Abstract
Background
Studies have suggested the chemopreventive effects of flavonoids on carcinogenesis. Yet numbers of epidemiologic studies assessing dietary flavonoids and breast cancer risk have yielded inconsistent results. The association between flavonoids, flavonoid subclasses (flavonols, flavan-3-ols, etc.) and the risk of breast cancer lacks systematic analysis.
Objective
We aimed to examine the association between flavonoids, each flavonoid subclass (except isoflavones) and the risk of breast cancer by conducting a meta-analysis.
Design
We searched for all relevant studies with a prospective cohort or case-control study design published before July 1st, 2012, using Cochrane library, MEDLINE, EMBASE and PUBMED. Summary relative risks (RR) were calculated using fixed- or random-effects models. All analyses were performed using STATA version 10.0.
Results
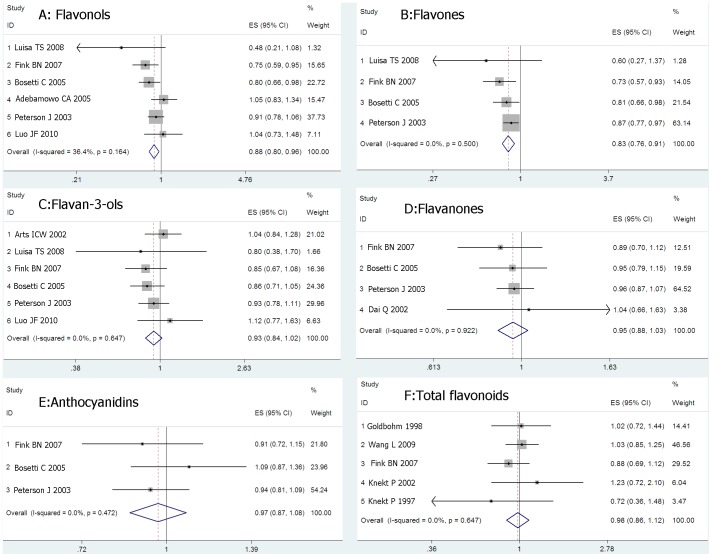
Twelve studies were included, involving 9 513 cases and 181 906 controls, six of which were prospective cohort studies, and six were case-control studies. We calculated the summary RRs of breast cancer risk for the highest vs lowest categories of each flavonoid subclass respectively. The risk of breast cancer significantly decreased in women with high intake of flavonols (RR = 0.88, 95% CI 0.80–0.98) and flavones (RR = 0.83, 95% CI: 0.76–0.91) compared with that in those with low intake of flavonols and flavones. However, no significant association of flavan-3-ols (RR = 0.93, 95% CI: 0.84–1.02), flavanones (summary RR = 0.95, 95% CI: 0.88–1.03), anthocyanins (summary RR = 0.97, 95% CI: 0.87–1.08) or total flavonoids (summary RR = 0.98, 95% CI: 0.86–1.12) intake with breast cancer risk was observed. Furthermore, summary RRs of 3 case-control studies stratified by menopausal status suggested flavonols, flavones or flavan-3-ols intake is associated with a significant reduced risk of breast cancer in post-menopausal while not in pre-menopausal women.
Conclusions
The present study suggests the intake of flavonols and flavones, but not other flavonoid subclasses or total flavonoids, is associated with a decreased risk of breast cancer, especially among post-menopausal women.
Introduction
Breast cancer is the leading cause of cancer death among women in Europe and North America. Almost 1.4 million women were diagnosed with breast cancer worldwide in 2008 and approximately 459,000 deaths were recorded [1], [2]. More than 2.5 million breast cancer survivors live in United States currently, and the number is expected to grow to 3.4 million by 2015 [3]. The National Cancer Institute (NCI) has recognized that prevention is a critical component in minimizing the number of individuals afflicted with cancer [4]. Recent reports suggest that approximately one-third of the most common cancers in western countries can be prevented by eating a healthy, plant-based diet; being physically active; and maintaining a healthy weight [5]. Epidemiologic studies and systematic analysis suggest diets rich in fruits and vegetables are associated with a reduced risk of cancer, in particular cancers of epithelial origin such as those of the mouth, colon, rectum [6], lung [7], and breast [8], [9]. As consumption of fruits and vegetables has been associated with a reduced risk of human cancers especially breast cancer [10], [11], dietary flavonoids, a group of more than 5 000 different polyphenolic compounds, have been identified as potential cancer-preventive components of fruits and vegetables [12], [13].
