Abstract
Auditory sensitivity in three species of woodpeckers was estimated using the auditory brainstem response (ABR), a measure of the summed electrical activity of auditory neurons. For all species, the ABR waveform showed at least two, and sometimes three prominent peaks occurring within 10 ms of stimulus onset. Also ABR peak amplitude increased and latency decreased as a function of increasing sound pressure levels. Results showed no significant differences in overall auditory abilities between the three species of woodpeckers. The average ABR audiogram showed that woodpeckers have lowest thresholds between 1.5 and 5.7 kHz. The shape of the average woodpecker ABR audiogram was similar to the shape of the ABR-measured audiograms of other small birds at most frequencies, but at the highest frequency data suggest that woodpecker thresholds may be lower than those of domesticated birds, while similar to those of wild birds.
INTRODUCTION
While there are numerous behavioral and physiological studies of auditory abilities in small birds, woodpeckers have been a neglected group in terms of their basic hearing ability. Woodpeckers are a unique group of birds that exhibit a number of specialized cranial adaptations serving to dampen forces on the bill and skull (Beecher, 1953; Bock, 1964, 1966; Spring, 1965). Because of these adaptations, a woodpecker's brain is protected from impact and vibration injury (Bock, 1964; May et al., 1976, 1979). This adaptive morphology involves unique modifications of the ear anatomy and musculature that include a narrowed round window and a specialized dual columellar footplate that, coupled with the round window membrane, may act to reduce transient force on the cochlear fluids (Kohllöffel, 1984). As a consequence, these adaptations to dampen mechanical shock during pecking could play a role in shaping the hearing sensitivities of these birds.
We used the auditory brainstem response (ABR) as a means of estimating hearing ability in woodpeckers. The ABR is a useful tool for studying the functionality of the auditory system in a wide variety of animals (e.g., Jewett, 1970; Corwin et al., 1982; Walsh et al., 1986; Burkard and Voigt, 1989; Donaldson and Rubel, 1990; Mills et al., 1990; Burkard et al., 1996; McFadden et al., 1996; Kenyon et al., 1998; Liu and Mark, 2001; Higgs et al., 2002). The derived audiogram is a good approximation of the shape of behavioral audiograms but does not necessarily predict absolute auditory sensitivity (e.g., Borg and Engström, 1983; Wenstrup, 1984; Stapells and Oates, 1997; Brittan-Powell et al., 2002, 2010). The ABR technique is also advantageous because the morphology of the ABR waveform is conserved in most vertebrates (e.g., Corwin et al., 1982; Walsh et al., 1992). Numerous studies now have examined the ABR specifically in birds, and it has proven a reliable technique for estimating basic auditory sensitivities across a range of species (Dooling and Walsh, 1976; Aleksandrov and Dmitrieva, 1992; Dmitrieva and Gottlieb, 1992; Brittan-Powell et al., 2002, 2005, 2010; Higgs et al., 2002; Lucas et al., 2002; Brittan-Powell and Dooling, 2004; Wright et al., 2004; Henry and Lucas, 2008, 2009; Noirot et al., 2011). The ABR and other evoked-potential techniques are particularly useful as a means of estimating auditory sensitivity because training of animals is not required, making these techniques faster and more efficient than behavioral tests.
Vocalizations in small birds are often correlated with auditory ability (Dooling et al., 2000). The peak power in the vocal signals of many species corresponds with the most sensitive part of the audiogram. In small woodpeckers, vocal signals have most energy in the frequency range 1–6 kHz (Winkler and Short, 1978; Jackson, 1994; Mahan, 1996; Jackson and Ouellet, 2002; Jackson et al., 2002). This frequency span is similar to that of other small–medium-sized birds (Fay, 1988; Dooling et al., 2000). Woodpecker drumming sounds, which may also serve as communication signals, are generally lower in frequency and broader in bandwidth than vocal signals, and may overlap with the frequencies typical of vocalizations (Winkler and Short, 1978; Short, 1982; Jackson, 1994; Mahan, 1996; Shackelford et al., 2000; Jackson and Ouellet, 2002; Jackson et al., 2002). Predictions of auditory abilities based on vocal signal structure would therefore suggest that auditory thresholds in woodpeckers should be typical of small birds in terms of bandwidth and frequency region of best sensitivity.
The main purpose for our tests with woodpeckers was to evaluate basic auditory abilities in this order of birds, including the estimation of a complete audiogram, as a comparison with other birds. To this end, we tested three species; two congenerics, the downy woodpecker (Picoides pubescens) and the hairy woodpecker (P. villosus), and a more distantly related species, the red-bellied woodpecker (Melanerpes carolinus). Woodpeckers have several unique anatomical adaptations to facilitate their pecking behavior, which is used both in foraging and long-range signaling, but the vocal repertoires in this group are not especially distinctive in terms of their spectral characteristics when compared with other small birds (Winkler and Short, 1978). We wished to determine whether either of these factors might carry some implications for auditory abilities in woodpeckers as a group. An additional benefit of our auditory estimates in woodpeckers involves conservation efforts with an endangered species, the red-cockaded woodpecker (P. borealis). There is a need to address the potential effects of noise on the hearing and breeding biology of red-cockaded woodpeckers, given the limited geographic range of this species and its relative proximity to potential noise sources. Data for auditory abilities in woodpeckers as a group will help inform noise-related conservation efforts in or near the habitats of this critically endangered bird.
