Abstract
The arbuscular mycorrhizal (AM) symbiosis, which forms between plant hosts and ubiquitous soil fungi of the phylum Glomeromycota, plays a key role for the nutrient uptake of the majority of land plants, including many economically important crop species. AM fungi take up nutrients from the soil and exchange them for photosynthetically fixed carbon from the host. While our understanding of the exact mechanisms controlling carbon and nutrient exchange is still limited, we recently demonstrated that (i) carbon acts as an important trigger for fungal N uptake and transport, (ii) the fungus changes its strategy in response to an exogenous supply of carbon, and that (iii) both plants and fungi reciprocally reward resources to those partners providing more benefit. Here, we summarize recent research findings and discuss the implications of these results for fungal and plant control of resource exchange in the AM symbiosis.
Keywords: arbuscular mycorrhiza, arginase, arginine, carbon, common mycelial network, interface, mutualism, nitrogen transport, urea cycle
Introduction
The arbuscular mycorrhizal (AM) symbiosis between fungi from the phylum Glomeromycota and the roots of approximately 65% of land plant species1 is characterized by an exchange of nutrients, such as phosphorus (P) and nitrogen (N), from the fungus for carbon (C) from the host. AM fungi are obligate biotrophs and depend almost exclusively on host derived C to complete their life-cycle and it has been estimated that the host transfers up to 20% of its photosynthetically fixed C to the fungus.2 This dependency of the fungus has led to the assumption that the host is in control of the symbiosis, and that the nutrient transport in the mycorrhizal symbiosis is primarily driven by host plant demand.3-5In contrast, recent results indicate that, despite its high host dependency, the fungus can gain control in the symbiosis by adjusting its nutrient transfer in response to the C supply from the host.6-8 Both plants and fungi are able to detect variation in the resources supplied by their partners, allowing them to adjust their own resource allocation accordingly. This reciprocal reward mechanism ensures ‘fair trade’ between the symbiosis partners.9 Here, we discuss these recent research findings in relation to strategies that both partners may use to regulate and maximize their nutritional benefit from the AM symbiosis.
Control of Nutrient Uptake Pathways in Mycorrhizal Roots
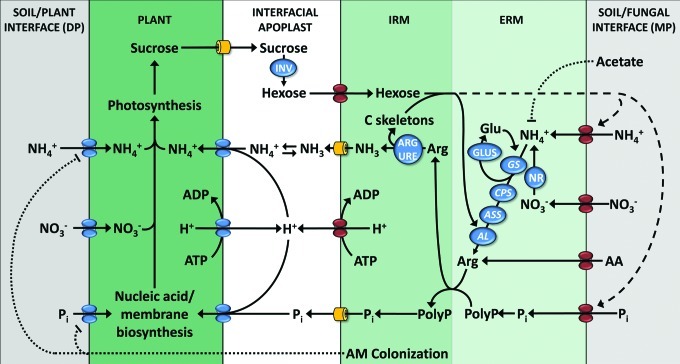
Mycorrhizal plants can acquire nutrients via two uptake pathways.10 The direct pathway (DP) involves the uptake of nutrients from the soil-root interface by high affinity P and N transporters located in the root epidermis and its root hairs. The mycorrhizal pathway (MP) involves the uptake of nutrients from the fungal-soil interface by the extraradical mycelium (ERM), translocation to the intraradical mycelium (IRM) and uptake by the host from the fungal-plant interface (Fig. 1) via mycorrhiza-inducible P and N transporters in the periarbuscular membrane.11,12 Plant uptake transporters of the DP are downregulated in mycorrhizal roots,13,14 and the MP can represent the main uptake pathway even in plants in which no positive growth benefit is observed.15 Whether the suppression of the DP in mycorrhizal roots is a host driven or a fungal mediated response is not known. The expression of plant uptake transporters of the DP is normally regulated by host plant demand, and the lower transcript levels in mycorrhizal roots could only be the result of an improved P supply.13 On the other hand, some transporters that are downregulated in mycorrhizal roots are not controlled by the P supply.16 It has been suggested that the suppression of the DP by AM fungi can even lead to growth depressions in mycorrhizal plants when the MP does not compensate for the reduced uptake of the DP.15 AM fungi differ in their efficiency with which they suppress the DP,14 and a strong suppression of the DP will shift the ratio between the two uptake pathways toward the MP and will result in a higher mycorrhizal dependency of the host. It is interesting to speculate that the AM fungus could use the downregulation of the DP to increase its C availability. A higher dependence on the MP for nutrient uptake has been shown to stimulate the C allocation to the root system.17,18
