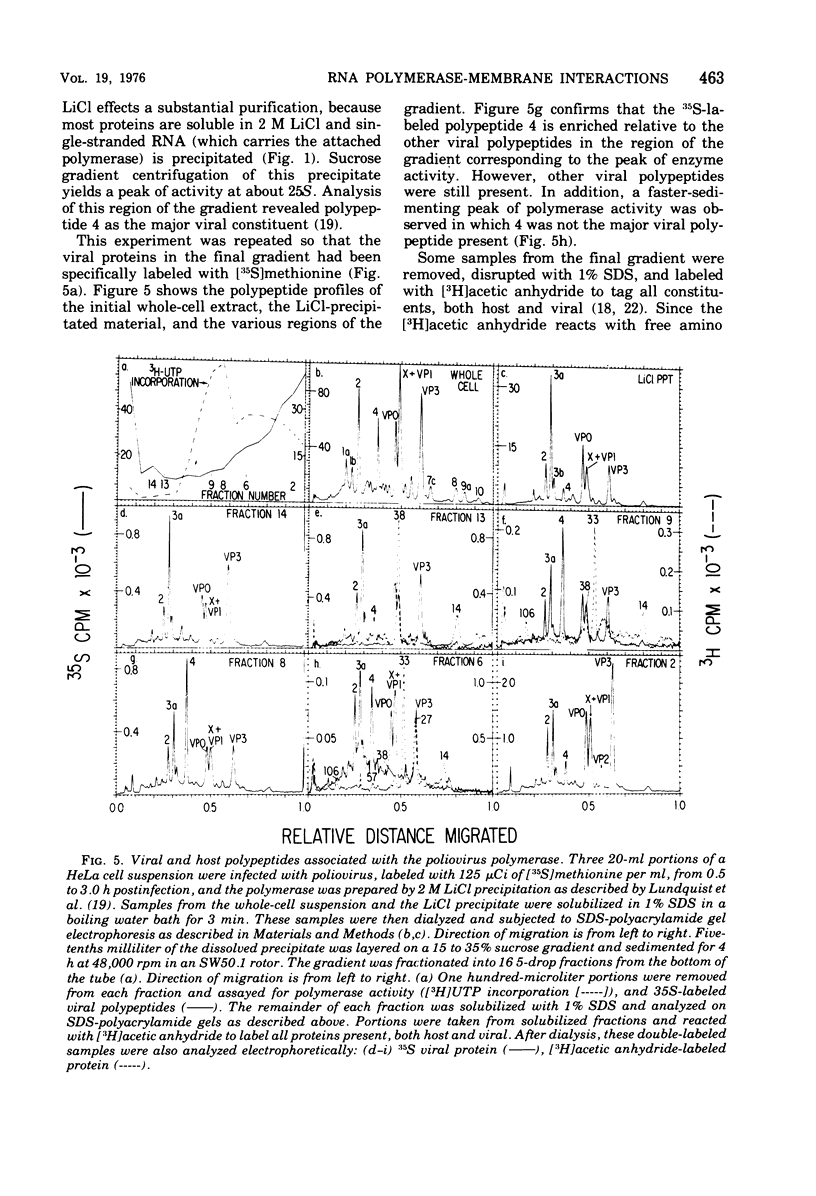
Abstract
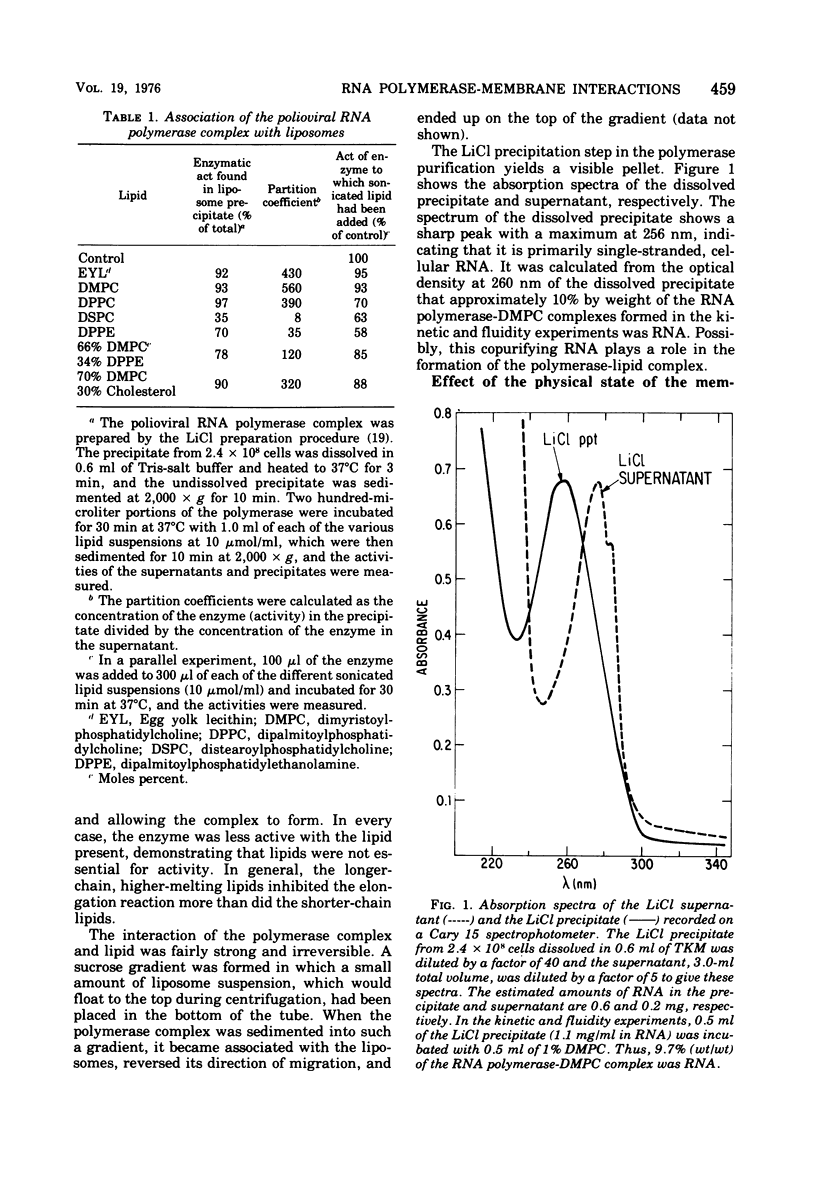
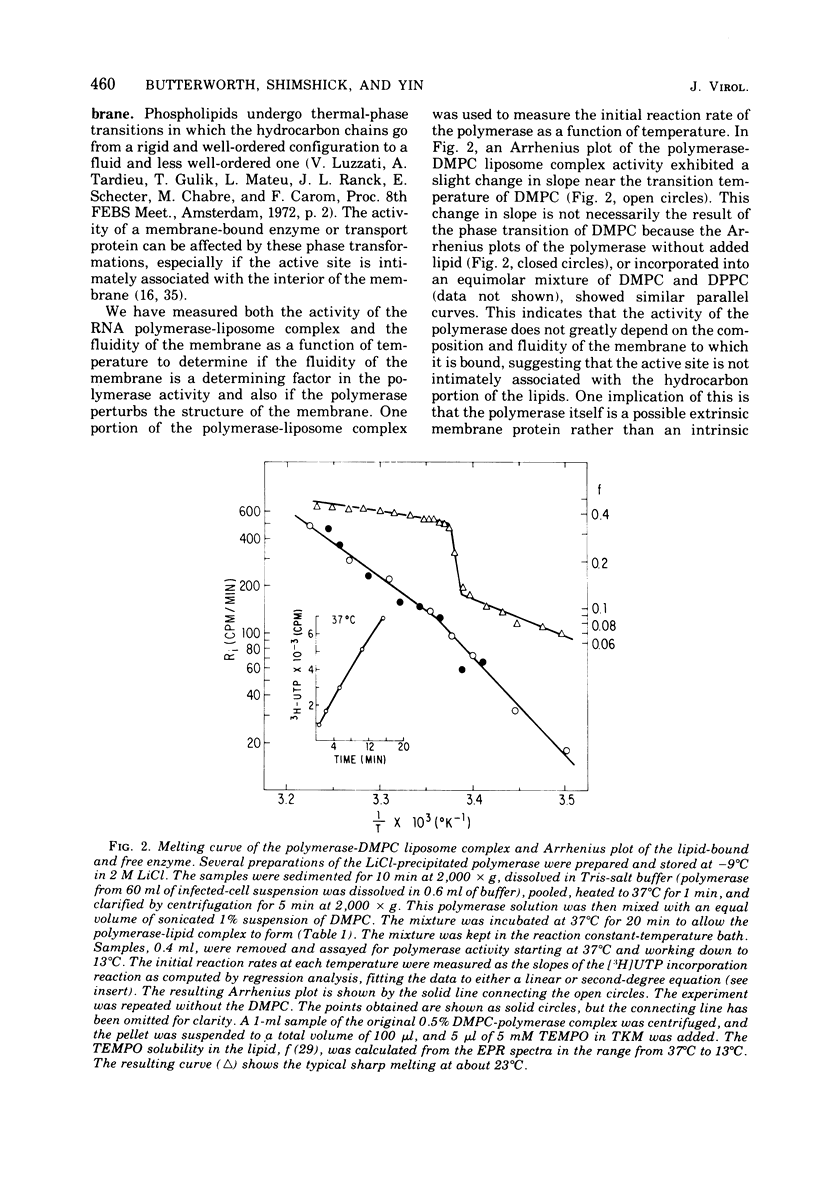
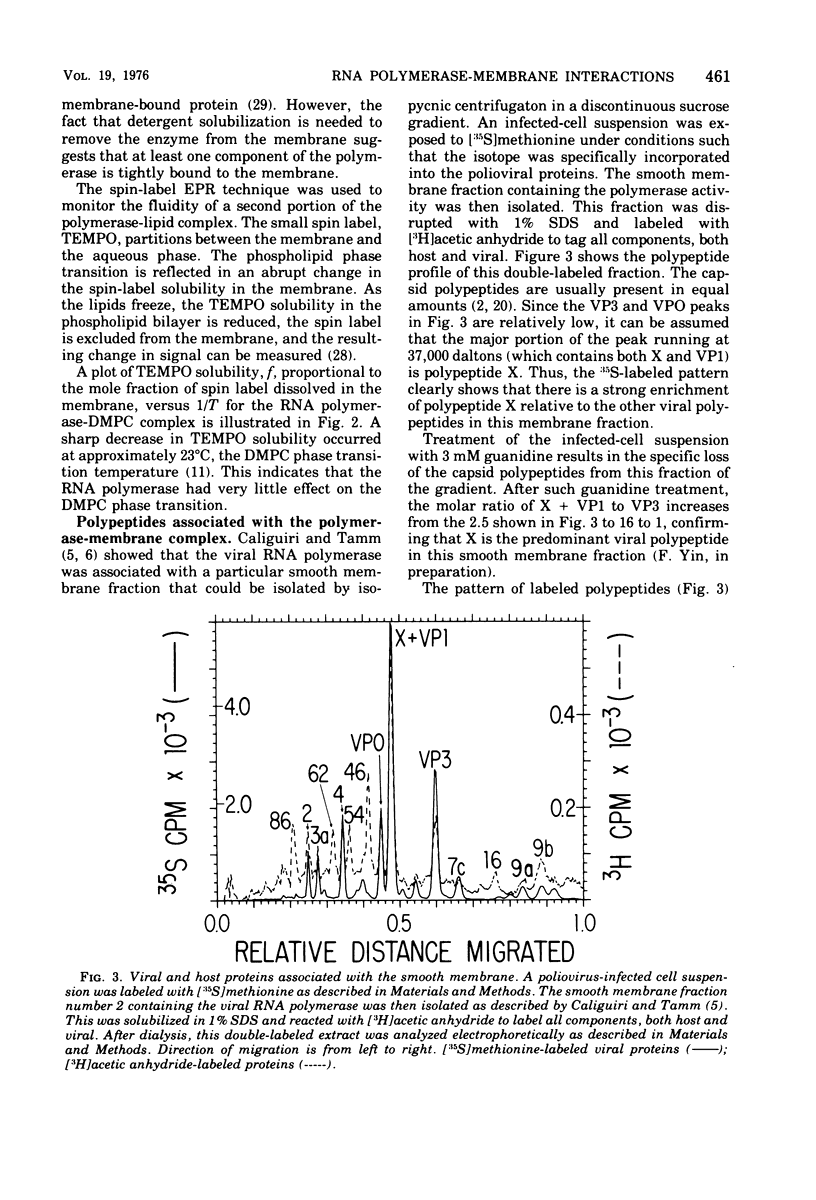
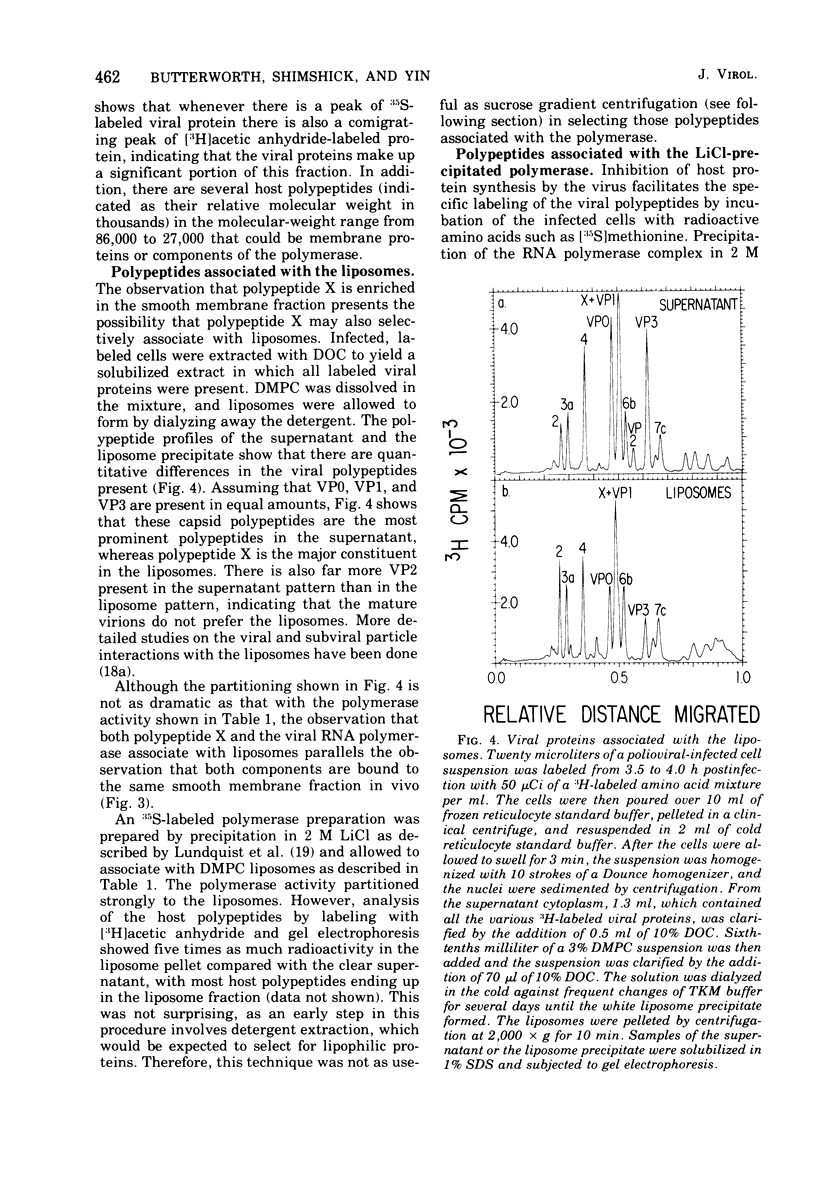
Polioviral RNA polymerase complex, which consists of enzyme, template, and nascent RNA, is membrane bound in vivo. The solubilized RNA polymerase complex associated spontaneously in vitro with phospholipid bilayer membranes (liposomes) of defined composition. The degree of association at 37 degrees C was greater for those membranes that were more fluid, suggesting that the binding involves the interaction of the RNA polymerase complex with the hydrocarbon chains in the interior of the lipid bilayer. The polymerase activity was not enhanced by addition of the lipid; in fact, the addition of some of the longer-chain lipids resulted in up to a 40% inhibition of the polymerase activity. Spin-label electron paramagnetic resonance experiments, which measured the membrane fluidity, and kinetic experiments on the rate of incorporation of tritiated UTP into RNA by the polymerase were performed as a function of temperature. The results indicated that the activity of the polymerase was not affected by the physical state of the phospholipid membrane and that its active site was not intimately associated