MATERIALS AND METHODS
Subjects
We captured fourteen woodpeckers: 9 downy woodpeckers (4 male, 5 female), 2 hairy woodpeckers (both female), and 3 red-bellied woodpeckers (1 male, 2 female) at field sites in Prince George's County, Maryland, during the late Winter and early Spring, and transported them to the University of Maryland. We performed ABR tests the same day under the direction of the Institutional Animal Care and Use Committee of the University of Maryland. We sedated all birds with intramuscular injections of a ketamine/diazepam mixture (50 mg/kg ketamine, 2 mg/kg diazepam).
Stimuli and ABR testing
We presented subjects with stimulus trains (see Brittan-Powell et al., 2002, 2005, 2010) that varied in frequency and level. Each train consisted of 9 single clicks or frequency tone bursts that increased successively in level (intensity) and were presented at a rate of 4/s. Each individual tone burst was 5 ms in duration (1 ms rise/fall cos2) with a 20 ms interstimulus interval. We used tone bursts of 0.5, 1.0, 1.5, 2.0, 2.86, 4.0, 5.7, and 8.0 kHz. The rectangular-pulse broadband clicks were 0.1 ms in duration with a 25 ms inter-stimulus interval. For all stimuli, the intensities presented spanned a 40-dB range in ascending order and increased in 5 dB steps (e.g., starting at 70 dB and increasing to 110 dB, or starting at 30 dB and increasing to 70 dB). Ranges were chosen to be sure that they encompassed the bird's threshold at that frequency and would provide an adequate number of amplitudes above threshold to estimate threshold accurately.
We measured stimulus levels in the free field of a large (2.24 m 2.13 m 2.03 m) anechoic room (Audio Suttle, AS-114, type 40 C, Norwalk, CT) by placing the 1/2-in. microphone of a sound level meter (System 824; Larson Davis, Inc., Provo, UT) at the approximate position of the animal's ear (30 cm from speaker). We played tones continuously and measured them using the fast weighting A scale on the sound level meter. For values below 1.0 kHz, the A-filter values were corrected for the filter. To determine the level of the short duration click, we used the peak equivalent SPL of the click (pSPL). A test tone, e.g., a 1000 Hz tone, was played and adjusted until the peak-to-peak voltage was the same as it was for the click. The SPL required to match the amplitude of the click, as indicated by the sound level meter, was the peak equivalent SPL (dB pSPL) of the click stimulus.
The ABR acquisition, equipment control, and data management have been described previously (see, e.g., Brittan-Powell et al., 2002, 2005, 2010; Higgs et al., 2002; Brittan-Powell and Dooling, 2004; Wright et al., 2004). The speaker (JBL Model 2105 H, James B Lansing Sounds, Inc., Northridge, CA) was 30 cm from the bird's right ear (90° azimuth; 0° elevation). Platinum alloy, subdermal needle electrodes (Grass F-E2; West Warwick, RI), with wires that were twisted together to reduce noise, were placed just under the skin at the vertex (active), directly behind the right ear canal (reference), and behind the canal of the ear contralateral to stimulation (ground). A Tucker-Davis Technologies (TDT, Gainesville, FL) modular rack-mount system was controlled by an optical cable-linked 350-MHz Pentium PC containing a TDT AP2 Digital Signal Process board and running TDT “BIOSIG” software. Sound stimuli were generated using TDT “SIGGEN” software, and fed through a DA1 digital-analog converter, a PA4 programmable attenuator, and a HB6 transducer which directly drove the JBL Model 2105 H speaker. The electrodes were connected to the TDT HS4 Headstage that amplifies and digitizes the signal before sending it over fiber optic cables to the TDT DB4 Digital Biological Amplifier. This amplifier allows additional filtering and gain to be added. A TDT TG6 timing generator synchronized the A/D and D/A conversion. Each ABR represents the average response of 300 stimulus presentations (150 averages for each polarity/phase were added together to cancel the cochlear microphonic), sampled at 20 kHz for 235 ms following onset of the stimulus (allows for 25 ms recording time for each stimulus). The biological signal was amplified (x 100K) and notch filtered at 60 Hz with the DB4 Digital Biological Amplifier during collection. The signal was bandpass filtered below 30 Hz and above 3000 Hz after collection using the BIOSIG program.
