Abstract
Background
Studies have associated thiazolidinedione (TZD) treatment with cardiovascular disease (CVD) and questioned whether the two available TZDs, rosiglitazone and pioglitazone, have different CVD risks. We compared CVD incidence, cardiovascular (CV) and all-cause mortality in type 2 diabetic patients treated with rosiglitazone or pioglitazone as their only TZD.
Methods
We analyzed survey, medical record, administrative, and National Death Index (NDI) data from 1999 through 2003 from Translating Research Into Action for Diabetes (TRIAD), a prospective observational study of diabetes care in managed care. Medications, CV procedures, and CVD were determined from health plan (HP) administrative data, and mortality was from NDI. Adjusted hazard rates (AHR) were derived from Cox proportional hazard models adjusted for age, sex, race/ethnicity, income, history of diabetic nephropathy, history of CVD, insulin use, and HP.
Results
Across TRIAD’s ten HPs, 1,815 patients (24%) filled prescriptions for a TZD, 773 (10%) for only rosiglitazone, 711 (10%) for only pioglitazone, and 331 (4%) for multiple TZDs. In the seven HPs using both TZDs, 1,159 patients (33%) filled a prescription for a TZD, 564 (16%) for only rosiglitazone, 334 (10%) for only pioglitazone, and 261 (7%) for multiple TZDs. For all CV events, CV and all-cause mortality, we found no significant difference between rosiglitazone and pioglitazone.
Conclusions
In this relatively small, prospective, observational study, we found no statistically significant differences in CV outcomes for rosiglitazone- compared to pioglitazone-treated patients. There does not appear to be a pattern of clinically meaningful differences in CV outcomes for rosiglitazone- versus pioglitazone-treated patients.
Keywords: Thiazolidinediones, rosiglitazone, pioglitazone, diabetes
INTRODUCTION
The first thiazolidinedione (TZD), troglitazone, was approved by the Food and Drug Administration (FDA) in January 1997 and withdrawn from the market in March 2000. The two available TZDs, rosiglitazone (FDA approved May 1999) and pioglitazone (FDA approved July 1999) have come under scrutiny because reports of potential adverse cardiovascular (CV) events and several meta-analyses have suggested differential risks.1-5
The purpose of this study was to compare the risk of adverse CV events and mortality in patients with type 2 diabetes treated with rosiglitazone or pioglitazone as their only TZD after adjustment for differences between groups. We used data from Translating Research Into Action for Diabetes (TRIAD), a large, prospective, observational study of diabetes care in managed care. In our analyses, we report the full range of outcomes previously reported in the literature: nonfatal myocardial infarction (MI); coronary revascularization; the combined outcome of nonfatal MI or coronary revascularization; nonfatal stroke; the combined outcome of nonfatal MI, coronary revascularization or nonfatal stroke; CV mortality; all-cause mortality; the combined outcome of nonfatal MI or all-cause mortality; the combined outcome of nonfatal MI, nonfatal stroke, or CV mortality; the combined outcome of nonfatal MI, nonfatal stroke, or all-cause mortality; and the combined outcome of nonfatal MI, coronary revascularization, nonfatal stroke, or all-cause mortality.
METHODS
The TRIAD methodology has been described in detail elsewhere.6 Six research centers collaborated with ten managed care health plans (HPs) and 68 provider groups that served approximately 180,000 geographically and ethnically diverse patients with diabetes. Institutional review boards at each participating center approved the study. All participants provided informed consent.
TRIAD enrolled 11,927 patients between July 2000 and August 2001. All were at least 18 years old, not pregnant, community-dwelling, English or Spanish speaking, and continuously enrolled in the HP for at least 18 months prior to the baseline patient survey. Medical record reviews were performed at baseline, HP administrative data were collected for 1999 through 2003, and the National Death Index (NDI) was searched for deaths occurring through 2003. We analyzed data for patients who had type 2 diabetes (excluding those with age at diagnosis under 30 years and treatment with insulin only) and with complete data for the variables investigated (N=7,439).
Across TRIAD’s ten HPs, 1,815 patients (24%) filled at least one prescription for a TZD, 773 (10%) for only rosiglitazone, 711 (10%) for only pioglitazone, and 331 (4%) for more than one TZD. In the seven HPs using both TZDs, 1,159 patients (33%) filled at least one prescription for a TZD, 564 (16%) for only rosiglitazone, 334 (10%) for only pioglitazone, and 261 (7%) for more than one TZD (Figure 1). Patients filling prescriptions for more than one TZD were excluded.
