Abstract
Ethylene controls photosynthesis and induces tolerance of plants to metal stress. However, little is known about the interaction between ethylene, photosynthesis and sulfur (S) availability under cadmium (Cd) stress. Recently, we reported that ethylene controls photosynthesis by increasing glutathione (GSH) synthesis with sufficient-S availability under Cd stress. Plants treated with Cd were less sensitive to ethylene and showed photosynthetic inhibition. Ethylene sensitivity of plants was increased with exogenously-sourced ethylene or with sufficient-S application resulting in induced GSH synthesis and alleviation of photosynthetic inhibition by Cd. In this addendum we present some additional data indicating that ethylene regulates photosynthesis by reducing glucose (Glc) sensitivity, thus reducing the Glc-mediated photosynthetic repression.
Keywords: ethylene, glucose sensitivity, glutathione, sugars, photosynthesis
Ethylene modifies the rate of photosynthesis depending on plants’ sensitivity and rate of ethylene production.1,2 Ethylene also increases tolerance of plants to cadmium (Cd) stress. It has been shown that blocking ethylene synthesis results in lower thiol contents and tolerance to Cd stress.3 We have recently published that ethylene is involved in sulfur (S)-mediated alleviation of Cd (200 mg Cd kg−1 soil) stress through increased glutathione (GSH) synthesis.4 Plants treated with Cd were less sensitive to ethylene despite high ethylene evolution and showed photosynthetic inhibition. Ethylene sensitivity of plants was increased with exogenously-sourced ethylene as ethephon (200 µl l−1) or with sufficient-S (100 mg S kg−1 soil) application. This resulted in the induction of GSH synthesis and antioxidant cycle leading to increased photosynthesis under Cd stress. The present work reports that in addition to the involvement of ethylene in alleviation of Cd stress with sufficient-S through GSH synthesis, there is a relationship between ethylene, glucose (Glc) sensitivity and sufficient-S in the alleviation of Cd stress in mustard (Brassica juncea) cv Varuna. We found that plants under Cd stress showed increased Glc sensitivity (Figs. 1 and 2) that resulted in the inhibition of Rubisco activity and photosynthesis.4 High endogenous Glc concentration results in the stronger repression of Rubisco mRNA levels and photosynthesis in ethylene insensitive genotypes.5 The inability to perceive ethylene results in increased sensitivity to Glc, which results in negative effects on Rubisco content and photosynthetic capacity.6 Therefore, even with high ethylene evolution under Cd stress the Glc-mediated photosynthetic suppression is not overcome. Supplementation of Cd-stressed plants with sufficient-S increased ethylene sensitivity, which reduced Glc sensitivity and reversed the negative effects of Cd on Rubisco and photosynthesis.
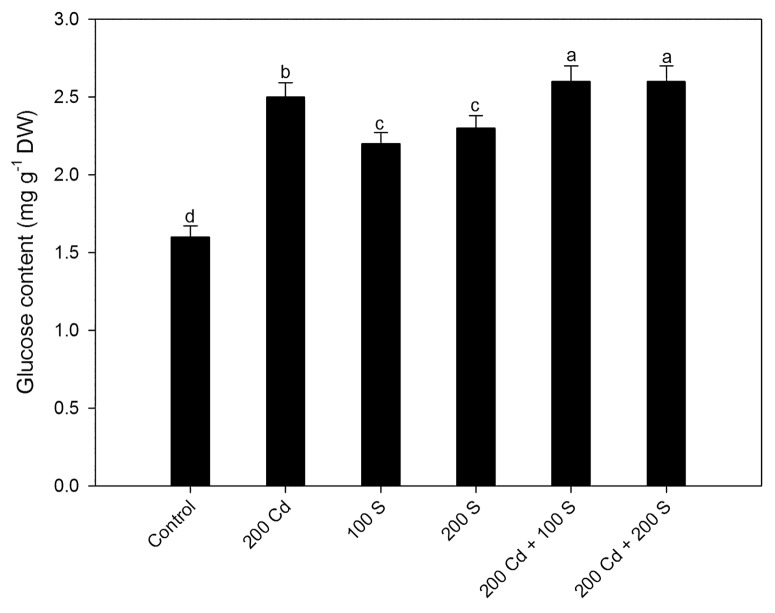
Figure 1. Leaf glucose content in mustard after treatment with Cd and/or S treatments at 30 d after sowing. Plants were grown with 0, 200 mg Cd kg−1 soil (200 Cd), 100 mg S kg−1 soil (200 S) or 200 mg S kg-1 soil (200 S) with combined Cd and S treatments. Means values ± SE are shown (n = 4). Means denoted by the same letter are not significantly different at p < 0.05 according to least significant difference test. Cd, cadmium; DW, dry weight; S, sulfur.
