Abstract
Plants have developed multifaceted defensive systems against adverse environmental factors. One such recognized system is the production of metabolites in plants. Jasmonic acid (JA) and its metabolite methyl jasmonate (MeJA) are known to play key roles in metabolites production. The role of MeJA as a mobile signal has been established in Arabidopsis and Solanaceae plants. However, it remains largely unclear how MeJA-based signaling is organized via its elicited metabolites. Here, we investigated the signaling ability of MeJA by means of vascular transport using Achyranthes bidentata as an experimental plant. Results showed that MeJA was transported and essentially metabolized into its active form JA-Ile in the distal undamaged leaves accompanied by emission of volatile organic compounds. Results presented and discussed therein provide convincing evidence that MeJA acts as a transportable inter-cellular mobile compound in plants self-defense scheme.
Keywords: methyl jasmonate, jasmonoyl isoleucine, deuterium labeling, plant signaling, long-distance signaling, VOC
Introduction
Plants produce volatile organic compounds (VOCs) as defensive metabolites to protect themselves against biotic and abiotic factors such as damage, injury, insects and pathogens.1-5 VOCs emissions and their pattern are influenced by exogenous application of jasmonic acid (JA) and its metabolite methyl jasmonate (MeJA).6,7 JA is metabolized into its bioactive compounds, loosely called jasmolites.7 One important JA metabolite is its conjugate jasmonoyl isoleucine (JA-Ile). JA-Ile elicits defensive reactions and its level increases locally in damaged leaves.7-9 Recent studies have provided convincing evidence that JA-Ile is the active compound, not the free JA, responsible for triggering gene expression.10,11 Hence, JA-Ile plays vital roles in regulation of the JA- and MeJA-based signaling in plants.
It has been reported that plant JA and MeJA possess transportable property from leaves to roots or to tissues.12,13 Hence, JA and MeJA are considered long-distance signaling compounds.14 Signaling compounds can be transported to distal plant sites via air (airborne) and vascular process to perform its function as a long-distance signal.14 Previously, it has been demonstrated that airborne MeJA is converted into JA-Ile in the receiver leaves accompanying the defensive VOCs emission.15 Nonetheless, direct evidence is still lacking for transport of MeJA to distal plant sites by vascular process to elicit defense responses.
Here, we used Achyranthes bidentata as an experimental plant system for studying MeJA transport by means of vascular process to distal sites, its metabolism and emission of VOCs as defensive metabolites. To track transport of MeJA and its metabolites, the deuterated MeJA (d2MeJA) was used. Evidence provided in this study demonstrates that MeJA is transported to distal leaves through vascular process and subsequently being metabolized into JA-Ile causing VOCs emission as defensive metabolites.
Materials and Methods
Chemicals and plant material
MeJA was purchased from Sigma-Aldrich. Standard compounds d2MeJA and d2JA-Ile were prepared as described previously.15,16 Other chemicals used in this study were of analytical grade. The d2MeJA solution in water (distilled H2O) was prepared by dissolving 3 mg of d2MeJA in 50 mL of H2O by vigorous stirring for 30 min. Achyranthes bidentata var tomentosa was used as an experimental plant as previously described.15 Achyranthes plants (ca. 25 cm tall) were cut at the 4th node from the top and used for all experiments.
Application of d2MeJA and analysis of JA metabolites and VOCs
The stem with leaves cut at the 4th node (length of ca. 20 cm; fresh weight of the whole plant material was about 4.6 g) of Achyranthes plant was placed in a way that the stem was touching the bottom of a glass bottle (20 mL capacity, 5.5 cm length, 2.2 cm width) containing 10 mL of the d2MeJA solution. The bottle neck was plugged with cotton and sealed with a flexible film (PARAFILM; American National Can) to prevent the d2-MeJA leakage (Fig. 1A). The entire experimental set up prepared was enclosed in a 2-L glass container (23.0 cm length, 10.5 cm width) for 24 h under light (intensity-50 μm/s/m2) as previously described.15 VOCs emitted in the headspace were collected by solid phase micro extraction (SPME) fibers (Stable Flex PDMS/DVB, Supelco). Collected VOCs were analyzed by gas chromatography-mass spectrometry (GC-MS; Perkin-Elmer Turbo Mass) or high performance liquid chromatography-tandem mass spectrometry [HPLC-MS/MS; TSQ Quantum Ultra-MS/MS equipped with Accela 600 HPLC system (Thermo Fisher Scientific Inc.).