Figure 1. Study Population.
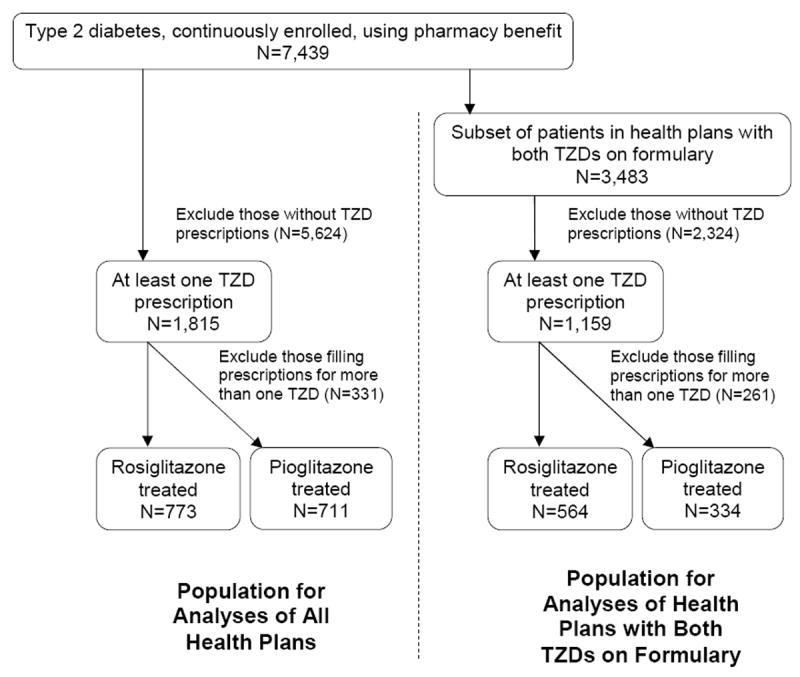
We used HP administrative data to determine TZD exposure and verify drug benefits. Patient surveys and medical record reviews were used to determine if patients were treated with insulin at baseline. Subsequent initiation of insulin was determined from analysis of administrative data. We ascertained nonfatal acute myocardial infarction, stroke, or percutaneous or surgical CV intervention from HP administrative data. We used the following International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes (with any 4th or 5th digit): 410 (acute myocardial infarction), 431 (intracerebral hemorrhage), 433 (occlusion and stenosis of precerebral arteries), and 434 (occlusion of cerebral arteries). Coronary revascularization procedures included operations on vessels of the heart (ICD-9-CM procedure code 36 with any 3rd or 4th digit), coronary artery repair procedures (CPT codes 33500-33572), intracoronary stents, coronary balloon angioplasty or atherectomy (CPT codes 92980-92984, 92995, 92996). Deaths and cause of death were ascertained from NDI.7
We performed time-to-event analyses. Our study window started with the first TZD prescription and ended with an event or censoring. Event dates were the first occurrence of a CV event or procedure after the first TZD prescription was filled. Patients without CV events or procedures were censored at whichever occurred first: the date the last TZD prescription was filled plus the days supply dispensed plus 90 days (to account for any persistent biological effects of the TZD); the date the person disenrolled from the HP; the last date of service recorded in the administrative data; or TRIAD’s administrative data cut-off date. The end of the study window was re-evaluated for each outcome. Because we were concerned that the use of a random effect in the model might not adequately adjust for differences in patients across HPs, we conducted analyses after excluding the three HPs that appeared to have only one TZD on formulary (as evidenced by prescriptions filled for only one TZD). For patients in these three plans, exposure to a specific TZD could not be distinguished from other unmeasured characteristics associated with membership in that HP.