Dietary flavonoids occur ubiquitously in plant foods, and can be categorized into six major subclasses based on their range and structural complexity: flavonols, flavones, flavan-3-ols, flavanones, anthocyanins and isoflavones (Figure 1) [14]–[16]. As reviewed before by Hooper L et al (Table 1), in western diet, major flavonoids included in these subclasses are quercetin, myricetin and kaempferol for flavonols, hesperitin and narigerin for flavanones, epicatechin and catechin for flavan-3-ols, apigenin and luteolin for flavones, cyanidin, delphinidin and malvidin for anthocyanidines, and genistein and daizeina for isoflavones [16]–[18]. Flavonols mainly exist in onions, broccoli, tea, and various common fruits, flavones in aromatic herbs, celery, and chamomile tea, flavan-3-ols in cocoa, red wine, grapes, apples, green tea, and other fruits, flavanones in oranges and other citrus fruits, anthocyanidines in colored berries, black currants, and isoflavones in soy food [19]–[21].
Figure 1. Structures of six flavonoid subclasses.
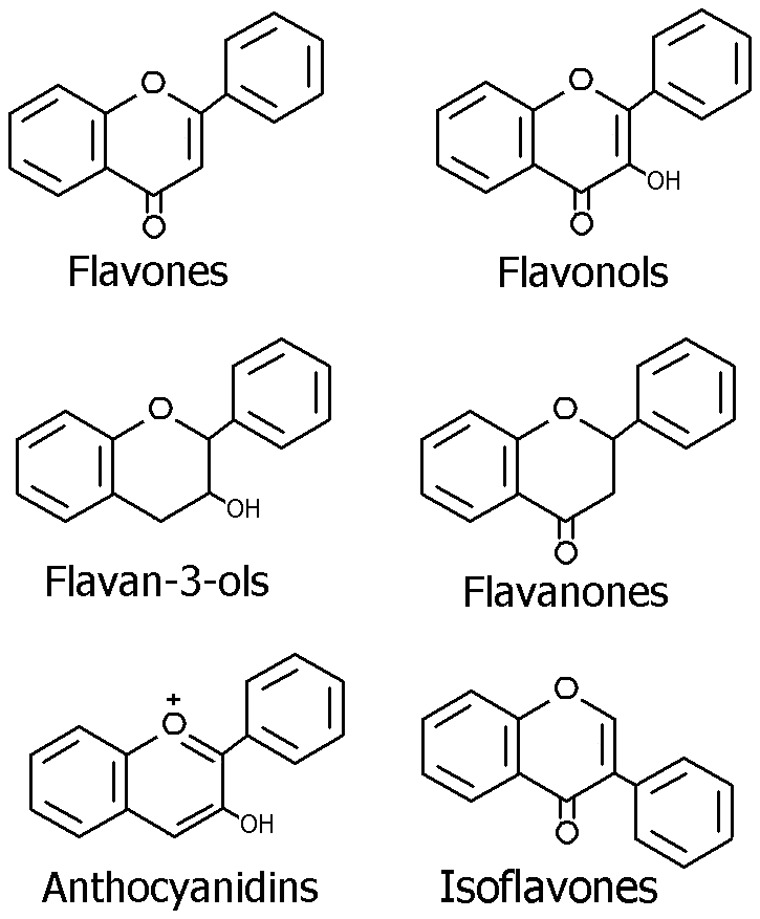
Table 1. Flavonoid subclasses, food sources and intakes [14].
| Flavonoid subclasses | Example compounds | Major dietary sources | Estimated daily intakes |
| Flavonols | Quercetin, kaempferol, myricetin, and isorhamnetin | Onions, broccoli, tea, and various fruits | mg/d12.9 |
| Flavones | Luteolin, apigenin, and tangeretin | Herbs (especially parsley), celery, and chamomile tea | 1.6 |
| Flavanones | Naringenin, hesperetin | Citrus fruit including oranges and grape fruit | 14.4 |
| Flavan-3-ols | Catechin, epicatechin, epigallocatechin | Cocoa or dark chocolate, apples, grape, red wine, and green tea | 156.9 |
| Anthocyanidins | Cyanidin, delphinidin, pelargonidin, andmalvidin | Colored berries and other fruit, especially cranberries, black currants, and blueberries | 3.1 |
| Isoflavones | Genistein, daidzein, and glycitein | Soy products including fermented products, eg, tofu, tempeh, miso, and soy protein isolate | 1.2 (US and Netherlands) 25–50 (Asia) |
In decades, studies have suggested the chemopreventive effects of flavonoids on carcinogenesis, the anticancer activity of dietary flavonoids has become an important and interesting topic. Yet in fact, a number of epidemiologic studies assessing the association between dietary flavonoid intake and the risk of breast cancer have yielded inconsistent results and have identified controversial evidence [22]–[34]. One possible explanation is that different flavonoid subclasses in diet may have different properties and effects in vivo. So it is important to elucidate the different role of each flavonoid subclass in the chemopreventive effect of these dietary compounds. However no systematic analysis has been performed to assess the association between dietary intake of flavonoid subclasses and the risk of breast cancer. Thus, we aimed to examine the association of each flavonoid subclass intake with the risk of breast cancer by performing a meta-analysis of epidemiologic studies. Given that many reviews have been conducted to assess the association between the dietary isoflavone intake and the breast cancer risk, we excluded this issue in the present study.
