.001 | ||||||||||||
| Normal | 314 | 83.1 | 5057 | 64.8 | 419 | 88.6 | 4335 | 77.9 | ||||||
| Mucus | 42 | 11.1 | 1657 | 21.2 | 51 | 10.8 | 1077 | 19.3 | ||||||
| Blood | 3 | 0.8 | 106 | 1.4 | 1 | 0.2 | 6 | 0.1 | ||||||
| Mucus + Blood | 19 | 5.0 | 979 | 12.6 | 2 | 0.4 | 150 | 2.7 | ||||||
| No. stools in 24 hours prior to arrival | 0.001 | <0.001 | ||||||||||||
| 3–5 | 38 | 10.1 | 792 | 10.2 | 39 | 8.2 | 501 | 9.0 | ||||||
| 6–10 | 187 | 49.5 | 4415 | 56.6 | 222 | 46.9 | 3225 | 57.9 | ||||||
| 11–14 | 81 | 21.4 | 1680 | 21.5 | 150 | 31.7 | 1199 | 21.5 | ||||||
| 15–20 | 46 | 12.2 | 565 | 7.2 | 36 | 7.6 | 400 | 7.2 | ||||||
| 21+ | 26 | 6.9 | 347 | 4.4 | 26 | 5.5 | 243 | 4.4 | ||||||
| Vomiting in 24 Hours prior to arrival | <0.001 | <0.001 | ||||||||||||
| None | 54 | 14.3 | 2604 | 33.4 | 39 | 8.2 | 1103 | 19.8 | ||||||
| <10 times | 256 | 67.7 | 4653 | 59.7 | 387 | 81.8 | 4249 | 76.3 | ||||||
| 10+ times | 68 | 18.0 | 542 | 6.9 | 47 | 9.9 | 216 | 3.9 | ||||||
Risk Factor Analysis
Rural and urban univariate analysis results are reported in Tables 3 and 4, respectively. The data for all risk factors were considered complete (≤5% missing), and we found no instances of collinearity among the assessed variables.
Table 3. Assessment of risk factors for severe cholera among rural children <5-years-old in Matlab, Bangladesh, 2000–2008.
| Univariate | Multivariatea | |||||||
| (n = 8,177) | (n = 8,159) | |||||||
| Cholera/Total (%) | RR | 95% CI | Cholera/Total (%) | RR | 95% CI | |||
| Sociodemographic | ||||||||
| Age (yr) b | ||||||||
| 0 | 65/4121 (2) | 1 | 65/4113 (2) | 1 | ||||
| 1 | 75/2703 (3) | 1.76 | (1.27–2.44) | 75/2698 (3) | 1.17 | (0.77–1.78) | ||
| 2 | 108/759 (14) | 9.02 | (6.70–12.20) | 108/758 (14) | 3.84 | (2.42–6.07) | ||
| 3 | 74/360 (21) | 13.03 | (9.51–17.90) | 74/358 (21) | 3.91 | (2.32–6.58) | ||
| 4 | 56/234 (24) | 15.17 | (10.88–21.2) | 54/232 (23) | 4.17 | (2.43–7.15) | ||
| Female sex | 154/3197 (5) | 1.07 | (0.88–1.31) | |||||
| Mother’s education (yr) c | ||||||||
| None | 142/1801 (8) | 1 | 142/1799 (8) | 1 | ||||
| 1–5 | 99/2082 (5) | 0.60 | (0.47–0.77) | 98/2076 (5) | 0.70 | (0.55–0.88) | ||
| 6–9 | 110/3084 (4) | 0.45 | (0.36–0.58) | 109/3078 (4) | 0.61 | (0.48–0.77) | ||
| 10–12 | 25/947 (3) | 0.33 | (0.22–0.51) | 25/945 (3) | 0.45 | (0.30–0.68) | ||
| >12 | 2/263 (1) | 0.10 | (0.02–0.39) | 2/261 (1) | 0.13 | (0.03–0.52) | ||
| Maternal newspaper readership | ||||||||
| Never | 347/7103 (5) | 1 | ||||||
| <7 days/week | 29/884 (3) | 0.67 | (0.46–0.98) | |||||
| Daily | 1/176 (1) | 0.12 | (0.02–0.82) | |||||
| Monthly household income (USD) | ||||||||
| 84+ | 142/3992 (4) | 1 | ||||||
| 50–84 | 169/3260 (5) | 1.46 | (1.17–1.81) | |||||
| 34–50 | 57/831 (7) | 1.93 | (1.43–2.60) | |||||
| <33 | 10/94 (11) | 2.99 | (1.63–5.49) | |||||
| Homeowner | 367/7925 (5) | 1.06 | (0.59–1.90) | |||||
| Concrete floors in home | 24/935 (3) | 0.52 | (0.35–0.79) | |||||
| Water & Sanitation | ||||||||
| Improved toilet facilities | 24/816 (3) | 0.61 | (0.41–0.92) | |||||
| Distance to drinking water d | – | 1.03 | (1.02–1.05) | |||||
| Water source | ||||||||