We compared groups using 2-tailed t-tests for continuous variables and χ2 tests for categorical variables. We tested the assumption of proportional hazards with graphical display and examination of the correlations between the ranked failure time variable and the Schoenfeld residuals of the independent variables. We used Cox proportional hazard multivariate models adjusted for baseline age, sex, race/ethnicity, income, history of diabetic nephropathy, history of CVD (transient ischemic attack, cerebrovascular accident, angina pectoris, myocardial infarction, coronary heart disease, congestive heart failure, or peripheral vascular disease), insulin use, and HP. Missing values for age, sex, race/ethnicity, income, and smoking were relatively infrequent (< 15% in all cases) and were imputed using single imputation with the transcan function in S-PLUS (edition 6.1; Insightful, Seattle, WA). Patients’ missing values for other variables used in this study were excluded. Statistical analyses were performed using SAS (version 9.1.3, SAS Institute, Cary, NC).
RESULTS
The first prescription for rosiglitazone was filled in June 1999 and the first prescription for pioglitazone was filled in August 1999. Subsequent uptake of TZD therapy was rapid. The cumulative number of patients with prescriptions for rosiglitazone was approximately 500, 970, 1,250, and 1,400 at one, two, three and four years after the first prescription was filled. For pioglitazone, the cumulative number of patients filling prescriptions was approximately 500, 800, 1,080, and 1,300 at one, two, three and four years.
The TRIAD HPs were ethnically, socio-economically, and geographically diverse. Among the ten HPs, seven had substantial numbers of prescriptions filled for both rosiglitazone and pioglitazone. In two other HPs, pioglitazone accounted for over 99% of TZD prescriptions and in one HP, rosiglitazone accounted for 100% of TZD prescriptions. Patients in the latter 3 HPs differed from each other with respect to demographic characteristics and socioeconomic position, and the differences in the characteristics of rosiglitazone-treated patients and pioglitazone-treated patients were greater when all ten HPs were included in the analyses. Limiting the analyses to the seven HPs with substantial numbers of patients filling prescriptions for both TZDs reduced the differences between rosiglitazone- and pioglitazone-treated patients. (Table 1).
Table 1.
Baseline Characteristics of the Study Population by Treatment Group
| All Health Plans (N = 10) | Health Plans with Both TZDs on Formulary (N = 7) | |||||
|---|---|---|---|---|---|---|
| Rosiglitazone Treated | Pioglitazone Treated | P Value | Rosiglitazone Treated | Pioglitazone Treated | P Value | |
| N | 773 | 711 | 564 | 334 | ||
| Mean age (years (SD)) | 58 (11) | 59 (11) | 0.01a | 59 (12) | 59 (11) | 0.92 |
| Sex (male) (%) | 330 (43) | 346 (49) | 0.02a | 276 (49) | 157 (47) | 0.58 |
| Race/Ethnicity (%) | <.001a | 0.63 | ||||
| Hispanic | 99 (13) | 113 (16) | 95 (17) | 47 (14) | ||
| Black | 170 (22) | 68 (10) | 76 (13) | 40 (12) | ||
| White | 341 (44) | 305 (43) | 246 (44) | 147 (44) | ||
| Asian/PI | 103 (13) | 158 (22) | 103 (18) | 71 (21) | ||
| Other | 60 (8) | 67 (9) | 44 (8) | 29 (9) | ||
| Annual household income (%) | <.001a | 0.29 | ||||
| < $15,000 | 299 (39) | 114 (16) | 122 (22) | 66 (20) | ||
| $15,000 to $40,000 | 221 (29) | 224 (32) | 200 (35) | 106 (32) | ||
| $40,000 to $75,000 | 153 (20) | 226 (32) | 143 (25) | 104 (31) | ||
| > $75,000 | 100 (13) | 147 (21) | 99 (18) | 58 (17) | ||
| History of diabetic nephropathy (%) | 93 (12) | 158 (22) | <.001a | 65 (12) | 53 (16) | 0.06 |
| History of cardiovascular disease (%) | 262 (34) | 238 (33) | 0.86 | 175 (31) | 127 (38) | 0.03a |
| Hypertension (%) | 557 (72) | 518 (73) | 0.73 | 397 (70) | 250 (75) | 0.15 |
| Dyslipidemia (%) | 412 (53) | 412 (58) | 0.07 | 340 (60) | 202 (60) | 0.95 |
| Current smoker (%) | 165 (21) | 125 (18) | 0.07 | 87 (15) | 75 (22) | 0.01a |
| On insulin at baseline (%) | 237 (31) | 176 (25) | 0.01a | 133 (24) | 75 (22) | 0.70 |
| Not on insulin at baseline, but started insulin during study (%) | 274 (35) | 233 (33) | 0.28 | 143 (25) | 94 (28) | 0.36 |
| Mean TZD duration (months (SD)) | 19 (13) | 18 (13) | 0.12 | 19 (14) | 18 (13) | 0.24 |
Statistically significant at 0.05.
