Abstract
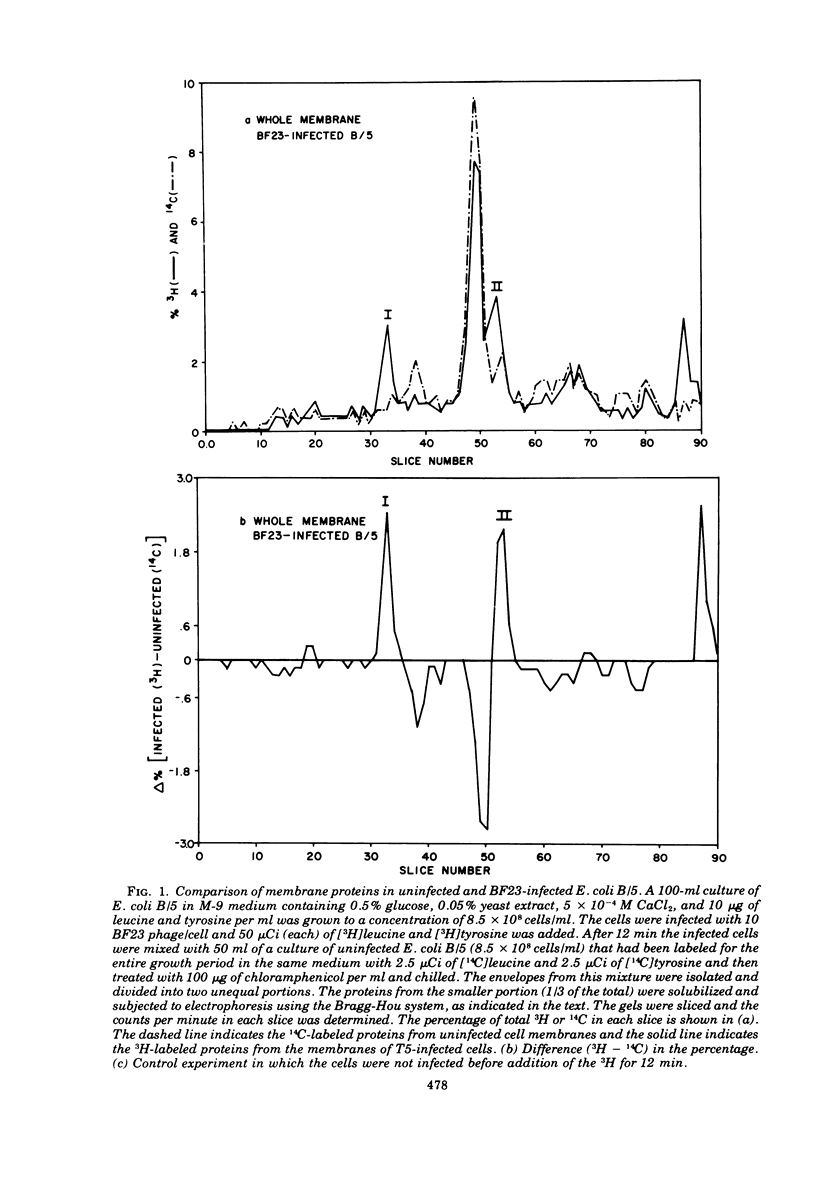
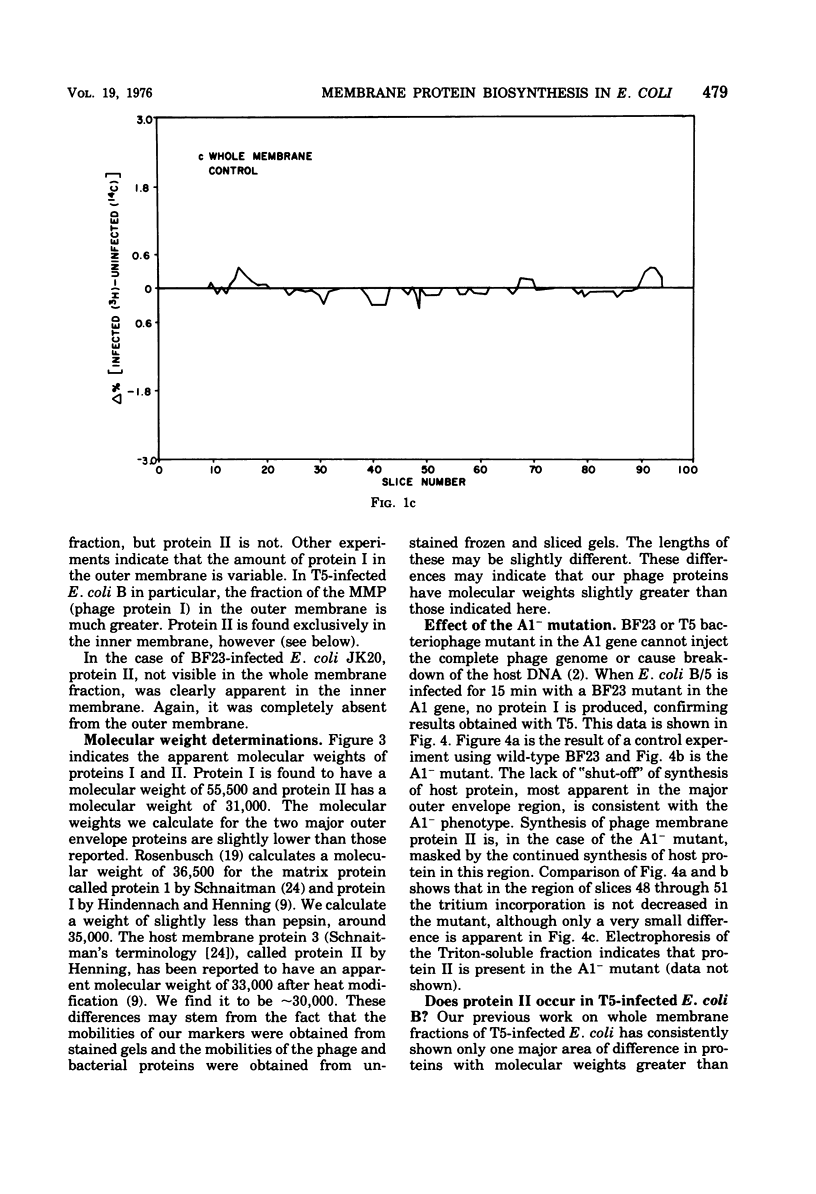
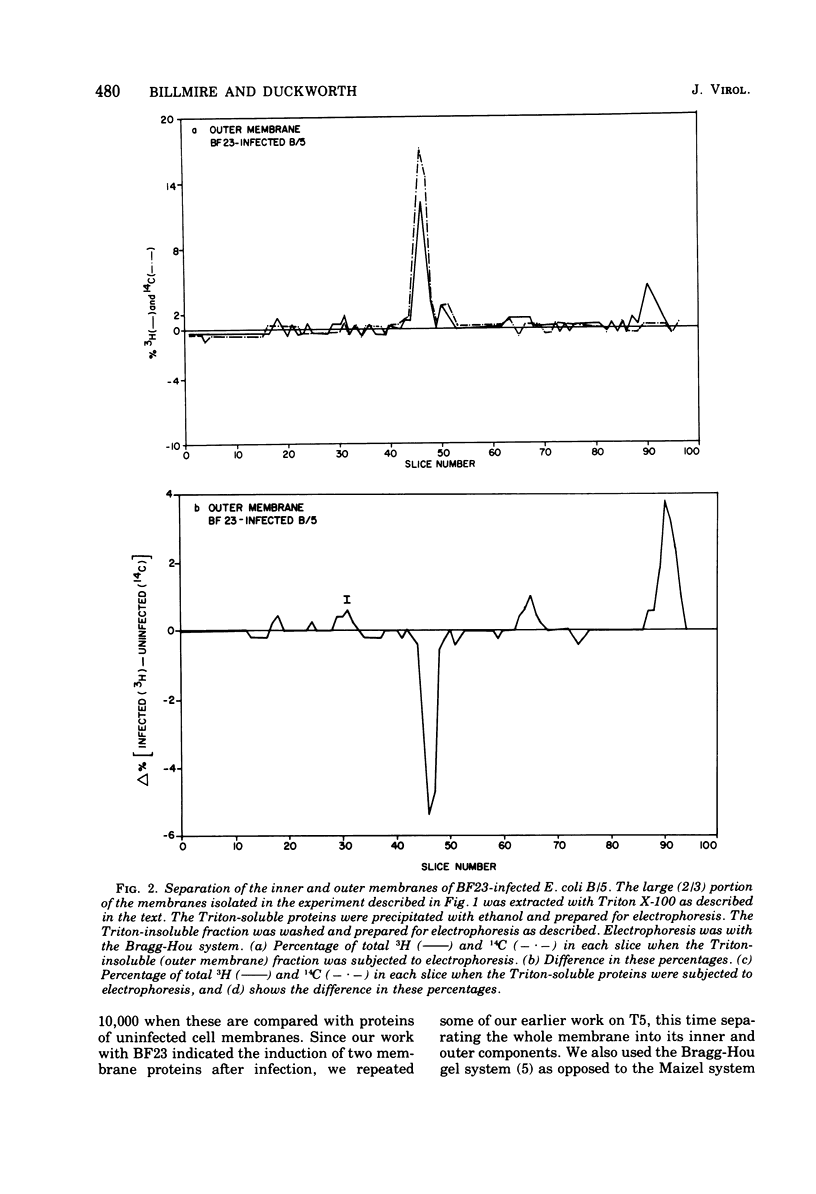
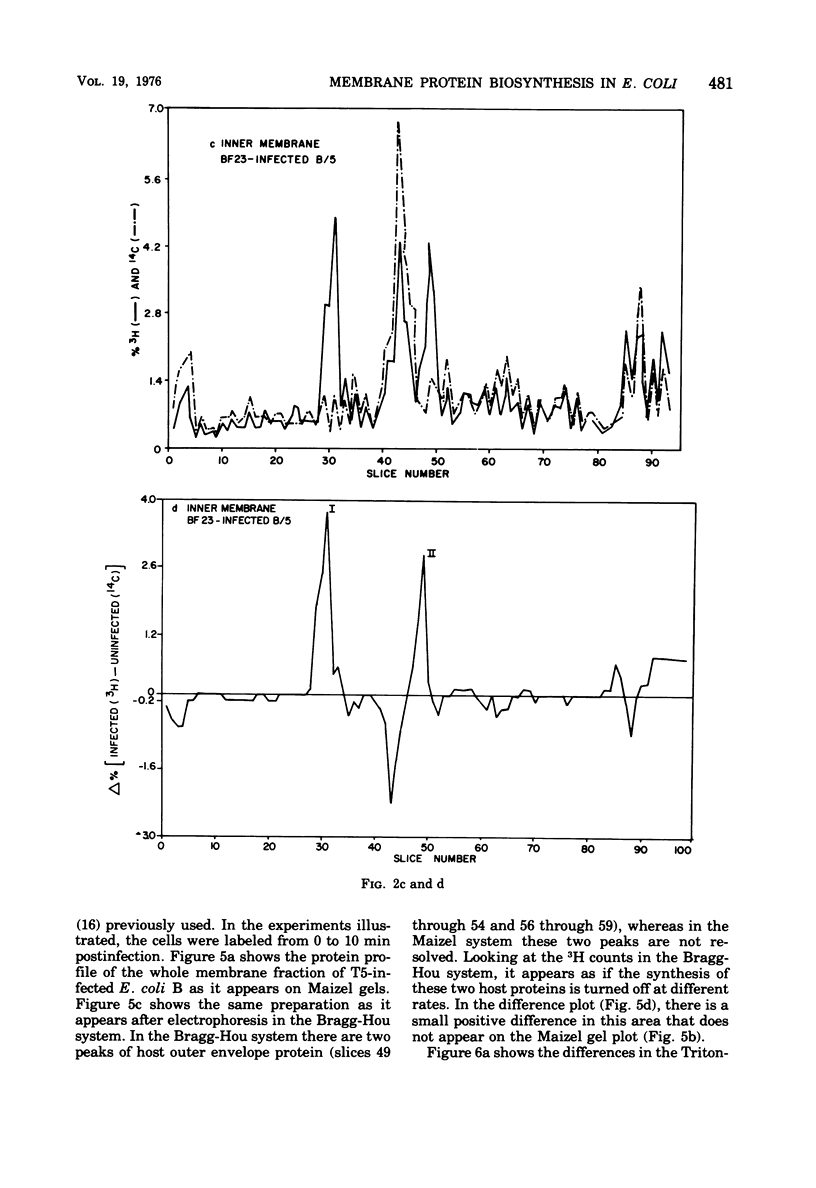
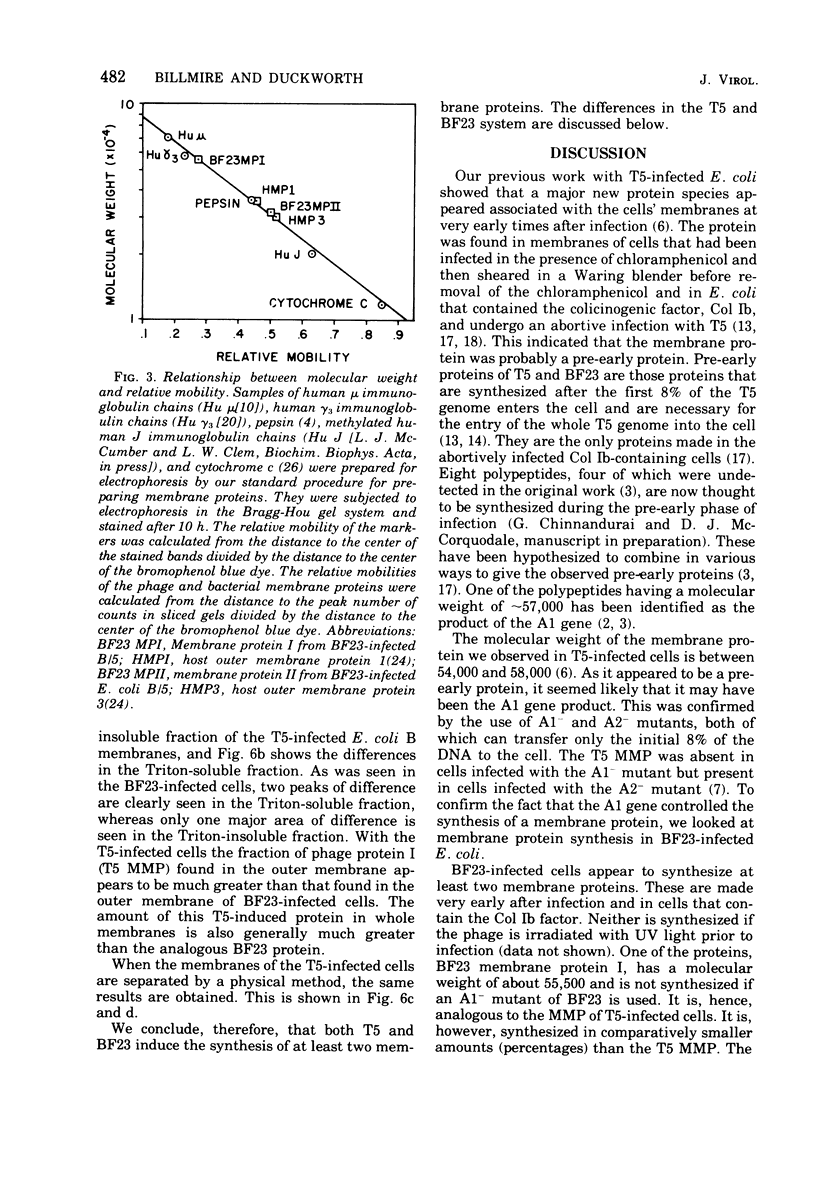
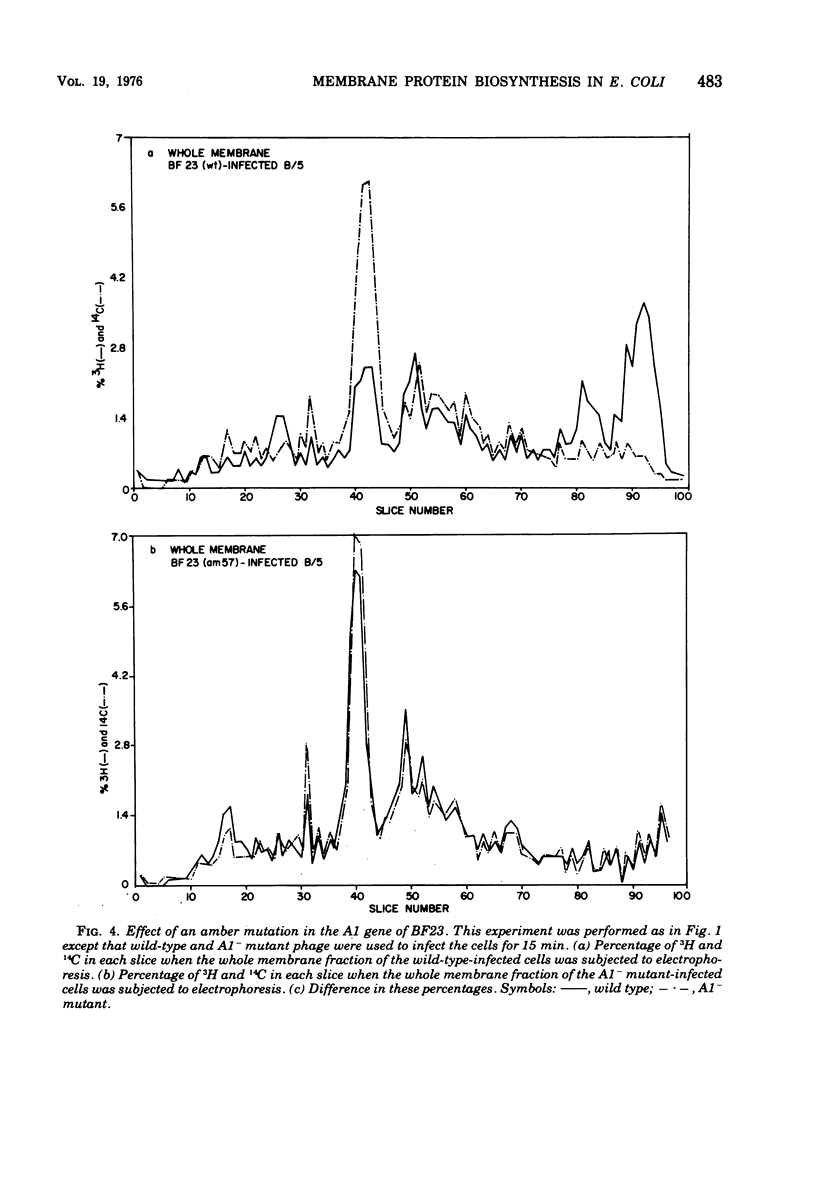
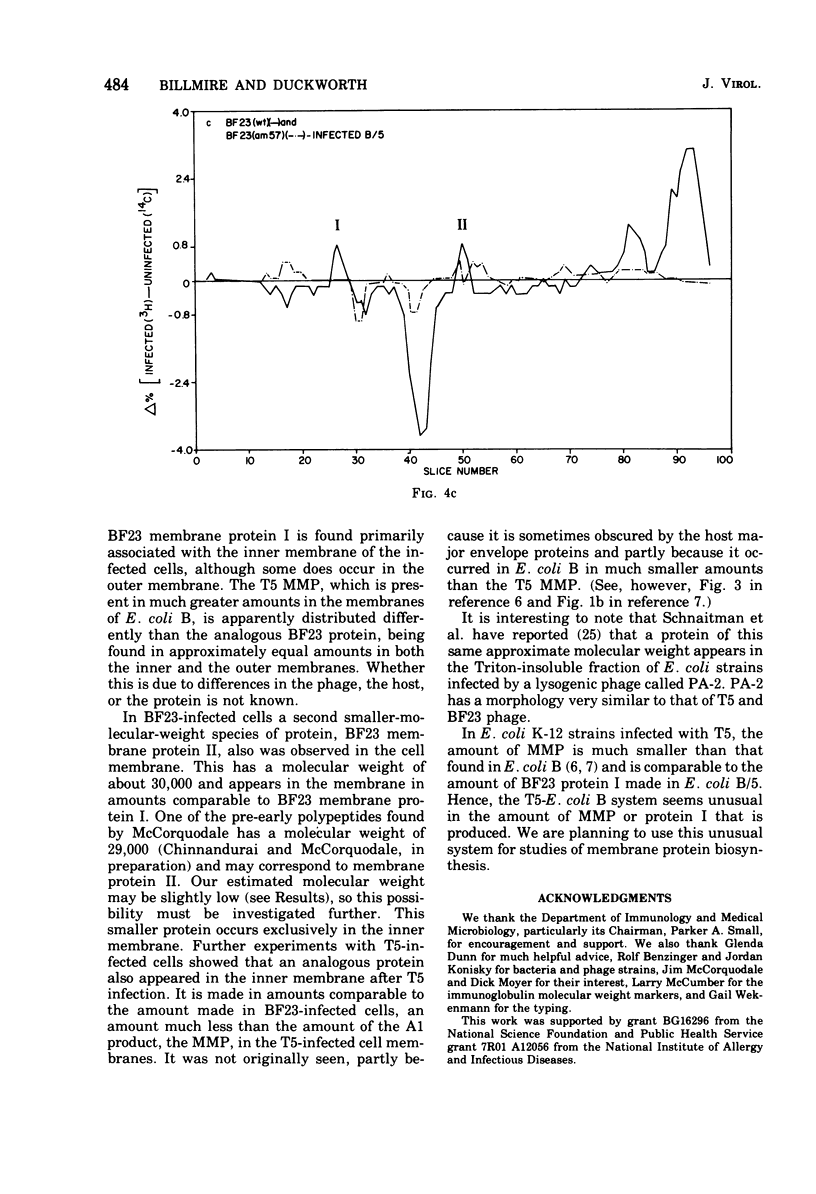
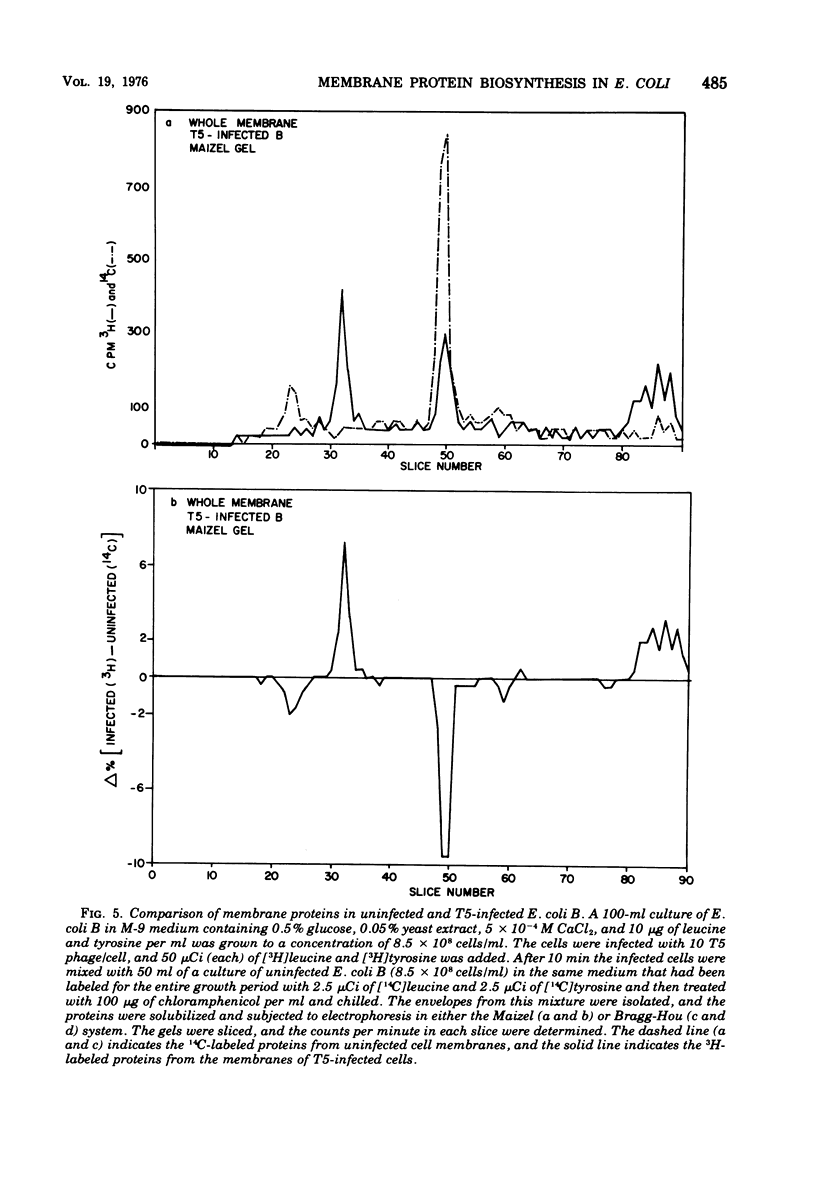
When Escherichia coli is infected with bacteriophage BF23, two new proteins with molecular weights greater than 10,000, as indicated