| Tape | 0/46 (0) | – | – | |||||
| Tube well | 30/1317 (3) | 1 | ||||||
| Surfacef | 341/6808 (5) | 1.78 | (1.28–2.49) | |||||
| Drinking water treatment | ||||||||
| None | 346/7680 (5) | 1 | ||||||
| Boiling | 7/134 (5) | 1.16 | (0.56–2.40) | |||||
| Other | 25/362 (7) | 1.53 | (1.04–2.27) | |||||
| Other Potential Risk Factors | ||||||||
| Family member with diarrhea in past week | 44/528 (8) | 1.91 | (1.41–2.58) | 44/527 (8) | 1.61 | (1.22–2.14) | ||
| Currently breastfed | 182/7060 (3) | 0.15 | (0.12–0.18) | 182/7060 (3) | 0.49 | (0.35–0.67) | ||
| Severe acute malnutrition g | 19/643 (3) | 0.62 | (0.39–0.97) | |||||
| Prior vitamin A supplementation | 311/4202 (7) | 4.39 | (3.38–5.70) | 309/4194 (7) | 1.65 | (1.12–2.43) | ||
| Distance to hospital h | – | 1.07 | (1.02–1.12) | |||||
Adjusted for sex, the number of household members, seasonality, and the other predictors in the model. The number of patients in the multivariate analysis was less than the number in the univariate analysis due to missing breast feeding data for 18 children.
Linear trend for increasing risk with age in the univariate and multivariate models (P<0.001).
Linear trend for decreasing risk with increasing maternal education in the univariate and multivariate models (P<0.001).
Per 10 meter increment.
Too few observations to develop a risk estimate for rural children.
Surface water includes ponds, rivers, and ditches.
Severe acute malnutrition defined as mid-upper arm circumference (MUAC) <11.5cm.
Per five kilometer increment.
Table 4. Assessment of risk factors for severe cholera among urban children <5–years-old in Dhaka, Bangladesh, 2000–2008.
| Univariate | Multivariatea | ||||||
| (n = 6,041) | (n = 6,008) | ||||||
| Cholera/Total (%) | RR | 95% CI | Cholera/Total (%) | RR | 95% CI | ||
| Sociodemographic | |||||||
| Age (yr) b | |||||||
| 0 | 103/3500 (3) | 1 | 101/3476 (3) | 1 | |||
| 1 | 112/1608 (7) | 2.37 | (1.82–3.07) | 111/1603 (7) | 2.33 | (1.80–3.02) | |
| 2 | 102/460 (22) | 7.53 | (5.83–9.73) | 102/459 (22) | 5.53 | (4.26–7.19) | |
| 3 | 88/286 (31) | 10.46 | (8.08–13.5) | 87/284 (31) | 5.86 | (4.38–7.83) | |
| 4 | 68/187 (36) | 12.36 | (9.45–16.2) | 68/186 (37) | 6.32 | (4.63–8.63) | |
| Female sex | 215/2467 (9) | 1.21 | (1.01–1.44) | ||||
| Mother’s education (yr) | |||||||
| None | 216/2058 (10) | 1 | |||||
| 1–5 | 105/1243 (8) | 0.80 | (0.64–1.01) | ||||
| 6–9 | 106/1516 (7) | 0.67 | (0.53–0.83) | ||||
| 10–12 | 27/748 (4) | 0.34 | (0.23–0.51) | ||||
| >12 | 19/476 (4) | 0.38 | (0.24–0.60) | ||||
| Maternal newspaper readership | |||||||
| Never | 440/5102 (9) | 1 | 438/5094 (9) | 1 | |||
| <7 days/week | 22/590 (4) | 0.43 | (0.28–0.66) | 22/590 (4) | 0.55 | (0.36–0.84) | |
| Daily | 9/324 (3) | 0.32 | (0.17–0.62) | 9/324 (3) | 0.41 | (0.21–0.79) | |
| Monthly household income (USD) | |||||||
| 84+ | 188/3077 (6) | 1 | 186/3062 (6) | 1 | |||
| 50–84 | 210/2175 (10) | 1.58 | (1.31–1.91) | 208/2161 (10) | 1.33 | (1.10–1.61) | |
| 34–50 | 62/605 (10) | 1.68 | (1.28–2.20) | 62/602 (10) | 1.34 | (1.03–1.75) | |
| <33 | 13/184 (7) | 1.16 | (0.67–1.99) | 13/183 (7) | 1.05 | (0.63–1.73) | |