Threshold estimation
We examined ABR waveforms produced in response to stimulus trains visually. We chose a range of 1–10 ms following the onset of the speaker stimulus to measure a response. Because test stimulus levels in the region of threshold differed by 5 dB, we defined ABR thresholds as the level 2.5 dB (one-half step) below the lowest stimulus level at which a response could be visually detected on the trace, regardless of wave (as in Brittan-Powell and Dooling, 2004; Brittan-Powell et al., 2005, 2010; Noirot et al., 2011). As a comparison with our visual threshold estimates, we estimated thresholds with linear regression (Brittan-Powell et al., 2002). To do this, we averaged the amplitudes between 0 and 1.95 ms after onset of stimulus and subtracted them from the largest positive peak between 1.0 and 4.5 ms (baseline-to-peak). This procedure resulted in obtaining the peak 1 amplitude across all stimulus levels. We used the amplitude-intensity functions generated from peak 1 amplitudes to estimate thresholds using linear regression and a 0 μVolt crossing as our threshold estimate (Brittan-Powell et al., 2002). For intensity-latency functions, we corrected latency to peak 1 for conduction delays between the sound source and the entrance of the ear canal of the animal (0.88 ms).
Statistical analyses
We used a two-way repeated measures analysis of variance (ANOVA) to test for differences in tone thresholds across frequencies and between species. We tested for differences in the click thresholds of different species using a one-way ANOVA. We compared thresholds between sexes for downy woodpeckers using an independent samples t test, and we compared techniques for estimating thresholds (visual detection vs linear regression) using paired t tests. Statistical tests were performed using the SYSTAT statistical software (Wilkinson, 1999).
RESULTS
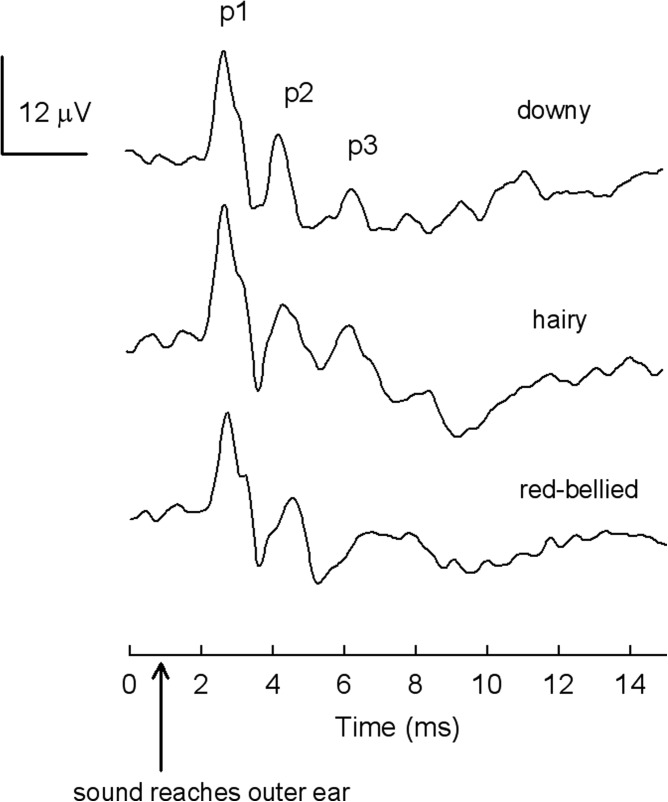
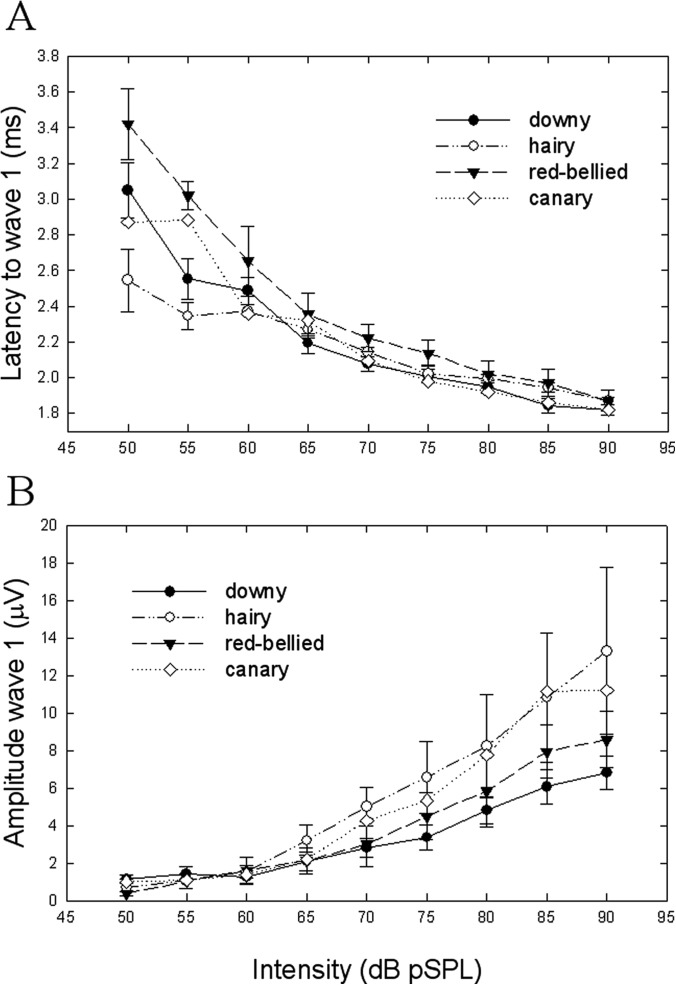
Waveform morphology was very similar across the three woodpecker species (Fig. 1). Visual examination of the waveforms showed at least two prominent peaks that occurred within the first 4–5 ms after sound reached the bird's external ear canal. As the level of stimulation increased, ABR amplitudes increased and peak latencies decreased. Figure 2 shows amplitude and latency-intensity functions for peak 1 in response to a click for the three species of woodpeckers compared with the canary, a small passerine (data from Brittan-Powell et al., 2010). There was little difference in latency or amplitude among the species. The average click threshold for all 14 woodpeckers estimated visually was 51.0 +/− 0.98 dB pSPL (mean +/− SE), with no differences between species [F(2,11) = 0.42, p > 0.05; one-way ANOVA]. Downy woodpeckers, for which we had the largest sample, showed no difference in ABR click threshold between sexes [t(9) = −0.056, p > 0.05].