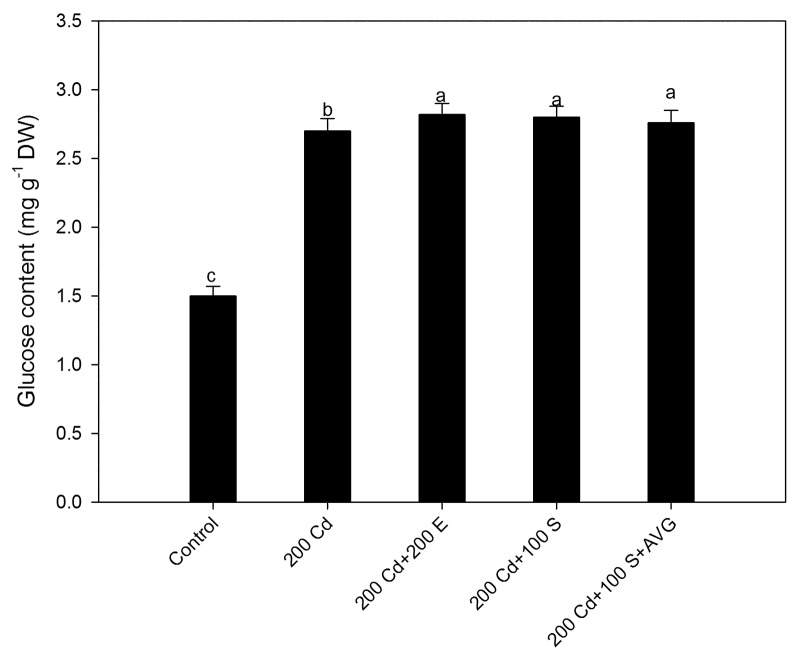
Figure 2. Leaf glucose content after treatment with 200 mg Cd kg−1 soil (200 Cd), 200 Cd in combination with 100 mg S kg−1 soil (100 S) or 20 μL L−1 ethephon (200 E) or 200 Cd + 100 S + 50 μL L−1 aminoethoxyvinylglycine (AVG) at 30 d after sowing. Ethephon or AVG was sprayed along with 0.5% teepol as surfactant at 20 d after sowing. Mean values ± SE are shown. (n = 4). Means denoted by the same letter are not significantly different at p < 0.05 according to the least significant difference test. Cd, cadmium; E, ethephon; DW, dry weight; S, sulfur.
The content of Glc increased with Cd in comparison to control and application of both sufficient and excess-S (200 mg S kg−1 soil) increased Glc content equally under no Cd in comparison to control but this increase was lesser than Cd stressed plants. The pathway of S-assimilation leads to ethylene synthesis through the formation of cysteine and methionine, which is perceived by plants. The increase in Glc with S was due to increase in photosynthesis and there was no Glc hypersensitivity. Under Cd stress, high Glc was synthesized and due to lesser ethylene perception resulted in photosynthetic inhibition. With the supplementation of sufficient-S to Cd stressed plants ethylene perception increased reversing the adverse effects of high Glc on photosynthesis. However, excess-S supplementation might have resulted in excess phytochelatins that caused damage to cells4 resulting in evolution of stress ethylene. Sufficient-S given to Cd-treated plants resulted in high Glc synthesis even more than plants under Cd stress, but the photosynthetic inhibition was overcome because sufficient-S led to the increased ethylene perception, which reduced Glc sensitivity. However, reverse occurred in plants receiving excess S where due to lesser ethylene perception Glc-mediated photosynthetic repression was observed. This study clearly focuses that ethylene besides executing its role in Cd tolerance via increased GSH synthesis also does it via reducing Glc sensitivity. Research has demonstrated that constitutive ethylene biosynthesis and signaling mutants show insensitivity to glucose.7-9 Prolonged glucose treatment revealed that gin4 is allelic to ctr1, while ethylene-insensitive mutants, such as ein2 and etr1–1 exhibit glucose hypersensitivity. These results confirm that ethylene could overcome the glucose-dependent developmental arrest.10
For the experimental verification of the role of ethylene in tolerance to Cd via Glc sensitivity, we tested whether S and Cd are related to Glc content, and further to confirm the role of ethylene in Cd alleviation via reducing Glc sensitivity we used ethephon as ethylene source and 1-aminoethoxy vinyl glycine (AVG) as ethylene biosynthesis inhibitor. The content of Glc increased with Cd alone or in the presence of S but the Glc sensitivity was reduced only with sufficient S under Cd stress where the ethylene evolved was perceived by the plants and thus promoted photosynthesis by reducing the negative effect of Glc on Rubisco gene expression. However, in the presence of AVG even with sufficient S photosynthetic inhibition occurred because of lesser ethylene evolution that could not suppress Glc sensitivity (Fig. 2). An interaction between ethylene and the Glc has been shown in genomic studies.11,12 Leon and Sheen10 show antagonistic relation between ethylene and glucose, as ethylene insensitive mutants were found to exhibit glucose hypersensitivity, whereas the constitutively responsive mutant ctr1 was shown to possess glucose insensitivity. The reduction in Glc sensitivity by ethephon has been reported,6 but the role of ethylene in reducing Glc mediated photosynthetic inhibition under Cd stress needs to be elucidated. High Glc causes feedback inhibition of transcription of photosynthetic genes and decreasing Glc content or sensitivity might increase photosynthesis. Seneweera et al.13 suggested that ethylene production promoted growth under circumstances where leaf Glc concentration was high. The developmental arrest by high sugar levels can be overcome by applying the ethylene precursor 1-aminocyclopropane-1-carboxylic acid.14 Ethylene reduces the negative feedback of carbohydrates on photosynthetic gene expression. Although high ethylene evolution occurs under stress it is stress ethylene that is not perceived by the plants and the inability to perceive ethylene results in increased sensitivity to Glc and negative effects on Rubisco content and photosynthetic capacity.15