| Residence in a slum | 101/928 (11) | 1.50 | (1.21–1.84) | ||||
| Homeowner | 49/872 (6) | 0.69 | (0.52–0.91) | ||||
| Concrete floors in home | 342/4747 (7) | 0.71 | (0.59–0.86) | ||||
| Water & Sanitation | |||||||
| Improved toilet facilities | 285/4262 (7) | 0.63 | (0.53–0.75) | ||||
| Distance to drinking water c | – | 1.04 | (1.01–1.06) | ||||
| Water source | |||||||
| Tap | 392/5235 (7) | 0.73 | (0.58–0.93) | ||||
| Tube well | 77/755 (10) | 1 | |||||
| Surfaced | 4/48 (8) | 0.82 | (0.31–2.14) | ||||
| Drinking water treatment | |||||||
| None | 291/2949 (10) | 1 | |||||
| Boiling | 179/3030 (6) | 0.60 | (0.50–0.72) | ||||
| Other | 3/61 (5) | 0.50 | (0.16–1.51) | ||||
| Other Potential Risk Factors | |||||||
| Family member with diarrhea in past week | 76/636 (12) | 1.63 | (1.29–2.05) | ||||
| Currently breastfed | 235/4809 (5) | 0.25 | (0.21–0.30) | 235/4808 (5) | 0.51 | (0.41–0.62) | |
| Severe acute malnutrition e | 63/1049 (6) | 0.76 | (0.58–0.98) | ||||
| Prior Vitamin A supplementation | 252/2911 (9) | 1.23 | (1.03–1.46) | ||||
| Distance to hospital f | – | 1.01 | (0.99–1.03) | ||||
Adjusted for sex, the number of household members, seasonality, and the other predictors in the model. The number of patients in the multivariate analysis was less than the number in the univariate analysis due to missing breast feeding (n = 12) and maternal newspaper readership (n = 21) data for urban children.
Linear trend for increasing risk with age in the univariate and multivariate models (P<0.001).
Per 10 meter increment.
Surface water includes ponds, rivers, and ditches.
Severe acute malnutrition defined as mid-upper arm circumference (MUAC) <11.5cm.
Per five kilometer increment.
In the rural model, cholera risk increased with age (P-trend: <0.001) and decreased monotonically with higher levels of maternal education (P-trend: <0.001), when adjusted for a priori confounders and the other predictors in the final model (Table 3). Four-year-olds faced more than four times the risk of those less than one (adjusted risk ratio (aRR) = 4.17, 95% CI: 2.43–7.15). There was an 87% risk reduction for those with more than 12 years of education (aRR = 0.13, 95%CI: 0.03–0.52), compared to those with no formal education. Having a family member with diarrhea in the past week was associated with increased risk (aRR = 1.61, 95% CI: 1.22–2.14), whereas current breastfeeding (aRR = 0.49, 95% CI: 0.35–0.67) halved the risk. Prior vitamin A supplementation (aRR = 1.65, 95% CI: 1.12–2.43) was associated with increased risk for severe cholera.
In the urban multivariate model, cholera risk also increased with age (P–trend: <0.001) and was halved with current breastfeeding (aRR = 0.51, 95% CI: 0.41–0.62) (Table 4). Daily (aRR = 0.41, 95% CI: 0.21–0.79) and occasional (aRR = 0.55, 95% CI: 0.36–0.84) maternal newspaper readership were associated with reduced risk, compared to children whose mothers did not read newspapers. Children from households with incomes between 34 and 84 USD per month experienced a 30% increased risk, compared to those from households with monthly incomes greater than 84 USD.