Figure 1.
Comparison of ABR waveforms for 3 adult woodpeckers, one from each species, in response to the click stimulus at 85 dB pSPL. Here 0 ms equals stimulus onset; the arrow indicates when sound reaches the outer ear. In all 3 species, ABR waveforms showed similar morphology, with at least 2 prominent peaks (p1, p2), as is typical of other birds tested to date.
Figure 2.
(A) Latency and (B) amplitude in response to the click stimulus forthe 3 woodpecker species compared with a small passerine, the canary (canary data from Brittan-Powell et al., 2010). (A) For all birds, latency to wave 1 decreased as stimulus level (intensity) increased and values were similar across species. (B) Amplitude of wave 1 varied among the woodpecker species, especially at higher stimulus levels, though these results should be taken with caution due to the small sample sizes involved.
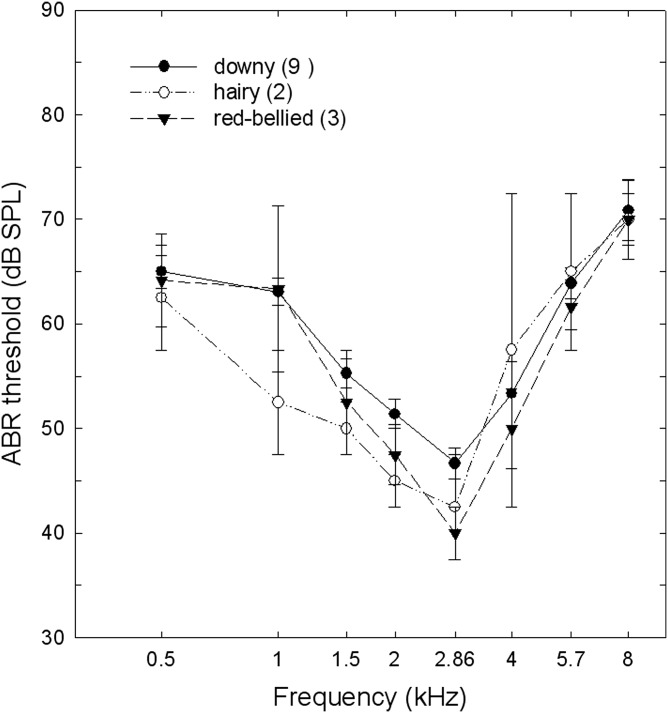
ABR audiograms using the visual method for estimating thresholds (Fig. 3) showed the typical U-shape found in other birds, with lowest thresholds between 1.5 and 4 kHz. Our results were independent of the method used for estimating thresholds from ABR waveform amplitudes. Threshold estimates did not differ in a comparison of the two techniques (visual, linear regression) across frequencies [ts(13) < 2.0, p > 0.05; paired t test]. A two-way repeated measures ANOVA comparing tone frequencies and woodpecker species found no significant differences between species [F(2,77) = 0.72, p > 0.05], or the interaction of species by frequency [F(14,77) = 0.62, p > 0.05]. As expected with a U-shaped audiogram, thresholds for different frequencies differed [F(7,77) = 21.13, p < 0.001], with birds being most sensitive (i.e. responding to tone bursts presented below 55 dB SPL) between 1.5 and 4 kHz. For downy woodpeckers, we also compared the audiograms to determine if there was a sex difference. A two-way ANOVA showed a main effect of frequency [F(7,56) = 19.53, p < 0.05], but no effect of sex [F(1,56) = 1.13, p > 0.05] or the interaction of sex by frequency.
Figure 3.