In general, rosiglitazone-treated patients were younger, more likely to be female, black and lower income than pioglitazone-treated patients. Rosiglitazone patients were less likely to have a history of diabetic nephropathy but were more likely to be insulin treated than pioglitazone-treated patients.
Table 2 shows the distribution of CV procedures, adverse CV events, and mortality by treatment group. The observed, unadjusted event rates were similar across treatment groups.
Table 2.
Unadjusted Adverse Cardiovascular Events and Mortality by Treatment Group
| All Health Plans (N = 10) | Health Plans with Both TZDs on Formulary (N = 7) | |||||||
|---|---|---|---|---|---|---|---|---|
| Rosia Treated | Piob Treated | P Value | Unadjusted Relative Risk of Rosi vs Pio | Rosi Treated | Pio Treated | P Value | Unadjusted Relative Risk of Rosi vs Pio | |
| N | 773 | 711 | 564 | 334 | ||||
| Nonfatal MI (%) | 17 (2) | 22 (3) | 0.28 | 0.71 | 12 (2) | 8 (2) | 0.79 | 0.89 |
| Coronary Revascularization (%) | 16 (2) | 16 (2) | 0.81 | 0.92 | 15 (3) | 11 (3) | 0.58 | 0.80 |
| Nonfatal MI or Coronary Revascularization (%) | 26 (3) | 30 (4) | 0.39 | 0.80 | 21 (4) | 15 (4) | 0.57 | 0.83 |
| Nonfatal Stroke (%) | 19 (2) | 13 (2) | 0.40 | 1.34 | 16 (3) | 8 (2) | 0.69 | 1.18 |
| Nonfatal MI, Coronary Revascularization, or Nonfatal Stroke (%) | 44 (6) | 42 (6) | 0.86 | 0.96 | 36 (6) | 23 (7) | 0.77 | 0.93 |
| CV Mortality (%) | 7 (1) | 12 (2) | 0.18 | 0.54 | 4 (1) | 3 (1) | 0.76 | 0.79 |
| All-Cause Mortality (%) | 14 (2) | 19 (3) | 0.26 | 0.68 | 10 (2) | 9 (3) | 0.35 | 0.66 |
| Nonfatal MI or All-Cause Mortality (%) | 31 (4) | 38 (5) | 0.22 | 0.75 | 22 (4) | 16 (5) | 0.52 | 0.81 |
| Nonfatal MI, Nonfatal Stroke or CV Mortality (%) | 42 (5) | 43 (6) | 0.61 | 0.90 | 31 (6) | 19 (6) | 0.90 | 0.97 |
| Nonfatal MI, Nonfatal Stroke or All-Cause Mortality (%) | 46 (6) | 48 (7) | 0.53 | 0.88 | 34 (6) | 23 (7) | 0.61 | 0.88 |
| Nonfatal MI, Coronary Revascularization, Nonfatal Stroke or All-Cause Mortality (%) | 54 (7) | 55 (8) | 0.58 | 0.90 | 42 (7) | 29 (9) | 0.51 | 0.86 |
Rosiglitazone
Pioglitazone
Figure 2 shows the adjusted hazard ratios (AHR) for CV procedures, adverse CV events, and mortality for rosiglitazone-treated patients compared to pioglitazone-treated patients after adjusting for age, sex, race/ethnicity, income, history of diabetic nephropathy, history of CVD, insulin use, and HP. Because of potential bias, we performed a sub-analysis excluding patients who filled TZD prescriptions before the baseline survey. We repeated the analyses for all outcomes and there were no differences in the results (not shown). For all CV events, CV and all-cause mortality, we found no statistically significant difference between rosiglitazone-treated patients and pioglitazone-treated patients.
Figure 2. Adjusted* Hazard Ratios among Patients with Type 2 Diabetes Treated with Rosiglitazone or Pioglitazone, TRIAD 1999-2003.