Discussion
Several factors emerged from this analysis that differentiated children with cholera from those with other types of diarrhea. Some findings were common to both the urban and rural setting, while others were limited to one setting or the other. Increasing age was strongly associated with cholera risk in both urban and rural settings, with a four-fold increased risk for rural four-year-olds and a six-fold increased risk for urban four-year-olds compared to those under one. Current breastfeeding, a behavior that can be successfully promoted [18], halved the risk in both settings. Socioeconomic status (SES) indicators were also key correlates of cholera risk in both settings: increasing maternal education was associated with decreasing cholera risk in rural children, and maternal newspaper readership and increasing family income was associated with decreased risk in urban children.
In the rural setting, children with a history of vitamin A supplementation or a family member with diarrhea had increased risk.
Cholera hospitalization risk increased after age two among rural children and after one among urban children. This finding is similar to that reported in a 1982 study in which rural children under two experienced hospitalization for cholera less frequently than those two to nine years old [19]. The delayed onset of risk among rural children may be explained, in part, by the greater proportion of rural mothers who breastfed their children. In addition, although we did not have data to assess this, women in rural settings may breastfeed longer than those in urban settings [20]. Early weaning may increase cholera risk through loss of cholera-specific IgA antibodies, which can be passed through breast milk and effectively protect against cholera disease in children who are colonized [21]. The protective effect of breastfeeding maybe especially pronounced in this dataset because breastfeeding does not appear to protect against rotavirus infection [22], which accounted for approximately one third of the other diarrhea in the DDSS.
In both settings, measures of maternal education – years of schooling or newspaper readership – were more strongly associated with reduced cholera risk than breastfeeding; similar findings were reported more than 35 years ago [23]. While the mechanism by which maternal education reduces cholera risk has not been specifically described, this finding underscores the importance of working toward Millennium Development Goal #2 (to achieve universal primary education) not only as a goal in its own right but also as a strategy to reduce child mortality (MDG #4).
The general lack of association of water and sanitation variables with cholera risk was surprising given the importance of water in cholera transmission. Rather than a true lack of association, it’s possible that our null results reflect the limitations of using self-reported water and sanitation measures, which may be unreliable.
The proper interpretation of the finding that rural children who received vitamin A were at higher cholera risk than those who did not is unclear, and the criteria by which children received vitamin A supplementation are unknown. Retinol deficiency is more common in children with cholera [24]. If supplementation was based on retinol deficiency, and those with prior supplementation are at continued risk for retinol deficiency, then the observed increased risk for cholera hospitalization among children who received vitamin A supplementation is to be expected. Alternatively, vitamin A deficiency may be a surrogate for malnutrition, which is also known to be associated with cholera severity or duration [25]. However, severe acute malnutrition was not associated with cholera risk in our data.
The increased risk associated with having a family member with diarrhea in the past week has also been found in studies of non-cholera diarrhea [29], [30]. In our study, the increased risk is likely due to shared primary exposures as well as genetic/familial susceptibility [26], [27] and secondary person-to-person transmission through environmental contamination [28].
The use of anonymized data prevented us from assessing repeat visits by the same patient. However, since cholera infection confers natural immunity [31], it is unlikely that an individual would contribute more than one cholera case to our study. This is confirmed by a previous study that found only three repeat cholera hospitalizations out of more than 7,000 cholera cases over a 15-year period [19]. Nonetheless, we cannot rule out the possibility that a patient classified in this study as having non-cholera diarrhea might have had cholera in the past. This possible misclassification might have led to over- or underestimation of associations. We were also unable to assess family clustering of diarrheal cases in the DDSS. Though clustering could lead to violations of underlying independent observation assumptions [26], [32], with a sample this large, any clustering effects are likely to be minimal. In addition, antibiotic use prior to hospitalization, which is known to occur in Bangladesh [33], could not be assessed. This could have skewed the DDSS data, since antibiotic treatment is highly efficacious. Despite these limitations, the large sample size, well-defined population, systematic sampling, and expert laboratory diagnosis of V. cholerae are strengths of this study, as is the fact that our referent group is comprised of hospital patients with other causes of diarrhea. Our study therefore highlights risk factors unique to cholera, as opposed to general diarrheal risks [34], [35].