ABR audiograms (mean +/− SE) for the 3 species of woodpeckers tested (number of individuals listed in parentheses).
DISCUSSION
The morphology of the ABR waveforms did not differ in shape between the woodpecker species tested here (Fig. 1). In general, ABR waveforms to click stimuli showed a pattern very similar to that exhibited in other birds (Moiseff et al., 1996; Brittan-Powell et al., 2002, 2005, 2010; Lucas et al., 2002; Brittan-Powell and Dooling, 2004; Wright et al., 2004; Henry and Lucas, 2008; Noirot et al., 2011), and more specifically, to downy woodpeckers tested with clicks previously (Lucas et al., 2002). Two peaks were evident in each waveform with the morphology of these peaks suggesting a direct analogy to peak 1 and peak 2 in budgerigars and other small birds (see Brittan-Powell et al., 2005, for differences from this pattern in owls).
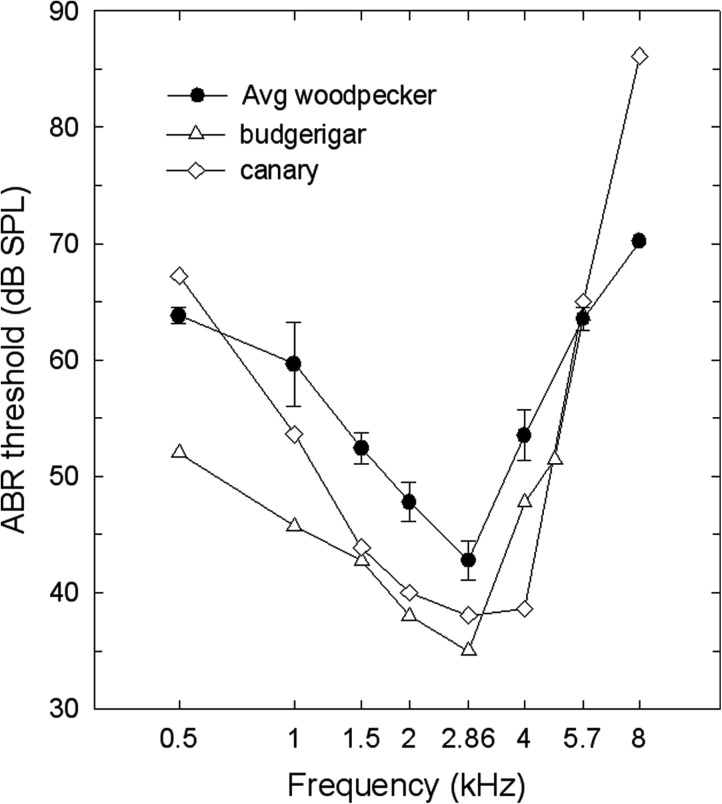
Based on ABR thresholds, woodpeckers have an audiogram that is similar in shape to that of other small birds, with lowest thresholds in the region of 1.5 to 4 kHz (Fig. 3). Waveform amplitudes in individual woodpeckers varied but their audiograms were close to those of several other small bird species (Fig. 4). Our results with woodpeckers did not conform to earlier predictions for woodpecker hearing based on their unique anatomy and physiology. In a prior study examining ABR responses to click stimuli, Lucas et al. (2002) found lower amplitudes and longer latencies in woodpeckers when compared with results to comparable stimuli in several passerine species. They predicted that high-frequency auditory sensitivity might be weaker in woodpeckers as a consequence. While some individual woodpeckers in our study also showed low peak amplitudes, we found that woodpeckers generally exhibited low- and high-frequency auditory abilities that were similar to those of canaries and budgerigars—domesticated birds—based on ABR thresholds (Fig. 4). It appears that woodpecker auditory thresholds correspond more closely to expectations based on size rather than on morphological specializations of the ear (Dooling et al., 2000).
Figure 4.
Average ABR audiogram for all 3 woodpecker species (mean +/− SE) as a comparison with 2 other species of birds whose ABR audiograms were obtained using the same stimuli and set up (budgerigars: Brittan-Powell et al., 2002; canaries: Brittan-Powell et al., 2010).
The region of peak energy in the vocalizations of woodpeckers in the genus Picoides (1–6 kHz) is similar to that of other small birds, perhaps ranging slightly higher when compared to some other species commonly used in laboratory auditory tests, such as canaries and budgerigars (Fay, 1988; Dooling et al., 2000; Delaney et al., 2011). Auditory sensitivities in these woodpeckers, as measured by the ABR, corresponded well in terms of the frequency of best sensitivity and the bandwidth of their best hearing range with the peak power in their vocal signals. As a contrast, red-bellied woodpecker vocalizations are generally lower in frequency than those of the two Picoides species (see, e.g., Wilkins, 1996; Shackelford et al., 2000). Neither the ABR waveforms nor the ABR audiograms for this species, however, showed any marked deviation in threshold or frequency range from those of the two Picoides species tested here (Fig. 3).