by polyacrylamide gel electrophoresis, are found associated with the cells' membranes. One of these, found associated with both the inner and outer membrane, has a molecular weight of about 55,000 and is regulated by the A1 gene of this phage, a gene found on the spontaneously injected 8% piece of BF23 DNA, DNA that codes for the synthesis of proteins necessary for the injection of the whole phage genome. The other protein, often undetected in whole membrane preparations, is found exclusively associated with the inner membrane. Evidence indicates that this protein is also regulated by the initially injected 8% piece of the DNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman L. D., Anderson G. C., McCorquodale D. J. Arrangement on the chromosome of the known pre-early genes of bacteriophages T5 and BF23. J Virol. 1973 Nov;12(5):1191–1194. doi: 10.1128/jvi.12.5.1191-1194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B., McCorquodale D. J. Identification of the gene controlling the synthesis of the major bacteriophage T5 membrane protein. J Virol. 1976 May;18(2):542–549. doi: 10.1128/jvi.18.2.542-549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B. Membrane protein biosynthesis in T5 bacteriophage-infected Escherichia coli. Arch Biochem Biophys. 1976 Feb;172(2):319–328. doi: 10.1016/0003-9861(76)90083-7. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. Preparation, purification, and properties of E. coli virus T2. J Gen Physiol. 1952 May;36(1):17–28. doi: 10.1085/jgp.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- McCorquodale D. J., Lanni Y. T. Patterns of protein synthesis in Escherichia coli infected by amber mutants in the first-step-transfer DNA of T5. J Mol Biol. 1970 Feb 28;48(1):133–143. doi: 10.1016/0022-2836(70)90224-x. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. II. Involvement of pre-early proteins. J Mol Biol. 1974 May 5;85(1):67–74. doi: 10.1016/0022-2836(74)90129-6. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Fu A. S., Szabo C. Regulation of bacteriophage T5 development by ColI factors. J Virol. 1972 May;9(5):804–812. doi: 10.1128/jvi.9.5.804-812.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Saluk P. H., Clem L. W. The unique molecular weight of the heavy chain from human IgG3. J Immunol. 1971 Jul;107(1):298–301. [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of rat liver mitochondrial and microsomal membrane proteins. Proc Natl Acad Sci U S A. 1969 Jun;63(2):412–419. doi: 10.1073/pnas.63.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Smith D., de Salsas M. F. Temperate Bacteriophage Which Causes the Production of a New Major Outer Membrane Protein by Escherichia coli. J Virol. 1975 May;15(5):1121–1130. doi: 10.1128/jvi.15.5.1121-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