Figure 1. Nutrient uptake via the direct pathway (DP) or mycorrhizal pathway (MP) in mycorrhizal roots. High affinity nutrient uptake transporters of the DP are downregulated in mycorrhizal roots (dotted line), and instead mycorrhiza-inducible transporters of the MP are expressed at the mycorrhizal interface. The ERM takes up inorganic N from the soil/fungal interface and N is assimilated and converted into arginine via glutamine synthetase (GS), carbamoyl-phosphate synthase glutamine chain (CPS), argininosuccinate synthase (ASS), and argininosuccinate lyase (AL). The basic amino acid arginine (Arg) acts as charge balance and is co-transported to the IRM with negatively charged polyphosphates (polyP) that are synthesized in the ERM from P taken up from the soil. PolyP are remobilized in the IRM and release inorganic P (PI) and Arg, which is re-converted into NH4+ via the catabolic arm of the urea cycle and the activity of a fungal arginase (ARG) and urease (URE). Plants transfer sucrose into the interfacial apoplast, which is hydrolyzed by the activity of a plant invertase (INV) into hexoses. The carbon supply of the host stimulates N and P uptake and transport via the MP in the AM symbiosis (hatched line). The supply of a carbon source (acetate) independent from the C supply of the host reduces N transport in the AM symbiosis.
Carbon as Trigger for Nutrient Uptake and Transport in the AM Symbiosis
The host provides the fungus with C in the form of sucrose (Fig. 1), which is broken down by plant derived acid invertase19,20 or sucrose synthase21 into hexoses which the fungus takes up via a high affinity monosaccharide transporter.22 AM fungi are unable to use sucrose as a C source23 so they induce the expression of the plant acid invertase in the mycorrhizal interface.19 It has previously been shown that an increase in the C availability for the AM fungus stimulates the P transport in the AM symbiosis.7,8 Our more recent work demonstrated that C also acts as a trigger for fungal N uptake and transport, and that the stimulation in N transport is driven by changes in fungal gene expression (Fig. 1).6
Woolhouse in 1975 was the first to speculate that C and P transport in the AM symbiosis are directly linked,24 and this hypothesis was recently supported by the demonstration that the mycorrhiza-inducible plant P transporter Pt4 and the fungal monosaccharide transporter MST2 are co-localized in the AM interface, and that their expression level is tightly linked.22 Phosphate transfer and the expression of Pt4 is essential for the AM symbiosis; the absence of this transporter in the periarbuscular membrane leads to a premature degradation of arbuscules and the symbiosis fails.25 Arbuscules in the AM symbiosis undergo a cycle of growth, degradation, senescence and recurrent growth, and it has been suggested that a consistent host-driven turnover of arbuscules provides the plant with an instrument to remove and ‘penalize’ inefficient AM fungal symbionts. This mechanism would also allow hosts to regulate its intracellular colonization according to changes in the exogenous nutrient supply conditions.26 Interestingly, the arbuscular phenotype of Pt4 mutants is rescued by N deprivation, indicating that the AM fungus can escape arbuscular degradation by N transport across the mycorrhizal interface. Does this mean that the host plant considers the sum of N and P benefits when regulating its intracellular colonization and the carbon supply to its fungal symbionts? More data are needed to answer this critical question.