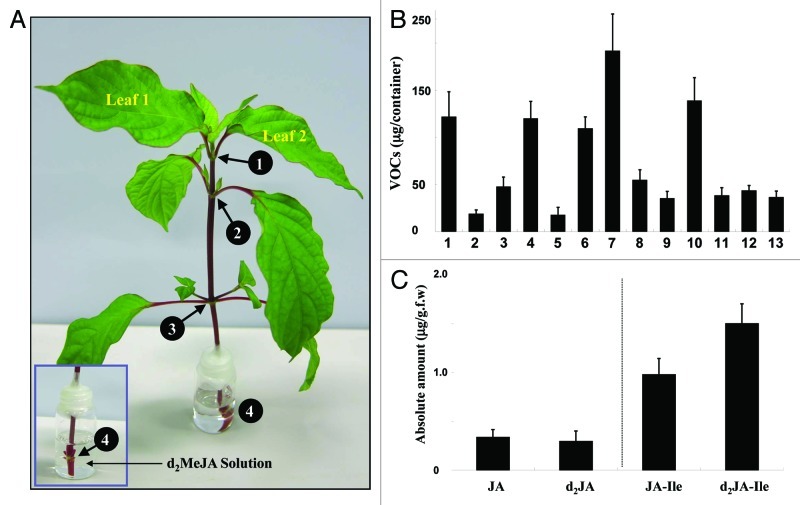
Figure 1. MeJA transport and metabolism in distal leaves. (A) The experimental set up includes Achyranthes plant and supply of the d2MeJA solution at the 4th node (number 4), as shown in an inset. Details are mentioned in the text. (B) Quantitative profiling of emitted VOCs. Numbers correspond to: 1, methyl 2-(E)-hexenoate; 2, 3-(Z)-hexenyl acetate; 3, 2-(E)-hexenyl acetate; 4, (E)-β-ocimene; 5, linalool; 6, (E)-4,8-dimethyl-1,3,7-nonatriene; 7, (E)-β-caryophyllene; 8, (E)-α-bergamotene; 9, sesquisabinene; 10, (E)-β-farnesene; 11, α-humulene; 12, (E,E)-α-farnesene; and 13, β-bisabolene. (C) Absolute quantification of exogenous/endogenous JA and JA-Ile in the upper developed leaves (Fig. 1A; leaf 1 and 2). Data shown in (B) and (C) are derived from eight independent biological experiments and were used to calculate means and SE. Error bars represent standard errors.
After VOCs collection and their analysis, the plant material was taken out from the glass container. Upper two leaves [Figure 1A; leaf 1 and 2 (fresh weight of 1.13 g)] were detached and used together for extraction and analysis of JA/JA-Ile as described previously.15 In brief, detached leaves were extracted with acetone (60 mL) and concentrated to give a crude extract, followed by extraction twice with chloroform (20 mL × 2) under acidic condition (pH 3). The chloroform extract was concentrated and dissolved in 2 mL of 80% (v/v) aqueous methanol. Prepared aqueous methanol solution was passed through the C-18 short column (Waters Sep-pak C-18 light) twice. Of the elutant, 5 μL was applied to GC-MS for JA/JA-Ile analysis and GC-FID quantification.