Methods
Search Strategy
We conducted a systematic search of literature published before July 1st 2012 using the Cochrane Library, MEDLINE, and EMBASE Databases and the following search terms: “flavonoid”, “flavonols”, “flavones”, “flavanones”, “flavan-3-ols”, “flavanols”, “anthocyanidins”, “phytoestrogens”, “polyphenolic compounds” and “breast cancer”. We also performed a manual search using reference lists of original articles and relevant reviews. Only full-length original journal articles were considered and no attempt was made to include abstracts or unpublished studies.
Study Selection
Studies were eligible for our analysis if: (1) the study design is cohort or case-control study; (2) data related to dietary consumption or exposure assessment (blood/urinary levels) of total flavonoids or one of flavonoid subclasses (isoflavones excluded) were available; (3) the association of flavonoids or one of flavonoid subclasses with breast cancer risk was specifically evaluated; (4) relatively complete assessment of total flavonoids or flavonoid subclass intake was performed; (5) relative risk (RR), hazard ratio (HR), or odds ratio (OR), and corresponding 95% confidence intervals (95% CI) were available. Because isoflavones have been studied extensively, including meta-analysises, studies focusing on isoflavones alone were not included in the present study. Originally, we included RCTs in our search criteria, but because there were no RCTs on flavonoids, no RCTs are included in the present study.
Data Extraction
We recorded study characteristics as follows: (1) name of the first author and publication year; (2) country or origin; (3) study design (cohort or case-control study); (4) mean length of follow-up; (5) number of cases and controls; (6) assessment of exposure, especially the database for assessment of flavonoid intake; (7) exposures to flavonoids; (8) media of flavonoids intakes; (9) RR, HR or OR from the most fully adjusted model for the highest versus the lowest flavonoids exposure and their 95% CI; (10) confounders adjusted for in multivariate analysis.
Statistical Analysis
We investigated the associations between intakes of each flavonoid subclass and the risk of breast cancer separately. Homogeneity of effect size across studies was tested by Q statistics (P<0.10). We also computed the I2, a quantitative measure of inconsistency across studies. If substantial heterogeneity exists, the random-effects model is appropriate; otherwise, the fixed-effects model is preferred [35]. A sensitivity analysis was conducted using both fixed- and random-effects models to evaluate the robustness of results. The potential publication bias was examined by the funnel plot and Egger’s test [36] (P<0.10). All analyses were performed using STATA version 10.0 (Stata Corp, College Station, Texas, USA). A P value <0.05 was considered statistically significant, except where otherwise specified.
Results
Characteristics of the Included Studies
The twelve studies [22]–[34] met the inclusion criteria after our complete review. Characteristics of these studies are presented in Table 2. The studies included in the final analysis had 9 513 cases and 181 906 controls.
Table 2. Characteristics of the included studies.
| Author, year and region | Study design | Mean follow-up | Cases/controls | Assessment of exposure | Flavonoids exposure and media of intake | OR or RR(95% CI) | Adjustments | ||
| (year) | (mg/d) | Total | Premenopausal | Postmenopausal | |||||
| Wang L 2009, U.S.A | Cohort | 1995–2007 | 1351 (38408) | SFFQ, Databases published in US and Europe | Total flavonoids(19.13) | 1.03(0.85 1.25) | age, race, energy intake, menopausal status, hormone replacement therapy, intake of fruit and vegetables et al. | ||
| Arts ICW 2002,U.S.A | Cohort | 1986–1998 | 1069 (34651) | SFFQ, Database from Netherlands | Flavan-3-ols(14.8) | 1.04(0.84 1.28) | age, education level, race, multivitamin use, menopausal status, BMI, energy intake, smoking habit, physical activity. | ||
| Adebamowo CA 2005, U.S.A | Cohort | 1991–1999 | 710 (90630) | FFQ, Databasepublished inEurope | Flavonols(17.1) | 1.05(0.83 1.34) | age, parity, age at first pregnancy, age at menarche, menopausal status, BMI, energy intake, alcohol consumption, height, smoking, et al. | ||