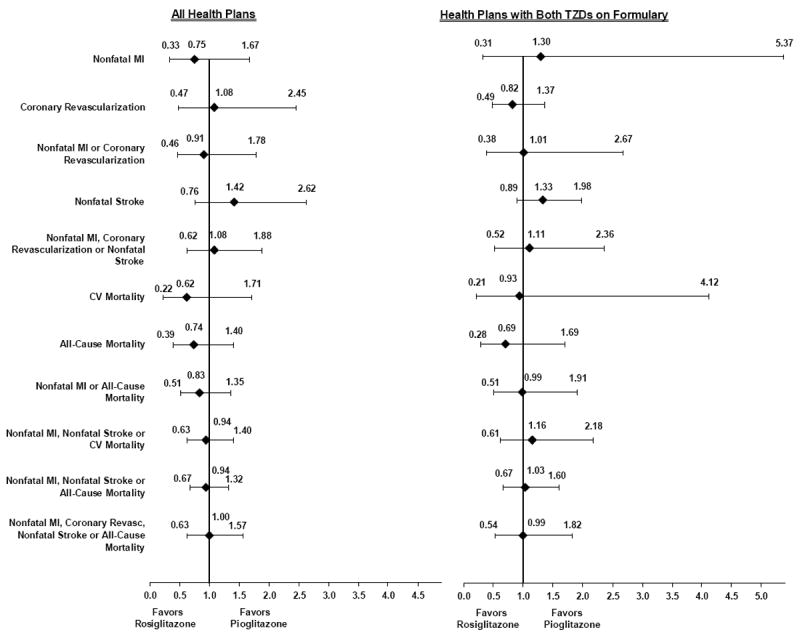
*Adjusted for age, sex, race/ethnicity, income, history of diabetic nephropathy, history of cardiovascular disease (transient ischemic attack, cerebrovascular accident, angina pectoris, myocardial infarction, congestive heart failure, coronary heart disease, peripheral vascular disease), insulin use, and health plan.
DISCUSSION
In this relatively small, prospective, observational study of diabetes care in managed care, we found that CV risk was similar for patients with type 2 diabetes treated with rosiglitazone and pioglitazone and observed no pattern of differential risk.
Previous studies that have directly compared rosiglitazone- to pioglitazone-treated patients have not found consistent, clinically meaningful differences in CV outcomes, except for a trend favoring pioglitazone treatment to rosiglitazone treatment with respect to all-cause mortality. Table 3 summarizes the published literature. A rosiglitazone-to-pioglitazone comparison conducted in 2007 by the manufacturer of pioglitazone focused on acute myocardial infarction after initiating TZD treatment and reported that pioglitazone-treated patients had a reduced risk of hospitalization for myocardial infarction (AHR 0.78, 95% CI 0.63-0.96) and for the composite outcome of nonfatal myocardial infarction and coronary revascularization (AHR 0.85, 95% CI 0.75-0.98).8 This retrospective observational analysis relied on administrative claims data, which reduced the investigators’ ability to adjust for potential confounders. A 2007 study of elderly patients (mean age 75 years) using a nested case-control methodology showed worse outcomes for patients treated with rosiglitazone with respect to acute myocardial infarction and all-cause mortality.9 However, the study was not powered to directly compare rosiglitazone- to pioglitazone-treated patients, and indeed, compared rosiglitazone- and pioglitazone-treated patients to patient treated with other oral antidiabetic medications. In addition, patients receiving insulin were excluded from the analysis. A 2008 study that compared rosiglitazone- and pioglitazone-treated Medicare beneficiaries also relied on claims and enrollment data, and found reduced risk of all-cause mortality for the pioglitazone-treated patients (AHR 0.87, 95% CI 0.79-0.95), but no difference with respect to myocardial infarction or stroke.10 A recent retrospective study of patients using oral anti-diabetic agents that excluded patients using insulin or multiple oral anti-diabetic agents found no significant difference between rosiglitazone and pioglitazone for the composite outcome of myocardial infarction or coronary revascularization or for all-cause mortality.11 Another recent study comparing rosiglitazone to pioglitazone treatment drew subjects from a single HMO treated within a single health system, included time-varying medication use and adjusted for propensity to treat based on medication history and clinical function, and estimated household income from the census.12 It found that pioglitazone treatment was associated with a reduction in all-cause mortality (AHR 0.60, 95% CI 0.42-0.96) compared to rosiglitazone treatment but found no significant differences with respect to acute myocardial infarction or stroke.12 A 2009 study of patients 66 years of age and older compared those initiating rosiglitazone and pioglitazone and found no significant difference in the risk of acute myocardial infarction. The CV mortality outcome in this study included heart failure and was therefore not directly comparable to ours. The study found that pioglitazone-treated patients had lower risk of all-cause mortality than rosiglitazone-treated patients (AHR 0.86, 95% CI 0.75-0.98). Besides excluding younger patients, this study excluded patients receiving insulin and included residents of long-term care facilities.13
Table 3.