The MeJA metabolite analysis was performed by HPLC-MS/MS. The HPLC column (Waters Atlantis T3, 3 μm, 2.1 × 15 mm) was adjusted to the flow rate of 0.2 mL/min with 80% aqueous methanol. Analytes were detected by a combination of the masses (m/z) 208.941 and 59.355, 210.831 and 59.356, 322.001 and 130.442, and 324.031 and 130.443 for JA, d2JA, JA-Ile, and d2JA-Ile, respectively. Dehydro-JA (monitored with 206.911 and 59.342) was used as an internal standard; analytical conditions used were the same as described previously.15
Results and Discussion
MeJA is transported to distal leaves via vascular process leading to emission of VOCs as defensive metabolites
To investigate whether MeJA is transported and elicits defensive metabolites in distal leaves, the d2MeJA solution was supplied to the 4th node stem of Achyranthes (Fig. 1A; see inset). Stem absorbed 5 mL, at 24 h post-feeding, of the d2MeJA solution (10 mL). A combined GC-MS and GC-FID analysis of collected VOCs at 24 h post-feeding resulted in identification of multiple VOCs (Fig. 1B). Those VOCs were methyl 2-(E)-hexenoate, 3-(Z)-hexenyl acetate, 2-(E)-hexenyl acetate, (E)-β-ocimene, linalool, (3E)-4,8-dimethyl-1,3,7-nonatriene, (E)-β-caryophyllene, (E)-α-bergamotene, sesquisabinene, (E)-β-farnesene, α-humulene, (E,E)-α-farnesene and β-bisabolene corresponding to numbers on the x-axis starting 1 through 13, respectively. The quantitative profile of VOCs indicates differential emission of these VOCs, implying their potential differentially regulation and production. No VOCs were detected from appropriate control plants under the applied experimental conditions (data not shown). All these VOCs have previously been reported to be emitted by Achyranthes plant-exposed to airborne MeJA.15
Next, to know whether d2MeJA was transported to upper leaves of the Achyranthes plant, metabolites of d2MeJA in the upper leaves (Fig. 1A; leaf 1 and 2) were analyzed by HPLC-MS/MS. Results manifested peaks of d2JA and d2JA-Ile along with their natural (non-deuterated) endogenous JA and JA-Ile. Their quantifications further revealed efficient conversion of JA and d2JA into their Ile conjugates (Fig. 1C). These results suggest that the supplied d2MeJA at the lower 4th node stem is transported to the upper leaves (leaf 1 and 2) through the vascular process and converted into the d2JA-Ile metabolite.
In a separate experiment, Achyranthes plant was supplied with normal water via the 4th node stem as done for the d2MeJA treatment. The whole experimental set up was kept side-by-side with the d2MeJA–fed Achyranthes plant in the same glass container. Analysis of the reference leaves (i.e., control) gave a small peak of d2JA (12 ng/g.f.w), which is most likely derived from possible leakage of airborne d2MeJA in the glass container. The calculated d2JA amount accounted only 4% of the total d2JA (298 ng/g.f.w) obtained through the vascular process. Moreover, no detectable d2JA-Ile peak could be identified in the reference leaves (i.e., control). Hence, identified VOCs in Figure 1B are mainly due to d2MeJA supply and its conversion into an active d2JA-Ile metabolite.
Conversion to JA-Ile is perhaps one simple and efficient way to defensive responses
To understand plant’s systemic defensive reactions, it is important to consider the processes by which mobile signals are produced at the damaged site, transported and perceived by target cells.17 It has been suggested that factors qualifying as transportable signals have the properties to: (1) induce defensive response; (2) be produced or released at the site of attack; (3) be translocated from the site of attack to systemic tissue; and (4) accumulate in systemic tissue before conferring resistance reactions.14 To date, mounting evidence implies that MeJA are likely candidates to act as long-distance signaling compounds.6,7,12-14,17 But, the perception of MeJA at distal sites in plants remains to be demonstrated.
The d2JA-Ile detection in the distal leaves shows that d2MeJA is transportable via vascular process and is metabolized into an active signaling component d2JA-Ile/JA-Ile. Present findings concur with the previous reported result suggesting that: (1) JA conversion/synthesis is required and (2) jasmonate action is involved in signal recognition in responding leaves by reciprocal grafting experiments.17 It appears that the JA-Ile-based signaling and perception is a simple and efficient defense strategy in Achyranthes, and perhaps in other plants. Inactive MeJA is transported to the distal sites and converted into active JA-Ile, which is proven to be recognized by the receptor COI1 leading to transcription of JA-responsive genes.11 Moreover, it has been shown that cis-JA-Ile binds more tightly to the receptor COI1/JAZ,18 suggesting that stereochemistry of a metabolite has an important role on its biological activity. To note, the upper leaves (Fig. 1A; leaf 1 and 2) contained both exogenous d2JA-Ile and endogenous JA-Ile. As far as stereochemistry of JA-Ile is concerned, our previous report in Achyranthes has shown that cis-JA-Ile is a major component in endogenous JA-Ile,16 which will be essentially involved in the VOCs elicitation in distal leaves.