In conclusion, we report that increasing age, measures of SES, maternal education, and current breastfeeding status are key correlates of risk for cholera hospitalization among children under five in rural and urban Bangladesh. In addition, a history of vitamin A supplementation and having a family member with diarrhea in the past week were associated with increased risk among rural children. The lack of association with water and sanitation measures highlights the need for a more thorough assessment of potential waterborne exposures. Continued attention should be directed to promotion of breastfeeding, female education, securing viable livelihoods, and promulgation of safe water sources. Finally, the risk faced by family members of cholera cases may warrant renewed research regarding the use of targeted chemoprophylaxis in endemic rural settings [33], [36].
Acknowledgments
We would like to extend our gratitude to Mr. Abdul Malek for his compilation of the data files and Dr. Dilruba Nasrin for providing essential background information.
Funding Statement
This work was supported by a Washington Global Health Alliance travel grant [2009 to DVC (http://www.wghalliance.org/)]; the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, National Eye Institute, National Heart, Blood, and Lung Institute, National Institute of Dental and Craniofacial Research, National Institute On Drug Abuse, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, and National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University [grant number R24 TW007988 to DVC (https://fogartyscholars.org/)]; the National Cancer Institute [grant number R25 CA94880 to DVC (http://www.cancer.gov/)]; and the American Relief and Recovery Act. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ali M, Lopez AL, Ae You Y, Eun Kim Y, Sah B, et al. (2012) The global burden of cholera. Bull World Health Organ 90: 209–218 doi:10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mosley WH, Benenson AS, Barui R (1968) A serological survey for cholera antibodies in rural east Pakistan. 2. A comparison of antibody titres in the innunized and control populationd of a cholera-vaccine field-trial area and the relation of antibody titre to cholera case rate. Bull World Health Organ 38: 335–346. [PMC free article] [PubMed] [Google Scholar]
- 3. Gunn Ra, Kimball aM, Pollard Ra, Feeley JC, Feldman Ra, et al. (1979) Bottle feeding as a risk factor for cholera in infants. Lancet 2: 730–732. [DOI] [PubMed] [Google Scholar]
- 4. Clemens JD, Sack DA, Harris JR, Khan MR, Chakraborty J, et al. (1990) Breast feeding and the risk of severe cholera in rural Bangladeshi children. Am J Epidemiol 131: 400–411. [DOI] [PubMed] [Google Scholar]
- 5. Qureshi K, Mølbak K, Sandström A, Kofoed P-E, Rodrigues A, et al. (2006) Breast milk reduces the risk of illness in children of mothers with cholera: observations from an epidemic of cholera in Guinea-Bissau. Pediatr Infect Dis J 25: 1163–1166 doi:10.1097/01.inf.0000246977.58697.a5. [DOI] [PubMed] [Google Scholar]
- 6. Guerrant RL, Kirchhoff L V, Shields DS, Nations MK, Leslie J, et al. (1983) Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis 148: 986–997. [DOI] [PubMed] [Google Scholar]
- 7. Strand T a, Sharma PR, Gjessing HK, Ulak M, Chandyo RK, et al. (2012) Risk factors for extended duration of acute diarrhea in young children. PLoS ONE 7: e36436 doi:10.1371/journal.pone.0036436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Reilly CE, Jaron P, Ochieng B, Nyaguara A, Tate JE, et al. (2012) Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural western Kenya, 2005–2007: a cohort study. PLoS Med 9: e1001256 doi:10.1371/journal.pmed.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitra a K, Rahman MM, Fuchs GJ (2000) Risk factors and gender differentials for death among children hospitalized with diarrhoea in Bangladesh. J Health Popul Nutr 18: 151–156. [PubMed] [Google Scholar]
- 10.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM (2011) Oral vaccines for preventing cholera. Cochrane Database Syst Rev: CD008603. doi:10.1002/14651858.CD008603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay B, Bopp C, Wells J (1994) Isolation and identification of Vibrio cholerae O1 from fecal specimens. In: Wachsmuth I, Blake P, Olsvik O, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, DC: American Society for Microbiology Press. 3–25. [Google Scholar]
- 12. Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack D a, et al. (2006) Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg 74: 1067–1073. [PMC free article] [PubMed] [Google Scholar]
- 13.WHO (1987) Programme for control of diarrhoeal diseases (CDD/83.3 Rev 1). Manual for laboratory investigation of acute enteric infections. Geneva: WHO. [Google Scholar]
- 14.XE Currency Table: Mid-market rates as of 2004-07-01 (n.d.). Available: http://www.xe.com/currencytables/?from=USD&date=2004-07-01. Accessed 2012 Nov 13.
- 15.WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation (2010) Types of drinking-water sources and sanitation. Available: http://www.wssinfo.org/definitions-methods/watsan-categories/. Accessed 2012 Oct 9.