Adjusted Hazard Ratiosa (Rosib vs. Pioc) for Adverse Cardiovascular Events in TRIAD Compared to the Literature
| Outcome | TRIAD | Gerrits8 | Lipscombe9 | Winkelmayer10 | Pantalone11 | Habib12 | Juurlink13 |
|---|---|---|---|---|---|---|---|
| Nonfatal Myocardial Infarction (MI) | NS | Favors PIO AHR 0.78 (0.63-0.96) | Favors PIO vs other oral antidiabetics | NS | — | NS | NS |
| Coronary Revascularization | NS | — | — | — | — | — | — |
| Nonfatal MI or Coronary Revascularization | NS | Favors PIO AHR 0.85 (0.75-0.98) | — | — | NS | — | — |
| Nonfatal Stroke | NS | — | — | NS | — | NS | NS |
| Cardiovascular Mortality | NS | — | — | — | — | — | — |
| All-cause Mortality | NS | — | Favors PIO vs other oral antidiabetics | Favors PIO AHR 0.87 (0.79-0.95) | NS | Favors PIO AHR 0.60 (0.42-0.96) | Favors PIO AHR 0.86 (0.75-0.98) |
| Nonfatal MI or All-cause Mortality | NS | — | — | — | — | — | — |
| Nonfatal MI, Nonfatal Stroke or Cardiovascular Mortality | NS | — | — | — | — | — | — |
| Nonfatal MI, Nonfatal Stroke, or All-cause Mortality | NS | — | — | — | — | — | — |
Adjusted for age, sex, race/ethnicity, income, history of diabetic nephropathy, history of CVD (transient ischemic attack, cerebrovascular accident, angina pectoris, myocardial infarction, congestive heart failure, coronary heart disease, or peripheral vascular disease), insulin use, and managed care health plan.
Rosiglitazone
Pioglitazone
NS = Not significant
— = Not reported
Our analyses directly compared rosiglitazone-treated patients to pioglitazone-treated patients. All were patients with type 2 diabetes enrolled in TRIAD’s managed care HPs and were geographically, ethnically, and socioeconomically diverse. We included younger patients and those treated with insulin and/or other oral anti-diabetic agents. We used multiple sources of data including survey responses, medical record reviews, administrative data, and NDI. We performed time-to-event analyses and adjusted for potential confounders and reported the full range of outcomes previously reported in the literature. Data collection was completed before publication of any reports of the favorable or unfavorable impact of TZDs on CV events which might have impacted prescribing patterns.
Despite these strengths, our analyses had several limitations. First, if a patient’s HP coverage included a limit on pharmacy benefits, patients may have filled prescriptions that we did not detect. We expect this potential problem to be minor because two large HPs submitted pharmacy utilization, not just claims, capturing prescriptions that were not covered. In addition, most HPs included denied claims, further capturing prescriptions that were not covered. Second, TRIAD participants showed a wide range of length of TZD exposure and number of TZDs used, potentially confounding the results. We mitigated this risk by excluding patients who used multiple TZDs. Third, using a model with a random effect for HP might not adequately adjust for differences in TZD prescribing patterns and population characteristics across HPs. We addressed this potential bias by performing sub-analyses limited to HPs with substantial numbers of prescriptions of both rosiglitazone and pioglitazone. Fourth, it is possible that those with a recent CV event were less likely to participate in TRIAD; however, this is unlikely to cause a bias in rosiglitazone vs. pioglitazone treatment. Finally, we acknowledge that our sample size was relatively small. We estimate that we had 80% power to detect a 5% difference in absolute risk between treatment groups. Our overall event rates of 7-9% were, however, much higher than the event rate of < 1% reported in the meta-analysis by Nissen and Wolski.1 As recently suggested by Hennekens and DeMets, the meta-analysis may be most useful for hypothesis generation. Value of meta-analyses is dependent on the quality and comparability of the data analyzed, and large and long-term, prospective randomized controlled clinical trials will be needed to conclusively demonstrate small to moderate harm.14
In conclusion, in this relatively small, prospective, observational study, we found no statistically significant differences in CV risk for rosiglitazone- compared to pioglitazone-treated patients. There did not appear to be clinically meaningful pattern of differences in CV outcomes for rosiglitazone- versus pioglitazone-treated patients.
