Abstract
Stem cells in the vegetative shoot apical meristem proliferate to produce more stem cells (self-renewal) and are simultaneously consumed to form leaf promordia. Therefore, to keep a stable number of stem cells, regulation of the balance between their proliferation and consumption is important. Recently we reported that stem cell population is increased in mutant plants lacking the entire ERECTA (ER) receptor kinase family. Here we describe that loss of function of the entire ER-family causes a decrease in leaf number in spite of the increase in stem cell population. This suggests that stem cell consumption might be decreased in the mutant, and this could be one of reasons why stem cell population appears to be increased. This situation is in sharp contrast to clv3 mutant, which also shows an increase in stem cell population but does not show a decrease in leaf production. We briefly discuss differences between the er-family mutant and the clv3 mutant.
Keywords: CLAVATA3, ERECTA family, WUSCHEL, Shoot apical meristem, cytokinin, leaf, stem cells
Loss Of Function of the Entire ERECTA-Family Causes a Decrease in Leaf Production in Spite of an Increase in Stem Cell Population
Mechanisms that regulate the homeostasis of stem cells in the shoot apical meristem (SAM) have been intensively investigated and some important regulators have been reported.1 WUSCHEL (WUS) is expressed at the organizing center below the stem-cell region and is required for the stem-cell proliferation and maintenance.2,3 WUS activates expression of CLAVATA3 (CLV3) in the stem cells,4 which encodes a secreted small peptide5,6 and serves as a useful stem cell marker gene.7 The secreted CLV3 peptides in turn repress the WUS expression.5,6,8 This WUS-CLV3 feedback loop ensures a constant number of stem cells.9,10
Arabidopsis ERECTA (ER) receptor kinase family consists of ER, ERECTA-LIKE 1 (ERL1) and ERECTA-LIKE 2 (ERL2)11,12 and play significant roles in various aspects of plant development.11,13,14 Previously we reported that the ER-family members are redundantly involved in phenotypes of a mutant displaying SAM-related defects,15,16 and our recent paper demonstrated that they redundantly regulate stem cell homeostasis.17CLV3 expression domain was expanded in the er erl1 erl2 triple mutant (hereafter, er-family mutant), indicating that stem cell population was increased in the mutant. Interestingly, though the CLV3 expression was highly upregulated, WUS expression did not show such remarkable change. In this mutant, the SAM morphology was flattened and broadened compared with the wild-type SAM (Fig. 1A and B).17 In addition to this defect in the SAM morphology, the number of formed leaves appeared to be decreased in the er-family mutant (Fig. 1A and B, triangles). This decrease in leaf production might indicate that stem cell consumption to form leaves is slower in the er-family mutant than in wild-type plants. Given that the balance between stem cell proliferation and consumption is important to determine the stem cell population in each situation, the slower consumption of stem cells in the er-family mutant could be one of reasons why stem cell population is increased in the mutant. Detailed analysis on rates of both proliferation and consumption of stem cells in the er-family mutant would be an important issue in the future research.
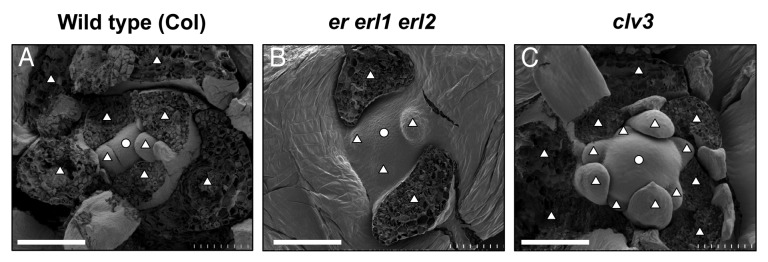
Figure 1. SEM images of shoot apex regions. The SAMs of 10-d-old seedlings with the genotypes of (A) wild type (Col), (B) er-105 erl1–2 erl2–1 triple mutant and (C) clv3–2 mutant were observed by SEM. Circles indicate the SAMs. Triangles indicate leaf primordia and traces of developing leaves that were removed to observe the SAM. Bars = 100 µm
Comparison Between the er-Family Mutant and the clv3 Mutant
Loss-of-function mutations in CLV3 induce the expansion of WUS expression, resulting in a dramatic increase in stem cell population.4,9 Because both the er-family mutant and the clv3 mutant show an increase in stem cell population, we compared phenotypes of the shoot apex regions between them. The clv3 mutant displayed a large dome-shaped SAM (Fig. 1C), which was different from a flattened SAM in the er-family mutant (Fig. 1B). This might be due to a difference in WUS expression. The WUS expression is drastically expanded in the clv3 mutant,9 while it is not the case in the er-family mutant.17 Another apparent difference between the er-family mutant and the clv3 mutant was related to leaf production. As described above, though the stem cell population was increased in the er-family mutant,17 