| Knekt P 2002, Finland | Cohort | 1967–1994 | 125 (4647) | QFIQ, Databases published in Finland | Total flavonoids(24.2) | 1.23(0.72 2.10) | age, geographic area, occupation, smoking, BMI | ||
| Goldbohm 1998, Netherlands | Cohort | 1986–1991 | 605(2 203) | SFFQ, Database from Netherlands | Total flavonoids(29.1) | 1.02(0.72 1.44) | age, education level, race, multivitamin use, menopausal status, BMI, energy intake, smoking habit. | ||
| Knekt P 1997, Finland | Cohort | 1967–1991 | 87 (4699) | QFIQ, Databasepublished inNetherland | Total flavonoids(nd) | 0.72(0.36 1.48) | sex, age, geographic area, occupation, BMI, energy intake, smoking, vit C and E, cholesterol, β-carotene, fiber, SFA, MUFA,PUFA | ||
| Luo JF 2010, Shanghai China | Nested case-control | 1997–2004 | 352/701 | Urinary excretion analysis | Flavonols(nd) Flavan-3-ols(nd) | 1.04(0.73 1.48)1.12(0.77 1.63) | age, education, age at menarche, age at 1st live birth, months of breastfeeding, smoking, et al. | ||
| Dai Q 2002, Shanghai China | Population- based case-control | 1996–1998 | 250/250 | Urinary excretion analysis | Flavanones(nd) | 1.04(0.66 1.63) | 1.53(0.77 3.04) | 0.79(0.41 1.51) | age at first live birth, ever diagnosed with fibroadenoma, total meat intake, and physical activity level. |
| Luisa TS 2008, Mexico | Hospital- based case-control | 1994–1996 | 141/141 | SFFQ, Databases published in Mexico | Flavonols(27.8) Flavones(2.5) Flavan-3-ols(7.9) | 0.48(0.21 1.08) 0.60(0.27 1.37) 0.80(0.38 1.70) | 0.49(0.19 1.23) 0.49(0.19 1.29) 1.22(0.48 3.08) | 0.21(0.07 0.60) 0.29(0.10 0.82) 0.63(0.25 1.62) | age, energy intake, lifetime lactation |
| Fink BN 2007, New York | Population- based case-control | 1996–1997 | 1434/1440 | FFQ, Database from USDA | Total flavonoids Flavonols(9.8) Flavones(0.13) Flavan-3-ols(162) Flavanones(31.2) Anthocyanidins(3.15) | 0.88(0.69 1.12) 0.75(0.59 0.95) 0.73(0.57 0.93) 0.85(0.67 1.08) 0.89(0.70 1.12) 0.91(0.72 1.15) | 1.12(0.72 1.74) 1.38(0.88 2.15) 1.07(0.70 1.65) 1.21(0.78 1.86) 0.80(0.53 1.21) 1.08(0.71 1.63) | 0.75(0.56 1.01) 0.54(0.40 0.73) 0.61(0.45 0.83) 0.74(0.55 0.99) 1.00(0.75 1.34) 0.85(0.64 1.14) | age,energy intake. |
| Bosetti C 2005, Italy | Hospital- based case-control | 1991–1994 | 2569/2588 | FFQ, Database from USDA | Flavonols(18.6) Flavones(0.5) Flavan-3-ols(36.4) Flavanones(33.7) Anthocyanidins(10.4) | 0.80(0.66 0.98) 0.81(0.66 0.98) 0.86(0.71 1.05) 0.95(0.79 1.15) 1.09(0.87 1.36) | 0.90(0.80 1.02) 0.87(0.76 0.99) 0.94(0.85 1.05) 0.98(0.85 1.13) 1.14(1.00 1.31) | 0.97(0.89 1.05) 0.90(0.81 1.00) 0.92(0.84 1.00) 0.93(0.82 1.05) 1.04(0.93 1.17) | age,study center, education, parity, alcohol consumption, nonalcohol energr intake. |
| Peterson J 2003, Athens, Greece | Hospital- based case-control | 1989–1991 | 820/1548 | SFFQ, Database from USDA | Flavonols(19.4) Flavones(0.4) Flavan-3-ols(23.5) Flavanones(33.5) Anthocyanidins(20.9) | 0.91(0.78 1.06) 0.87(0.77 0.97) 0.93(0.78 1.11) 0.96(0.87 1.07) 0.94(0.81 1.09) | age, place of birth, parity, age at first pregnancy, age at menarche, menopausal status, BMI, energy intake, alcohol consumption. |
BMI: body mass index; 95% CI: 95% confidence intervals; FFQ: food frequency questionnaire; nd: no detection; QFIQ: quantitative food intake questionnaire; SFFQ: semiquantitative food frequency questionnaire; USDA: U.S.Department of Agriculture; SFA: saturated fatty acids, MUFA: monounsaturated fatty acids, PUFA: polyunsaturated fatty acids.
The selected studies were published between 1997 and 2010 spanning 13 years, and all of them were published in English. Among these 12 studies, 6 were prospective cohort studies, 1 was nested case-control study, 2 were population-based case-control studies, and 3 were hospital-based case-control studies; moreover, 4 studies were from USA, 2 from Finland, 2 from China, and the rest were respectively from Netherlands, Mexico, Italy and Greece. The exposure assessments of flavonoids in 10 studies were made by food frequency questionnaire or by quantitative food intake questionaire, and in 2 studies were measured by urinary excretion analysis. Most individual studies were adjusted for a wide range of potential confounders, including age, race, education, energy intake, BMI, physical activity, parity, smoking, alcohol, and hormone replacement therapy.