KEY POINTS.
In this relatively small, prospective, observational study, we found no statistically significant differences in cardiovascular risk for rosiglitazone-compared to pioglitazone-treated patients.
There does not appear to be a pattern of clinically meaningful differences in cardiovascular outcomes for rosiglitazone- versus pioglitazone-treated patients.
Acknowledgments
FUNDING/SUPPORT: This study was jointly funded by Program Announcement number 04005 from the Centers for Disease Control and Prevention (Division of Diabetes Translation) and the National Institute of Diabetes and Digestive and Kidney Diseases. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding organizations. Significant contributions to this study were made by members of the Translating Research Into Action for Diabetes (TRIAD) Study Group. We acknowledge the participation of our health plan partners.
Footnotes
FINANCIAL DISCLOSURES: Dr. Herman has served as a consultant to and received honoraria from GlaxoSmithKline. The other authors report no conflicts of interest.
References
- 1.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761.NEJMoa072761 [DOI] [PubMed] [Google Scholar]
- 2.Mele J. Avandia® (rosiglitazone maleate), GlaxoSmithKline, NDA 21-071 supplement 022, FDA meta-analysis; Joint Meeting of Metabolic & Endocrine Advisory Committee and Drug Safety & Risk Management Advisory Committee; Jul 30, [November 6, 2007]. Available at: www.fda.gov/ohrms/dockets/ac/07/slides/2007-4308s1-05-fda-mele.ppt. [Google Scholar]
- 3.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298(10):1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 4.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 5.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370(9593):1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 6.The Translating Research Into Action for Diabetes (TRIAD) study: a multicenter study of diabetes in managed care. Diabetes Care. 2002;25(2):386–389. doi: 10.2337/diacare.25.2.386. [DOI] [PubMed] [Google Scholar]
- 7.McEwen LN, Kim C, Haan M, et al. Diabetes reporting as a cause of death: results from the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2006;29(2):247–253. doi: 10.2337/diacare.29.02.06.dc05-0998.29/2/247 [DOI] [PubMed] [Google Scholar]
- 8.Gerrits CM, Bhattacharya M, Manthena S, Baran R, Perez A, Kupfer S. A comparison of pioglitazone and rosiglitazone for hospitalization for acute myocardial infarction in type 2 diabetes. Pharmacoepidemiol Drug Saf. 2007;16(10):1065–1071. doi: 10.1002/pds.1470. [DOI] [PubMed] [Google Scholar]
- 9.Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 2007;298(22):2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmayer WC, Setoguchi S, Levin R, Solomon DH. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch Intern Med. 2008;168(21):2368–2375. doi: 10.1001/archinte.168.21.2368. [DOI] [PubMed] [Google Scholar]
- 11.Pantalone KM, Kattan MW, Yu C, et al. The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta Diabetol. 2009;46(2):145–154. doi: 10.1007/s00592-008-0090-3. [DOI] [PubMed] [Google Scholar]
- 12.Habib ZA, Tzogias L, Havstad SL, et al. Relationship between thiazolidinedione use and cardiovascular outcomes and all-cause mortality among patients with diabetes: a time-updated propensity analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):437–447. doi: 10.1002/pds.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juurlink DN, Gomes T, Lipscombe L, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ. 2009;339:b2942. doi: 10.1136/bmj.b2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennekens CH, DeMets D. Commentary: The need for large-scale randomized evidence without undue emphasis on small trials, meta-analyses, or subgroup analyses. JAMA. 2009;302(21):2361–2362. doi: 10.1001/jama.2009.1756. [DOI] [PubMed] [Google Scholar]