- 16. Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706 doi:10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 17. Greenland S (1995) Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 6: 356–365. [DOI] [PubMed] [Google Scholar]
- 18. Haider R, Ashworth a, Kabir I, Huttly SR (2000) Effect of community-based peer counsellors on exclusive breastfeeding practices in Dhaka, Bangladesh: a randomised controlled trial [see commments]. Lancet 356: 1643–1647. [DOI] [PubMed] [Google Scholar]
- 19. Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, et al. (1982) Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol 116: 959–970. [DOI] [PubMed] [Google Scholar]
- 20. Giashuddin MS, Kabir M (2004) Duration of breast-feeding in Bangladesh. Indian J Med Res 119: 267–272. [PubMed] [Google Scholar]
- 21. Glass RI, Svennerholm AM, Stoll BJ, Khan MR, Hossain KM, et al. (1983) Protection against cholera in breast-fed children by antibodies in breast milk. N Engl J Med 308: 1389–1392 doi:10.1056/NEJM198306093082304. [DOI] [PubMed] [Google Scholar]
- 22. Glass RI, Stoll BJ, Wyatt RG, Hoshino Y, Banu H, et al. (1986) Observations questioning a protective role for breast-feeding in severe rotavirus diarrhea. Acta Paediatr Scand 75: 713–718. [DOI] [PubMed] [Google Scholar]
- 23. Levine RJ, Khan MR, D’Souza S, Nalin DR (1976) Failure of sanitary wells to protect against cholera and other diarrhoeas in Bangladesh. Lancet 2: 86–89. [DOI] [PubMed] [Google Scholar]
- 24. Chowdhury F, Khan AI, Harris JB, LaRocque RC, Chowdhury MI, et al. (2008) A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr Infect Dis J 27: 986–992 doi:10.1097/INF.0b013e3181783adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer DL, Koster FT, Alam a K, Islam MR (1976) Nutritional status: a determinant of severity of diarrhea in patients with cholera. J Infect Dis 134: 8–14. [DOI] [PubMed] [Google Scholar]
- 26. Rahman KM, Duggal P, Harris JB, Saha SK, Streatfield PK, et al. (2009) Familial aggregation of Vibrio cholerae-associated infection in Matlab, Bangladesh. J Health Popul Nutr 27: 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glass RI, Holmgren J, Haley CE, Khan MR, Svennerholm AM, et al. (1985) Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol 121: 791–796. [DOI] [PubMed] [Google Scholar]
- 28. Giebultowicz S, Ali M, Yunus M, Emch M (2011) A comparison of spatial and social clustering of cholera in Matlab, Bangladesh. Health Place 17: 490–497 doi:10.1016/j.healthplace.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blake PA, Ramos S, MacDonald KL, Rassi V, Gomes TA, et al. (1993) Pathogen-specific risk factors and protective factors for acute diarrheal disease in urban Brazilian infants. J Infect Dis 167: 627–632. [DOI] [PubMed] [Google Scholar]
- 30.Rowe PC, Orrbine E (1994) Epidemic Escherichia coil O157: H7 gastroenteritis and hemolytic-uremic syndrome in a Canadian Inuit community: Intestinal illness in family members as a risk factor: 1–6. [DOI] [PubMed] [Google Scholar]
- 31. Ali M, Emch M, Park JK, Yunus M, Clemens J (2011) Natural cholera infection-derived immunity in an endemic setting. J Infect Dis 204: 912–918 doi:10.1093/infdis/jir416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, et al. (2008) Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2: e221 doi:10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque a SG, et al. (2009) Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis 49: 1473–1479 doi:10.1086/644779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mccarthy N, Giesecke J (1999) Case-case comparisons to study causation: 764–768. [DOI] [PubMed] [Google Scholar]
- 35. Wilson N, Baker M, Edwards R, Simmons G (2008) Case-case analysis of enteric diseases with routine surveillance data: Potential use and example results. Epidemiol Perspect Innov 5: 6 doi:10.1186/1742-5573-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reveiz L, Chapman E, Ramon-Pardo P, Koehlmoos TP, Cuervo LG, et al. (2011) Chemoprophylaxis in contacts of patients with cholera: systematic review and meta-analysis. PLoS ONE 6: e27060 doi:10.1371/journal.pone.0027060. [DOI] [PMC free article] [PubMed] [Google Scholar]