Flavonoid Subclasses and Breast Cancer Risk
We identified 6 studies of flavonols intake and breast cancer risk, 4 studies of flavones, 6 studies of flavan-3-ols, 4 studies of flavanones, 3 studies of anthocyanins, and 5 studies of total flavonoids. We calculated the summary RR using fixed- or random-effects models respectively. As shown in Figure 2, no substantial heterogeneity existed across studies of the flavonoid subclasses. Overall, the risk of breast cancer significantly decreased in women with highest intakes of flavonols (summary RR = 0.88, 95% CI: 0.80–0.98) and flavones (summary RR = 0.83, 95% CI: 0.76–0.91) by 12% and 17% respectively, compared with that in those with lowest intakes of flavonols and flavones. However, no significant association of flavan-3-ols (summary RR = 0.93, 95% CI: 0.84–1.02), flavanones (summary RR = 0.95, 95% CI: 0.88–1.03), anthocyanins (summary RR = 0.97, 95% CI: 0.87–1.08) or total flavonoids (summary RR = 0.98, 95% CI: 0.86–1.12) with breast cancer risk was observed.
Figure 2. Meta-analysis of studies examining association between flavonoids consumption and risk of breast cancer.
Effect of Menopausal Status on Association between Flavonoid and Breast Cancer
Summary RRs of 4 case-control studies were stratified by menopausal status [20], [22], [23]. As shown in Table 3, significant associations of flavonols, flavones and flavan-3-ols intakes with reduced risk of breast cancer were observed in post-menopausal while not in pre-menopausal women. Menopausal status may contribute to the association between flavonoids and breast cancer risk. However, there were significant heterogeneities among studies of flavonols and flavones in post-menopausal women, and of flavan-3-ols in pre-menopausal women. Furthermore, no significant association between flavanones intake and breast cancer risk was observed in either post-menopausal or pre-menopausal women.
Table 3. Results of stratified analyses by menopausal status.
| Menopause status | Summary RR(95% CI) | P for heterogeneity | I2, % |
| Flavonols | |||
| Pre-menopause | 0.92 (0.82 1.03) | 0.081 | 60.3 |
| Post-menopause | 0.92 (0.85 0.99) | 0.000 | 90.4 |
| Flavones | |||
| Pre-menopause | 0.88 (0.77 1.00) | 0.323 | 11.5 |
| Post-menopause | 0.86 (0.77 0.94) | 0.008 | 79.3 |
| Flavan-3-ols | |||
| Pre-menopause | 0.96 (0.86 1.06) | 0.000 | 0.474 |
| Post-menopause | 0.90 (0.83 0.98) | 0.286 | 20.2 |
| Flavanones | |||
| Pre-menopause | 0.98 (0.86 1.11) | 0.281 | 21.3 |
| Post-menopause | 0.94 (0.84 1.05) | 0.791 | 0.0 |
Publication Bias
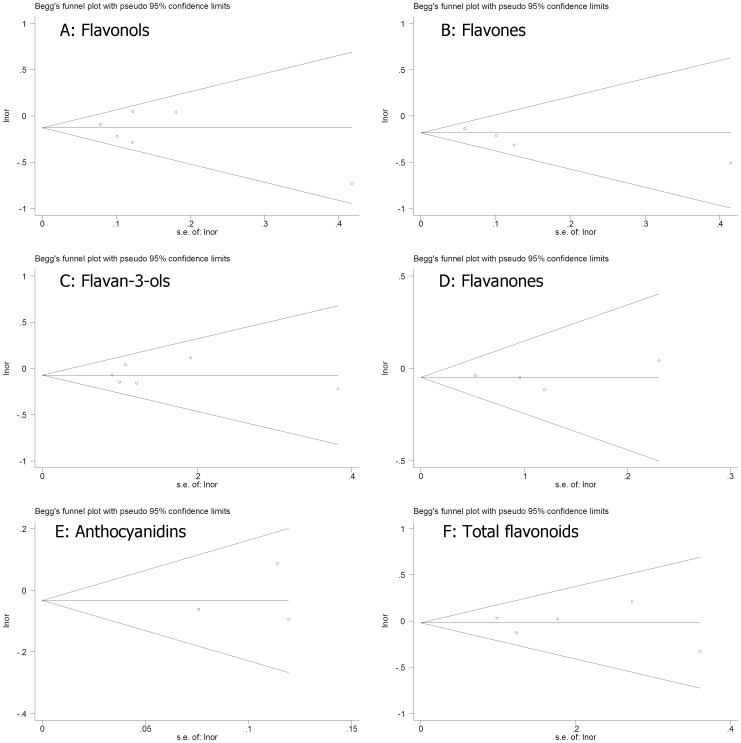
As shown in Figure 3, results from Egger’s tests indicated little evidence of publication bias in these studies (flavonols: P = 0.571, flavones: P = 0.106, flavan-3-ols: P = 0.890, flavanones: P = 0.964, anthocyanins: P = 0.449, and total flavonoids: P = 0.853).