leaf production was decreased, compared with wild-type plants (Fig. 1A and B, triangles). On the other hand, in the clv3 mutant, leaf production was apparently increased (Fig. 1C). It is likely that, in the clv3 mutant, the stem cell population is increased by enhanced stem cell proliferation triggered by drastic upregulation of WUS expression.9 This is in sharp contrast to the case of the er-family mutant where slower consumption of stem cells due to slower leaf production could be one of reasons of increased stem cell population. However, interestingly, the stem cell proliferation was remarkably activated by cytokinin even in the er-family mutant and, in this cytokinin-treated condition, leaf production was also drastically stimulated,17 which was similar to the clv3 phenotypes. It might be plausible that the ER-family regulates the balance between stem cell proliferation and consumption via modulation of cytokinin pathway. More detailed analysis on relationships among stem cell regulation by the WUS-CLV3 circuit, formation of leaf primordia, and cytokinin action in the er-family mutant might help to understand substantial roles for the ER-family in the SAM regulation.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22080
References
- 1.Besnard F, Vernoux T, Hamant O. Organogenesis from stem cells in planta: multiple feedback loops integrating molecular and mechanical signals. Cell Mol Life Sci. 2011;68:2885–906. doi: 10.1007/s00018-011-0732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laux T, Mayer KF, Berger J, Jürgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122:87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–15. doi: 10.1016/S0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science. 1999;283:1911–4. doi: 10.1126/science.283.5409.1911. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Sawa S, Kinoshita A, Mizuno S, Kakimoto T, Fukuda H, et al. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science. 2006;313:845–8. doi: 10.1126/science.1128439. [DOI] [PubMed] [Google Scholar]
- 6.Ohyama K, Shinohara H, Ogawa-Ohnishi M, Matsubayashi Y. A glycopeptide regulating stem cell fate in Arabidopsis thaliana. Nat Chem Biol. 2009;5:578–80. doi: 10.1038/nchembio.182. [DOI] [PubMed] [Google Scholar]
- 7.Yadav RK, Girke T, Pasala S, Xie M, Reddy GV. Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc Natl Acad Sci U S A. 2009;106:4941–6. doi: 10.1073/pnas.0900843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenhard M, Laux T. Stem cell homeostasis in the Arabidopsis shoot meristem is regulated by intercellular movement of CLAVATA3 and its sequestration by CLAVATA1. Development. 2003;130:3163–73. doi: 10.1242/dev.00525. [DOI] [PubMed] [Google Scholar]
- 9.Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–44. doi: 10.1016/S0092-8674(00)80700-X. [DOI] [PubMed] [Google Scholar]
- 10.Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science. 2000;289:617–9. doi: 10.1126/science.289.5479.617. [DOI] [PubMed] [Google Scholar]
- 11.Shpak ED, Berthiaume CT, Hill EJ, Torii KU. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development. 2004;131:1491–501. doi: 10.1242/dev.01028. [DOI] [PubMed] [Google Scholar]
- 12.Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, et al. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell. 1996;8:735–46. doi: 10.1105/tpc.8.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–3. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- 14.Uchida N, Lee JS, Horst RJ, Lai HH, Kajita R, Kakimoto T, et al. Regulation of inflorescence architecture by intertissue layer ligand-receptor communication between endodermis and phloem. Proc Natl Acad Sci U S A. 2012;109:6337–42. doi: 10.1073/pnas.1117537109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida N, Igari K, Bogenschutz NL, Torii KU, Tasaka M. Arabidopsis ERECTA-family receptor kinases mediate morphological alterations stimulated by activation of NB-LRR-type UNI proteins. Plant Cell Physiol. 2011;52:804–14. doi: 10.1093/pcp/pcr032. [DOI] [PubMed] [Google Scholar]
- 16.Uchida N, Tasaka M. Regulation of NB-LRR-type UNI and its related signaling pathway: signaling crosstalk and methodology for quick identification of related factors. Plant Signal Behav. 2011;6:1219–22. doi: 10.4161/psb.6.8.16181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida N, Shimada M, Tasaka M. ERECTA-family receptor kinases regulate stem-cell homeostasis via buffering its cytokinin responsiveness in the shoot apical meristem. Plant Cell Physiol published online August 9 2012; doi:10.1093/pcp/pcs109. [DOI] [PMC free article] [PubMed]