It is perhaps not surprising that the ABR audiograms of the three species of woodpeckers we tested did not differ significantly from one another given the overall similarity of general auditory sensitivities in small birds (Dooling et al., 2000). Our results are also similar to those of a preliminary audiogram published for downy woodpeckers using 3 individuals (Delaney et al., 2011). An ancillary goal of our work was to estimate auditory abilities of red-cockaded woodpeckers (P. borealis) given its endangered status and the proximity of its remaining habitats to anthropogenic noise sources. Red-cockaded woodpeckers reside in some areas, near military bases for example, that may be subjected to the effects of loud anthropogenic noises. Downy and hairy woodpeckers are close relatives of the red-cockaded woodpecker (Short, 1982; Jackson, 1994). They are therefore useful surrogates for this species, and our study will provide more complete information to better understand the potential effects of loud noise on the auditory thresholds of these birds. As our ABR thresholds were broadly similar across woodpecker species, we predict that hearing in the red-cockaded woodpecker would be most sensitive between 1 and 6 kHz and follow a pattern similar to that found in the three species of woodpeckers we tested.
We found that woodpecker audiograms, as estimated by the ABR, are broadly similar to those of other small birds, especially passerines. As in many other species of birds, the peak power in the vocal spectra of woodpeckers corresponds generally with the region of best sensitivity in their audiograms. The unique anatomy of the woodpecker head and ear suggest caution in estimating likely behavioral thresholds, but given that ABR waveform amplitudes and thresholds fall within the range of those for other small birds including canaries and budgerigars, their auditory abilities are comparable to those of other birds tested for their hearing thus far.
ACKNOWLEDGMENTS
We wish to thank Larry Pater and the U.S. Army CERL for support. We thank Mimi Ghim and Rebecca Duckworth Forkner for technical assistance in the field, and Amanda Lauer and Kevin Omland for comments on earlier drafts. This work was supported in part by P30DC004664 from the National Institute of Deafness and Communicative Disorders (NIDCD) of the National Institutes of Health, NIDCD grant R03DC04762 to B.L.; National Institutes of Health Grants R01DC00198 and R01DC001372 to R.J.D., and SERDP CS-1083. Experiments described here comply with the “Principles of animal care” publication No. 86-23, revised 1985 of the National Institutes of Health, the University of Maryland animal care and use committee, and the laws of the United States.
References
- Aleksandrov, L. I., and Dmitrieva, L. P. (1992). “Development of auditory sensitivity of altricial birds: Absolute thresholds of the generation of evoked potentials,” Neurosci. Behav. Physiol. 22, 132–137. 10.1007/BF01192385 [DOI] [PubMed] [Google Scholar]
- Beecher, W. J. (1953). “Feeding adaptations and systematics in the avian order Piciformes,” J. Wash. Acad. Sci. 43, 293–299. [Google Scholar]
- Bock, W. J. (1964). “Kinetics of the avian skull,” J. Morphol. 114, 1–42. 10.1002/jmor.1051140102 [DOI] [Google Scholar]
- Bock, W. J. (1966). “An approach to the functional analysis of bill shape,” Auk 83, 10–51. 10.2307/4082976 [DOI] [Google Scholar]
- Borg, E., and Engström, B. (1983). “Hearing thresholds in the rabbit,” Acta Oto-Laryngol. 95, 19–26. 10.3109/00016488309130911 [DOI] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., and Dooling, R. J. (2004). “Development of auditory sensitivity in budgerigars (Melopsittacus undulatus),” J. Acoust. Soc. Am. 115, 3092–3102. 10.1121/1.1739479 [DOI] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., Dooling, R. J., and Gleich, O. (2002). “Auditory brainstem responses (ABR) in adult budgerigars (Melopsittacus undulatus),” J. Acoust. Soc. Am. 112, 999–1008. 10.1121/1.1494807 [DOI] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., Dooling, R. J., Ryals, B. M., and Gleich, O. (2010). “Electrophysiological and morphological development of the inner ear in Belgian waterslager canaries,” Hear. Res. 269, 56–69. 10.1016/j.heares.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittan-Powell, E. F., Lohr, B., Hahn, D. C., and Dooling, R. J. (2005). “Auditory brainstem responses in the Eastern Screech Owl: An estimate of auditory thresholds,” J. Acoust. Soc. Am. 118, 314–321. 10.1121/1.1928767 [DOI] [PubMed] [Google Scholar]