Figure 3. Funnel plot of flavonoids consumption and risk of breast cancer.
Discussion
Studies have suggested that plant flavonoids have many biological benefits, such as the antioxidant, anti-inflammatory, anti-tumor [37] and anti-atherosclerosis effects [38], [39]. Cancer preventive phytochemicals, especially flavonoids, have been shown to suppress or block cancer progression by a variety of mechanisms [40], [41]. More attention is given to preventing colon, rectum, lung, prostate or breast cancer through daily diet because of the chemoprotective effects of dietary flavonoids and other phytochemicals. However, most of the cancer preventive effects of phytochemicals, including flavonoids, were shown in animal and cell culture studies; human clinical trials examining the chemopreventive potential of phytochemicals are lacking. In fact, some epidemiologic studies assessing the association between the flavonoid intake and the breast cancer risk have yielded inconsistent results. Moreover, different dietary flavonoid subclasses, which vary in chemical structures and bioactivities, may have different chemopreventive effects on breast cancer. The present meta-analysis of population studies supports a significant association of flavonols and flavones intake with a reduced risk of breast cancer. However, neither the total flavonoids nor the other flavonoid subclasses intake has been found to be associated with the breast cancer risk. More studies are warranted to confirm the results. The findings likely provide useful insight and evidence that can be used by registered dietitians and other healthcare professionals when discussing diet and cancer prevention with patients.
In establishing flavonoids as one of the contributors to the protective effects, the very first step is to estimate flavonoid intake from various dietary sources [21]. Yet dietary flavonoids are composed of a great variety of polyphenolic compounds which widely exist in plant foods, so it is difficult to assess the intake of total flavonoids and flavonoid subclasses. Part of the inconsistencies of epidemiological studies may be attributable to the difficulty in measuring intake levels of flavonoids. The estimated daily intake of total flavonoids in the same country may differ in different studies, suggesting that some heterogeneity may exist in dietary assessment of flavonoids intake. Estimation of flavonoid intake from dietary sources has been feasible since 2003 when the U.S. Department of Agriculture (USDA) released the database for the flavonoid content of selected foods. Since then, many articles have been published in which flavonoid intake in various subpopulation groups was estimated from relatively large, current databases of flavonoid concentration data. Furthermore, biomarkers such as urinary excretion or plasma metabolite levels could complement dietary assessment of the bioavailability of these dietary compounds. However, information is still limited on the intake of flavonoids and each flavonoid subclass in the United States and worldwide. More carefully designed studies should be performed to improve the method and database for assessing dietary flavonoids intake.
Menopausal status and estrogen-receptor (ER) status, as effect modifiers, may greatly effect the association between the flavonoid intake and breast cancer risk. Some studies showed that the association between the intake of soy isoflavone and the reduced risk of breast cancer incidence or recurrence was stronger in post-menopausal women than in premenopausal women [42], [43]. Although the other flavonoid subclasses have weaker phytoestrogen activity than isoflavones, the menopausal status and ER status also influence their association with breast cancer. The present analysis indicates a significant association of flavonol, flavone and flavan-3-ol intake with the reduced risk of breast cancer in post-menopausal but not in pre-menopausal women. The possible mechanism might partially lie in that flavonoids affect the ovarian synthesis of sex hormones or the alteration of other menstrual cycle characteristics [44], [45]. Although flaonoids, especially isoflavones, are most widely recognized for their weak estrogenic activity, they have a variety of other biologic activities that may influence cancer risk, such as antioxidant, antiproliferative, [46] and antiangiogenic activities [47] as well as inhibiting the effects of cytokines, growth factors, and several enzymes [48], [49]. The anticancer effects of flavonoids may be exerted by the combination of a variety of biologic activities, and would be influenced by some established risk factors for cancer such as alcohol consumption [50], smoking status, energy intake, menopausal status, use of hormonal treatment for menopause et al [51], [52]. Therefore, the chemoprevention of flavonoids may be varied among different subpopulation. More carefully designed studies should be performed to investigate the association of phytochemicals with cancer.
Conclusions
The present study suggests the intakes of flavonols and flavones, but not the other flavonoid subclasses or total flavonoids, can potentially contribute to breast cancer prevention, especially among post-menopausal women. More studies are needed to confirm the findings.
Funding Statement
This work was supported by the research grant from National Natural Science Foundation of China (30901194) and Youth Innovation Foundation of Third Military Medical University (2009XQN14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, et al.. (2010) GLOBOCAN 2008: cancer incidence and mortality worldwide. In: IARC CancerBase No. 10 (version 2.0). Lyon: IARC.