with the membrane. Analysis of both the viral and host polypeptides associated with the smooth membrane-bound polymerase indicated that X was the primary viral polypeptide present. In addition, host polypeptides of molecular weight 86,000, 62,000, 54,000, and 46,000 were also present. If the membrane was disrupted with detergent, polypeptide X was released from the polymerase activity, suggesting that X may play a role in binding the polymerase to the membrane. In an analogous manner, polypeptide X associated spontaneously with phospholipid membranes to a greater extent than the capsid polypeptides. Analysis of both the host and viral polypeptides associated with the viral RNA polymerase purified by precipitation in 2 M LiCl indicated that host polypeptides of molecular weight 106,000, 38,000, 33,000, and 14,000 were the major constituents, whereas relatively small amounts of the viral polypeptides were present. It was confirmed that of the viral polypeptides found, polypeptide 4 was present in the largest amount.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Hall L., Stoltzfus C. M., Rueckert R. R. Virus-specific proteins synthesized in encephalomyocarditis virus-infected HeLa cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3083–3087. doi: 10.1073/pnas.68.12.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Mosser A. G. Proteins associated with the poliovirus RNA replication complex. Virology. 1971 Nov;46(2):375–386. doi: 10.1016/0042-6822(71)90039-0. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Geissler E., Scotti P. D., Tannock G. A. Further characterization of the genetic map of poliovirus temperature-sensitive mutants. In: strategy of the viral genome. Ciba Found Symp. 1971:75–100. doi: 10.1002/9780470719824.ch5. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Maizel J. V., Summers D. F. Soluble RNA polymerase complex from poliovirus-infected HeLa cells. Virology. 1970 Apr;40(4):840–846. doi: 10.1016/0042-6822(70)90129-7. [DOI] [PubMed] [Google Scholar]
- Grant C. W., McConnell H. M. Glycophorin in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4653–4657. doi: 10.1073/pnas.71.12.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Lipid requirements for Rhodopsin regenerability. Biochemistry. 1973 Oct 23;12(22):4517–4523. doi: 10.1021/bi00746a033. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Capadil R. A., Vanderkooi G., Griffith O. H. Lipid-protein and lipid-lipid interactions in cytochrome oxidase model membranes. J Supramol Struct. 1973;1(4):269–280. doi: 10.1002/jss.400010404. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch W. T., Jr, Arlinghaus A. Stable polypeptides associated with the 250S mengovirus-induced RNA polymerase structure. Arch Virol. 1975;47(3):201–215. doi: 10.1007/BF01317808. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Gosser L. B., Shimshick E. J. Interaction of liposomes with subviral particles of poliovirus type 2 and rhinovirus type 2. J Virol. 1976 Aug;19(2):746–749. doi: 10.1128/jvi.19.2.746-749.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R. E., Ehrenfeld E., Maizel J. V., Jr Isolation of a viral polypeptide associated with poliovirus RNA polymerase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4773–4777. doi: 10.1073/pnas.71.12.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S. Evidence for the existence of protomers in the assembly of encephalomyocarditis virus. J Virol. 1975 May;15(5):1107–1120. doi: 10.1128/jvi.15.5.1107-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D. M., Raftery M. A. Purified acetylcholine receptor: its reconstitution to a chemically excitable membrane. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4768–4772. doi: 10.1073/pnas.71.12.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Rueckert R. R. Radiolabeling of proteins and viruses in vitro by acetylation with radioactive acetic anhydride. J Biol Chem. 1975 Feb 25;250(4):1413–1421. [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Scheid A. S., Tamm I. Chemical and enzymatic characteristics of cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):30–38. doi: 10.1016/0042-6822(72)90235-8. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Tamm I. Incorporation of lipid precursors into cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):39–47. doi: 10.1016/0042-6822(72)90236-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Diskin B., Oron L., Traub A. Isolation and subunit structure of polycytidylate-dependent RNA polymerase of encephalomyocarditis virus. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3815–3819. doi: 10.1073/pnas.69.12.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. III. In vitro addition of polyadenylic acid to poliovirus RNAs. J Virol. 1975 Jun;15(6):1432–1439. doi: 10.1128/jvi.15.6.1432-1439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub A., Duskin B., Rosenberg H., Kalmar E. Isolation and properties of the replicase of encephalomyocarditis virus. J Virol. 1976 May;18(2):375–382. doi: 10.1128/jvi.18.2.375-382.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Reversible lipid titrations of the activity of pure adenosine triphosphatase-lipid complexes. Biochemistry. 1974 Dec 31;13(27):5501–5507. doi: 10.1021/bi00724a008. [DOI] [PubMed] [Google Scholar]