- Burkard, R., McGee, J., and Walsh, E. J. (1996). “Effects of stimulus rate on feline brain-stem auditory evoked response during development. I. Peak latencies,” J. Acoust. Soc. Am. 100, 978–990. 10.1121/1.416209 [DOI] [PubMed] [Google Scholar]
- Burkard, R., and Voigt, H. F. (1989). “Stimulus dependencies of the gerbil brain-stem auditory-evoked response (BAER): I. Effects of click level, rate, and polarity,” J. Acoust. Soc. Am. 85, 2514–2525. 10.1121/1.397746 [DOI] [PubMed] [Google Scholar]
- Corwin, J. T., Bullock, T. H., and Schweitzer, J. (1982). “The auditory brain stem response in five vertebrate classes,” Electroencephalogr. Clin. Neurol. 54, 629–641. 10.1016/0013-4694(82)90117-1 [DOI] [PubMed] [Google Scholar]
- Delaney, D. K., Pater, L. L., Carlile, L. D., Spadgenske, E. W., Beaty, T. A., and Melton, R. H. (2011). “Response of red-cockaded woodpeckers to military training operations,” Wildlife Monogr. 177, 1–38. 10.1002/wmon.3 [DOI] [Google Scholar]
- Dmitrieva, L. P., and Gottlieb, G. (1992). “Development of brainstem auditory pathway in mallard duck embryos and hatchlings,” J. Comp. Physiol. A 171, 665–671. 10.1007/BF00194114 [DOI] [PubMed] [Google Scholar]
- Donaldson, G. S., and Rubel, E. W. (1990). “Effects of stimulus repetition rate on ABR threshold, amplitude and latency in neonatal and adult Mongolian gerbils,” Electroencephalogr. Clin. Neurophysiol. 77, 458–470. 10.1016/0168-5597(90)90006-Y [DOI] [PubMed] [Google Scholar]
- Dooling, R. J., Lohr, B., and Dent, M. L. (2000). “Hearing in birds and reptiles,” in Comparative Hearing: Birds and Reptiles, edited by Dooling R. J., Popper A. N., and Fay R. R. (Springer-Verlag, New York: ), pp. 308–359. [Google Scholar]
- Dooling, R. J., and Walsh, J. K. (1976). “Auditory evoked response correlates of hearing in the parakeet (Melopsittacus undulatus),” Physiol. Psychol. 4, 224–232. [Google Scholar]
- Fay, R. R. (1988). Hearing in Vertebrates: A Psychophysics Databook (Hill-Fay Associates, Winnetka, IL: ), pp. 1–621. [Google Scholar]
- Henry, K. S., and Lucas, J. R. (2008). “Coevolution of auditory sensitivity and temporal resolution with acoustic signal space in three songbirds,” Anim. Behav. 76, 1659–1671. 10.1016/j.anbehav.2008.08.003 [DOI] [Google Scholar]
- Henry, K. S., and Lucas, J. R. (2009). “Vocally correlated seasonal auditory variation in the house sparrow (Passer domesticus),” J. Exp. Biol. 212, 3817–3822. 10.1242/jeb.033035 [DOI] [PubMed] [Google Scholar]
- Higgs, D. M., Brittan-Powell, E. F., Soares, D., Souza, M. J., Carr, C. E., Dooling, R. J., and Popper, A. N. (2002). “Amphibious auditory responses of the American alligator (Alligator mississipiensis),” J. Comp. Physiol. A 188, 217–223. 10.1007/s00359-002-0296-8 [DOI] [PubMed] [Google Scholar]
- Jackson, J. A. (1994). “Red-cockaded woodpecker (Picoides borealis),” in The Birds of North America, edited by Poole A., and Gill F. (The Birds of North America, Philadelphia, PA: ), Vol. 85, pp. 1–20. [Google Scholar]
- Jackson, J. A., and Ouellet, H. R. (2002). “Downy woodpecker (Picoides pubescens),” in The Birds of North America, edited by Poole A., and Gill F. (The Birds of North America, Philadelphia, PA: ), Vol. 613, pp. 1–32. [Google Scholar]
- Jackson, J. A., Ouellet, H. R., and Jackson, B. J. S. (2002). “Hairy woodpecker (Picoides villosus),” in The Birds of North America, edited by Poole A., and Gill F. (The Birds of North America, Philadelphia, PA: ), Vol. 702, pp. 1–32. [Google Scholar]
- Jewett, D. (1970). “Volume-conducted potentials in response to auditory stimuli as detected by averaging in the cat,” Electroencephalogr. Clin. Neurol. 28, 609–618. 10.1016/0013-4694(70)90203-8 [DOI] [PubMed] [Google Scholar]
- Kenyon, T. N., Ladich, F., and Yan, H. Y. (1998). “A comparative study of hearing ability in fishes: The auditory brainstem response approach,” J. Comp. Physiol. A 182, 307–318. 10.1007/s003590050181 [DOI] [PubMed] [Google Scholar]
- Kohllöffel, L. U. (1984). “Notes on the comparative mechanics of hearing. I. A shock-proof ear,” Hear. Res. 13, 73–76. 10.1016/0378-5955(84)90096-0 [DOI] [PubMed] [Google Scholar]