- 2. Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, et al. (2012) The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 36: 237–48. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society Global cancer facts and figures (2007) American Cancer Society, Atlanta.
- 4.National Cancer Institute (NCI), US Department of Health and Human Services (2006) The NCI Strategic Plan for Leading the Nation to Eliminate the Suffering and Death Due to Cancer. Bethesda, MD: NCI.
- 5.American Institute for Cancer Research (AICR) and World Cancer Research Fund (WCRF). Policy and Action for Cancer Prevention. Washington, DC: AICR/WCRF; 2009.
- 6. Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, et al. (2011) Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 141: 106–18. [DOI] [PubMed] [Google Scholar]
- 7. Tang NP, Zhou B, Wang B, Yu RB, Ma J (2009) Flavonoids intake and risk of lung cancer: a meta-analysis. Jpn J Clin Oncol 39: 352–9. [DOI] [PubMed] [Google Scholar]
- 8. Aune D, Chan DS, Vieira AR, Rosenblatt DA, Vieira R, et al. (2012) Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 134: 479–93. [DOI] [PubMed] [Google Scholar]
- 9. Reiss R, Johnston J, Tucker K, Desesso JM, Keen CL (2012) Estimation of cancer risks and benefits associated with a potential increased consumption of fruits and vegetables. Food Chem Toxicol 50: 4421–7. [DOI] [PubMed] [Google Scholar]
- 10. Zhang CX, Ho SC, Chen YM, Fu JH, Cheng SZ, et al. (2009) Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer 125: 181–8. [DOI] [PubMed] [Google Scholar]
- 11. Butler LM, Wu AH, Wang R, Koh WP, Yuan JM, et al. (2010) A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr 91: 1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ni F, Gong Y, Li L, Abdolmaleky HM, Zhou JR (2012) Flavonoid ampelopsin inhibits the growth and metastasis of prostate cancer in vitro and in mice. PLoS One 7: e38802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang H, Fu Y, Yuan LJ, Yi L, Xu HX, et al. (2012) MicroRNA-34a and microRNA-21 play roles in the chemopreventive effects of 3,6-dihydroxyflavone on 1-methyl-1-nitrosourea-induced breast carcinogenesis. Breast Cancer Res 14: R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hooper L, Kroon1 PA, Rimm EB, Cohn JS, Harvey I, et al (2008) Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 88: 38–50. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Yang X, Coburn RA, Morris ME (2005) Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem Pharmacol 70: 627–39. [DOI] [PubMed] [Google Scholar]
- 16. Arts IC, Hollman PC (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81: 317S–25S. [DOI] [PubMed] [Google Scholar]
- 17. Neuhouser ML (2004) Dietary flavonoids and cancer risk: evidence from human population studies. Nutr Cancer 50: 1–7. [DOI] [PubMed] [Google Scholar]
- 18. Cho YA, Kim J, Park KS, Lim SY, Shin A, et al. (2010) Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr 64: 924–32. [DOI] [PubMed] [Google Scholar]
- 19. Ross JA, Kasum CM (2002) Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Ann Rev Nutr 22: 19–34. [DOI] [PubMed] [Google Scholar]
- 20. Gary R (2003) Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. Beecher J Nutr 133: 3248S–3254S. [DOI] [PubMed] [Google Scholar]
- 21. Chun OK, Lee SG, Wang Y, Vance T, Song WO (2012) Estimated flavonoid intake of the elderly in the United States and around the world. J Nutr Gerontol Geriatr 31: 190–205. [DOI] [PubMed] [Google Scholar]
- 22. Torres-Sanchez L, Galvan-Portillo M, Wolff MS, Lopez-Carrillo L (2009) Dietary consumption of phytochemicals and breast cancer risk in Mexican women. Publ Health Nutr 12: 825–831. [DOI] [PubMed] [Google Scholar]
- 23. Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR, et al. (2008) Dietary flavonoid intake and risk of cancer in postmenopausal women: The Iowa Women’s Health Study. Inter J Cancer 123: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, et al. (2007) Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol 165: 514–523. [DOI] [PubMed] [Google Scholar]
- 25. Bosetti C, Spertini L, Parpinel M, Gnagnarella P, Lagiou P, et al. (2005) Flavonoids and breast cancer risk in Italy. Cancer Epidemiol Biomar Prev 14: 805–808. [DOI] [PubMed] [Google Scholar]
- 26. Adebamowo CA, Cho E, Sampson L, Katan MB, Spiegelman D, et al. (2005) Dietary flavonols and flavonol-rich foods intake and the risk of breast Cancer. Inter J Cancer 114: 628–633. [DOI] [PubMed] [Google Scholar]
- 27. Peterson J, Lagiou P, Samoli E, Lagiou A, Katsouyanni K, et al. (2003) Flavonoid intake and breast cancer risk: A case-control study in Greece. Brit J Cancer 89: 1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knekt P, Kumpulainen J, Järvinen R, Rissanen, H Heliövaara M, et al. (2002) Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 76: 560–568. [DOI] [PubMed] [Google Scholar]
- 29. Knekt P, Järvinen R, Seppänen R, Heliövaara M, Teppo L, et al. (1997) Dietary Flavonoids and the Risk of Lung Cancer and Other Malignant Neoplasms. Am J Epidemiol 146: 223–230. [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, et al. (2009) Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr 89: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arts IC, Jacobs DR Jr, Gross M, Harnack LJ, Folsom AR (2002) Dietary catechins and cancer incidence among postmenopausal women: the Iowa Women’s Health Study (United States). Cancer Causes Control 13: 373–82. [DOI] [PubMed] [Google Scholar]
- 32.Goldbohm RA, Hertog MGL, Brants HAM, van Poppel G, van den Brandt PA (1998) Intake of flavonoids and cancer risk: a prospective cohort study. In: Armado R, Andersson H, Bardócz S, Serra F, eds. Polyphenols in food. Luxembourg: Office for Official Publications of the European Communities 159–66.