Nutrient Allocation in Common Mycelial Networks
AM fungi interact simultaneously within a common mycelial network (CMN) with multiple hosts from different plant species, and therefore do not rely on a single host for their C supply. Currently, it is not known how AM fungi allocate resources within a CMN or how host plants of the CMN are able to compete with other plants for limited nutrient resources. It has been shown that C to nutrient exchange ratios in CMN are fungal and plant species-dependent and that plant species differ in their contribution to the C availability of the CMN.27 Recently, we demonstrated that AM fungi, despite the coenocytic nature of their hyphae, are able to distinguish between a C source that is directly supplied to the ERM or host C delivered via the mycorrhizal interface.6 When an exogenous supply of C became available for the AM fungus and the fungus became less dependent on its host for its C supply, a fungal arginase gene in the ERM was upregulated, and the N transport to the mycorrhizal host was reduced.6 Consistently, a downregulation of two fungal ammonium transporters was observed when an exogenous C source became available for the fungus.28 This suggests that (i) there is a change in fungal strategy when the fungus has access to a C source independent from a single host and (ii) that the C supply of the host may play an important role for the allocation of nutrients within a CMN. Recent work from our lab in whole plant systems suggests that AM fungi allocate N and P resources in CMN according to the C benefit that different hosts are able to provide (Fellbaum et al., unpublished).
While significant progress has been made in understanding transport and allocation processes in the AM symbiosis, much more work is needed to understand the mechanistic strategies of both partners, and how these strategies are mediated by external resources. This will allow us to make predictions about mycorrhizal functioning under global change, and allow us to maximize the benefits of the mutualism to increase the nutrient efficiency of crops in environmentally sustainable agriculture.
Acknowledgments
We acknowledge the financial support of the National Science Foundation IOS Awards 0943338 and 1051397 and a Netherlands Organization for Scientific Research Vidi and Meervoud Grant (to E.T.K.).
Glossary
Abbreviations:
- AM
arbuscular mycorrhizal
- Arg
arginine
- C
carbon
- CMN
common mycelia network
- DP
direct pathway
- ERM
extraradical mycelium
- IRM
intraradical mycelium
- MP
mycorrhizal pathway
- N
nitrogen
- P
phosphate
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22015
References
- 1.Wang B, Qiu YL. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza. 2006;16:299–363. doi: 10.1007/s00572-005-0033-6. [DOI] [PubMed] [Google Scholar]
- 2.Wright DP, Read DJ, Scholes JD. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ. 1998;21:881–91. doi: 10.1046/j.1365-3040.1998.00351.x. [DOI] [Google Scholar]
- 3.Maldonado-Mendoza IE, Dewbre GR, Harrison MJ. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol Plant Microbe Interact. 2001;14:1140–8. doi: 10.1094/MPMI.2001.14.10.1140. [DOI] [PubMed] [Google Scholar]
- 4.Benedetto A, Magurno F, Bonfante P, Lanfranco L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza. 2005;15:620–7. doi: 10.1007/s00572-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 5.Thomson BD, Clarkson DT, Brain P. Kinetics of phosphorus uptake by the germ-tubes of the vesicular-arbuscular mycorrhizal fungus, Gigaspor magarita. New Phytol. 1990;116:647–53. doi: 10.1111/j.1469-8137.1990.tb00550.x. [DOI] [Google Scholar]
- 6.Fellbaum CR, Gachomo EW, Beesetty Y, Choudhari S, Strahan GD, Pfeffer PE, et al. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A. 2012;109:2666–71. doi: 10.1073/pnas.1118650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammer EC, Pallon J, Wallander H, Olsson PA. Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol Ecol. 2011;76:236–44. doi: 10.1111/j.1574-6941.2011.01043.x. [DOI] [PubMed] [Google Scholar]
- 8.Bücking H, Shachar-Hill Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 2005;165:899–911. doi: 10.1111/j.1469-8137.2004.01274.x. [DOI] [PubMed] [Google Scholar]
- 9.Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science. 2011;333:880–2. doi: 10.1126/science.1208473. [DOI] [PubMed] [Google Scholar]