- Liu, G. B., and Mark, R. F. (2001). “Functional development of the inferior colliculus (IC) and its relationship with the auditory brainstem response (ABR) in the tammar wallaby (Macropus eugenii),” Hear. Res. 157, 112–123. 10.1016/S0378-5955(01)00289-1 [DOI] [PubMed] [Google Scholar]
- Lucas, J. R., Freeberg, T. M., Krishnan, A., and Long, G. R. (2002). “A comparative study of avian auditory brainstem responses: Correlations with phylogeny and vocal complexity, and seasonal effects,” J. Comp. Physiol. A 188, 981–992. 10.1007/s00359-002-0359-x [DOI] [PubMed] [Google Scholar]
- Mahan, T. A. (1996). “Analysis of acoustic signals of adult male and female downy woodpeckers (Picoides pubescens),” M.Sc. thesis, Eastern Kentucky University, Richmond, KY, pp. 1–78. [Google Scholar]
- May, P. R. A., Fuster, J. M., Haber, J., and Hirschman, A. (1979). “Woodpecker drilling behavior,” Arch. Neurol. 36, 370–373. 10.1001/archneur.1979.00500420080011 [DOI] [PubMed] [Google Scholar]
- May, P. R. A., Fuster, J. M., Newman, P. A., and Hirschman, A. (1976). “Woodpeckers and head injury,” Lancet 1, 454–455. 10.1016/S0140-6736(76)91477-X [DOI] [PubMed] [Google Scholar]
- McFadden, S. L., Walsh, E. J., and McGee, J. (1996). “Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus),” Hear. Res. 100, 68–79. 10.1016/0378-5955(96)00108-6 [DOI] [PubMed] [Google Scholar]
- Mills, J. J., Schmiedt, R. A., Kulish, L. F. (1990). “Age-related changes in auditory potentials of Mongolian gerbil,” Hear. Res. 46, 201–210. 10.1016/0378-5955(90)90002-7 [DOI] [PubMed] [Google Scholar]
- Moiseff, A., Haresign, T., and Wang, J. (1996). “Sound localization from binaural cues by the barn owl auditory system,” in Neuroethological Studies of Cognitive and Perceptual Processes, edited by Moss C. F. and Shettleworth S. J. (Westview Press, Boulder, CO: ), pp. 305–323. [Google Scholar]
- Noirot, I., Brittan-Powell, E. F., and Dooling, R. J. (2011). “Masked auditory thresholds in three species of birds, as measured by the auditory brainstem response,” J. Acoust. Soc. Am. 129, 3445–3448. 10.1121/1.3578452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford, C. E., Brown, R. E., and Conner, R. N. (2000). “Red-bellied woodpecker (Melanerpes carolinus),” in The Birds of North America, edited by Poole A. and Gill F. (The Birds of North America, Philadelphia, PA: ), Vol. 500, pp. 1–24. [Google Scholar]
- Short, L. L. (1982). “Woodpeckers of the world,” Del. Mus. Nat. Hist. Monogr. Ser. No. 4, pp. 1–676. [Google Scholar]
- Spring, L. W. (1965). “Climbing and pecking adaptations in some North American woodpeckers,” Condor 67, 457–488. 10.2307/1365612 [DOI] [Google Scholar]
- Stapells, D. R., and Oates, P. (1997). “Estimation of pure-tone audiogram by the auditory brainstem response: A review,” Audiol. Neurootol. 2, 257–280. 10.1159/000259252 [DOI] [PubMed] [Google Scholar]
- Walsh, E. J., Gorga, M., and McGee, J. (1992). “Comparisons of the development of auditory brainstem response latencies between cats and humans,” Hear. Res. 60, 53–63. 10.1016/0378-5955(92)90058-U [DOI] [PubMed] [Google Scholar]
- Walsh, E. J., McGee, J., and Javel, E. (1986). “Development of auditory-evoked potentials in the cat. I. Onset of response and development of sensitivity,” J. Acoust. Soc. Am. 79, 712–724. 10.1121/1.393461 [DOI] [PubMed] [Google Scholar]
- Wenstrup, J. J. (1984). “Auditory sensitivity in the fish-catching bat, Noctilio leporinus,” J. Comp. Physiol. A 155, 91–101. 10.1007/BF00610934 [DOI] [Google Scholar]
- Wilkins, H. D. (1996). “The acoustic signals of male and female red-bellied woodpeckers: Description and causation,” M.Sc. thesis, Eastern Kentucky University, Richmond, KY, pp. 1–73. [Google Scholar]
- Wilkinson, L. (1999). SYSTAT, Version 9 (SPSS, Chicago, IL: ). [Google Scholar]
- Winkler, H., and Short, L. L. (1978). “A comparative analysis of acoustical signals in pied woodpeckers (Aves, Picoides),” Bull. Am. Mus. Nat. Hist. 160, 1–109. [Google Scholar]
- Wright, T. F., Brittan-Powell, E. F., Dooling, R. J., Mundinger, P. C. (2004). “Sex-linked inheritance of hearing and song in the Belgian Waterslager canary,” Proc. Biol. Sci. 271 Suppl 6, S409–412. 10.1098/rsbl.2004.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]