- 33. Luo J, Gao YT, Chow WH, Shu XO, Li H, et al. (2010) Urinary polyphenols and breast cancer risk: results from the Shanghai Women’s Health Study. Breast Cancer Res Treat 120: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, et al. (2002) Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev 11: 815–21. [PubMed] [Google Scholar]
- 35. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 36. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang H, Yu B, Yu X, Yi L, Chen C, et al. (2010) Anti-cancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr Cancer 62: 1–9. [DOI] [PubMed] [Google Scholar]
- 38. Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, et al. (2012) Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr 96: 781–8. [DOI] [PubMed] [Google Scholar]
- 39. Wang W, Wang F, Yang YJ, Hu ZL, Long LH, et al. (2011) The flavonoid baicalein promotes NMDA receptor-dependent long-term potentiation and enhances memory. Br J Pharmacol 162: 1364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang PM, Tseng HH, Peng CW, Chen WS, Chiu SJ (2012) Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int J Oncol 40: 469–478. [DOI] [PubMed] [Google Scholar]
- 41. Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL (2012) The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem Pharmacol 83: 6–15. [DOI] [PubMed] [Google Scholar]
- 42. Dong JY, Qin LQ (2011) Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat 125: 315–23. [DOI] [PubMed] [Google Scholar]
- 43. Guha N, Kwan ML, Quesenberry CP Jr, Weltzien EK, Castillo AL, et al. (2009) Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the life after cancer epidemiology study. Breast Cancer Res Tr 118: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cassidy A, Bingham S, Setchell KD (1994) Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr 60: 333–340. [DOI] [PubMed] [Google Scholar]
- 45. Lu LJ, Anderson KE, Grady JJ, Nagamani M (1996) Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev 5: 63–70. [PubMed] [Google Scholar]
- 46. Thomas CM, Wood RC 3rd, Wyatt JE, Pendleton MH, Torrenegra RD, et al (2012) Anti-neoplastic activity of two flavone isomers derived from Gnaphalium elegans and Achyrocline bogotensis. PLoS One 7: e39806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim KK, Singh AP, Singh RK, Demartino A, Brard L, et al. (2012) Anti-angiogenic activity of cranberry proanthocyanidins and cytotoxic properties in ovarian cancer cells. Int J Oncol 40: 227–35. [DOI] [PubMed] [Google Scholar]
- 48.Toledo AC, Sakoda CP, Perini A, Pinheiro NM, Magalhães RM, et al.. (2012) Flavonone treatment reverses airway inflammation and remodeling in an asthma murine model. Br J Pharmacol, ahead of print. [DOI] [PMC free article] [PubMed]
- 49.Berger A, Venturelli S, Kallnischkies M, Böcker A, Busch C, et al.. (2012) Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J Nutr Biochem, ahead of print. [DOI] [PubMed]
- 50.Touvier M, Druesne-Pecollo N, Kesse-Guyot E, Andreeva VA, Fezeu L, et al.. (2012) Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: a prospective cohort study. Breast Cancer Res Treat, ahead of print. [DOI] [PubMed]
- 51. Luo J, Gao YT, Chow WH, Shu XO, Li H, et al. (2012) Urinary polyphenols, glutathione S-transferases copy number variation, and breast cancer risk: results from the Shanghai women’s health study. Mol Carcinog 51: 379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christensen KY, Naidu A, Parent MÉ, Pintos J, Abrahamowicz M, et al. (2012) The risk of lung cancer related to dietary intake of flavonoids. Nutr Cancer 64: 964–74. [DOI] [PubMed] [Google Scholar]