- 10.Smith SE, Jakobsen I, Grønlund M, Smith FA. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011;156:1050–7. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison MJ, Dewbre GR, Liu JY. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell. 2002;14:2413–29. doi: 10.1105/tpc.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guether M, Neuhäuser B, Balestrini R, Dynowski M, Ludewig U, Bonfante P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009;150:73–83. doi: 10.1104/pp.109.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiou T-J, Liu H, Harrison MJ. The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. Plant J. 2001;25:281–93. doi: 10.1046/j.1365-313x.2001.00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Grunwald U, Guo W, Fischer K, Isayenkov S, Ludwig-Müller J, Hause B, et al. Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormone-treated Medicago truncatula roots. Planta. 2009;229:1023–34. doi: 10.1007/s00425-008-0877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2011;62:227–50. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Versaw WK, Pumplin N, Gomez SK, Blaylock LA, Harrison MJ. Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. J Biol Chem. 2008;283:24673–81. doi: 10.1074/jbc.M802695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen KL, Bouma TJ, Lynch JP, Eissenstat DM. Effects of phosphorus availability and vesicular–arbuscular mycorrhizas on the carbon budget of common bean (Phaseolus vulgaris) New Phytol. 1998;139:647–56. doi: 10.1046/j.1469-8137.1998.00242.x. [DOI] [Google Scholar]
- 18.Postma JA, Lynch JP. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol. 2011;156:1190–201. doi: 10.1104/pp.111.175489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaarschmidt S, Roitsch T, Hause B. Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J Exp Bot. 2006;57:4015–23. doi: 10.1093/jxb/erl172. [DOI] [PubMed] [Google Scholar]
- 20.Schaarschmidt S, González MC, Roitsch T, Strack D, Sonnewald U, Hause B. Regulation of arbuscular mycorrhization by carbon. The symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity. Plant Physiol. 2007;143:1827–40. doi: 10.1104/pp.107.096446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohnjec N, Perlick AM, Pühler A, Küster H. The Medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi. Mol Plant Microbe Interact. 2003;16:903–15. doi: 10.1094/MPMI.2003.16.10.903. [DOI] [PubMed] [Google Scholar]
- 22.Helber N, Wippel K, Sauer N, Schaarschmidt S, Hause B, Requena N. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell. 2011;23:3812–23. doi: 10.1105/tpc.111.089813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrent JL, James TY, Vasaitis R, Taylor AF. Friend or foe? Evolutionary history of glycoside hydrolase family 32 genes encoding for sucrolytic activity in fungi and its implications for plant-fungal symbioses. BMC Evol Biol. 2009;9:148. doi: 10.1186/1471-2148-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolhouse HW. Membrane structure and transport problems considered in relation to phosphorus and carbohydrate movements and the regulation of endotrophic mycorrhizal associations. In: Sanders FET, Mosse B, Tinker PB, eds. Endomycorrhizas. London, UK: Academic Press, 1975:209–39. [Google Scholar]
- 25.Javot H, Penmetsa RV, Breuillin F, Bhattarai KK, Noar RD, Gomez SK, et al. Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. Plant J. 2011;68:954–65. doi: 10.1111/j.1365-313X.2011.04746.x. [DOI] [PubMed] [Google Scholar]
- 26.Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, et al. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 2010;64:1002–17. doi: 10.1111/j.1365-313X.2010.04385.x. [DOI] [PubMed] [Google Scholar]
- 27.Walder F, Niemann H, Natarajan M, Lehmann MF, Boller T, Wiemken A. Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol. 2012;159:789–97. doi: 10.1104/pp.112.195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Tienda J, Testillano PS, Balestrini R, Fiorilli V, Azcón-Aguilar C, Ferrol N. GintAMT2, a new member of the ammonium transporter family in the arbuscular mycorrhizal fungus Glomus intraradices. Fungal Genet Biol. 2011;48:1044–55. doi: 10.1016/j.fgb.2011.08.003. [DOI] [PubMed] [Google Scholar]