Concluding Remarks
Evidence provided in this study suggests that MeJA is transportable via vascular process and metabolized into JA-Ile in the distal leaves, accompanying endogenous JA-Ile production and VOCs emission as defensive metabolites. Deuterium labeling approach and experimental design might be applied to better understand the systemic reactions and responses in plants. An idea of transportation with successive activation might be a key concept to understand the MeJA signaling. To strengthen the role of MeJA as a transportable signal, further study needs to investigate whether MeJA produced in the wounded local tissues is loaded onto the vascular system.
Acknowledgments
G.K.A. appreciates Japan Society for the Promotion of Science (JSPS; ID Number S-10182) for research at NIAS (with Dr. Shoshi Kikuchi) and collaborations therein with S.T. and R.R., which has resulted in the completion of this present study. R.R. acknowledges the great support of Professors Yoshihiro Shiraiwa (Chairperson, Faculty of Life and Environmental Sciences, University of Tsukuba) and Seiji Shioda and Dr. Tetsuo Ogawa (Department of Anatomy I, Showa University School of Medicine) in promoting interdisciplinary research and unselfish encouragement. Finally, we appreciate the Editor at Scientific and English Editing (SEE) [IMPROVE Consultancy (Pvt.) Ltd.; http://improveconsultant.com/] for a final edit and rewriting some part of the text of this manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/21762
References
- 1.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science. 2006;311:812–5. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 2.Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci. 2006;25:417–40. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 3.Dicke M, van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol. 2009;5:317–24. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- 4.Arimura G-I, Kost C, Boland W. Herbivore-induced, indirect plant defences. Biochim Biophys Acta. 2005;1734:91–111. doi: 10.1016/j.bbalip.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Takabayashi J, Sabelis MW, Janssen A, Shiojiri K, van Wijk M. Can plants betray the presence of multiple herbivore species to predators and parasitoids? The role of learning in phytochemical information networks. Ecol Res. 2006;21:3–8. doi: 10.1007/s11284-005-0129-7. [DOI] [Google Scholar]
- 6.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–97. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamogami S, Rakwal R, Agrawal GK. Jasmonates to Jasmolites in Plants: Past, Present, and Future. Adv Bot Res. 2011;60:309–48. doi: 10.1016/B978-0-12-385851-1.00006-8. [DOI] [Google Scholar]
- 8.Suza WP, Staswick PE. The role of JAR1 in Jasmonoyl-L: -isoleucine production during Arabidopsis wound response. Planta. 2008;227:1221–32. doi: 10.1007/s00425-008-0694-4. [DOI] [PubMed] [Google Scholar]
- 9.Suza WP, Rowe ML, Hamberg M, Staswick PE. A tomato enzyme synthesizes (+)-7-iso-jasmonoyl-L-isoleucine in wounded leaves. Planta. 2010;231:717–28. doi: 10.1007/s00425-009-1080-6. [DOI] [PubMed] [Google Scholar]
- 10.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–27. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci U S A. 2008;105:7100–5. doi: 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z-P, Baldwin IT. Transport of [2-14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta. 1997;203:436–41. doi: 10.1007/s004250050211. [DOI] [Google Scholar]
- 13.Thorpe MR, Ferrieri AP, Herth MM, Ferrieri RA. 11C-imaging: methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta. 2007;226:541–51. doi: 10.1007/s00425-007-0503-5. [DOI] [PubMed] [Google Scholar]
- 14.Heil M, Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13:264–72. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Tamogami S, Rakwal R, Agrawal GK. Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Biophys Res Commun. 2008;376:723–7. doi: 10.1016/j.bbrc.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 16.Tamogami S, Agrawal GK, Rakwal R. An in planta technique for cis-/trans-stereochemical analysis of jasmonoyl isoleucine. J Plant Physiol. 2010;167:933–7. doi: 10.1016/j.jplph.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Li C, Lee GI, Howe GA. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci U S A. 2002;99:6416–21. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol. 2009;5:344–50. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]