Our work shows that ethylene has a role in inducing S-mediated tolerance to Cd stress and alleviation of photosynthetic inhibition through maintaining reduced Glc sensitivity. Under Cd stress, plants exhibited less sensitivity to ethylene which increased with sufficient-S.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22079
References
- 1.Khan NA. An evaluation of the effects of exogenous ethephon, an ethylene releasing compound, on photosynthesis of mustard (Brassica juncea) cultivars that differ in photosynthetic capacity. BMC Plant Biol. 2004;4:21. doi: 10.1186/1471-2229-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ. The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci. 2006;11:176–83. doi: 10.1016/j.tplants.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.di Toppi LS, Lambardi M, Pazzagli L, Cappugi G, Durante M, Gabbrielli R. Response to cadmium in carrot in vitro plants and cell suspension cultures. Plant Sci. 1998;137:119–29. doi: 10.1016/S0168-9452(98)00099-5. [DOI] [Google Scholar]
- 4.Masood A, Iqbal N, Khan NA. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012;35:524–33. doi: 10.1111/j.1365-3040.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- 5.Tholen D, Pons TL, Voesenek LACJ, Poorter H. The role of ethylene perception in the control of photosynthesis. Plant Signal Behav. 2008;3:108–9. doi: 10.4161/psb.3.2.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal N, Nazar R, Syeed S, Masood A, Khan NA. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J Exp Bot. 2011;62:4955–63. doi: 10.1093/jxb/err204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo A, Bossi F, Finkelstein RR, León P. Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol. 2003;133:231–42. doi: 10.1104/pp.103.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price J, Laxmi A, St Martin SK, Jang JC. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell. 2004;16:2128–50. doi: 10.1105/tpc.104.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 10.León P, Sheen J. Sugar and hormone connections. Trends Plant Sci. 2003;8:110–6. doi: 10.1016/S1360-1385(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 11.Zhong GY, Burns JK. Profiling ethylene-regulated gene expression in Arabidopsis thaliana by microarray analysis. Plant Mol Biol. 2003;53:117–31. doi: 10.1023/B:PLAN.0000009270.81977.ef. [DOI] [PubMed] [Google Scholar]
- 12.De Paepe A, Vuylsteke M, Van Hummelen P, Zabeau M, Van Der Straeten D. Transcriptional profiling by cDNA-AFLP and microarray analysis reveals novel insights into the early response to ethylene in Arabidopsis. Plant J. 2004;39:537–59. doi: 10.1111/j.1365-313X.2004.02156.x. [DOI] [PubMed] [Google Scholar]
- 13.Seneweera S, Aben SK, Basra AS, Jones B, Conroy JP. Involvement of ethylene in the morphological and developmental response of rice to elevated atmospheric CO2 concentrations. Plant Growth Regul. 2003;39:143–53. doi: 10.1023/A:1022525918305. [DOI] [Google Scholar]
- 14.Zhou L, Jang JC, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci U S A. 1998;95:10294–9. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tholen D, Pons TL, Voesenek LACJ, Poorter H. Ethylene insensitivity results in down-regulation of rubisco expression and photosynthetic capacity in tobacco. Plant Physiol. 2007;144:1305–15. doi: 10.1104/pp.107.099762. [DOI] [PMC free article] [PubMed] [Google Scholar]