Abstract
Infection of MPC-11 mouse plasmacytoma cells by vesicular stomatitis virus results in 30 to 35% reduction in [35S]methionine incorporation into total proteins within 30 min postinfection. By 6 h postinfection, total protein synthesis is reduced by 80 to 90%. However, even by 30 min postinfection, a differential suppression of the synthesis of individual host protein is observed. The synthesis of the immunoglobin G (IgG) heavy chain (H), and, in particular, the synthesis of IgG light chain (L), is considerably more resistant to vesicular stomatitis virus-induced inhibition than is the synthesis of the non-IgG proteins as a whole; e.g., when the synthesis of non-IgG proteins was reduced by 41%, the synthesis of the H and L chains was reduced by 28 and 7%, respectively. Furthermore, these alterations in the relative synthesis of the L chain, H chain, and non-IgG are comparable to the alterations previously observed in uninfected MPC-11 cells when the overall rate of polypeptide chain initiation was selectively reduced (D.L. Nuss and G. Koch, 1976). These results are discussed in terms of the strategy of virus-directed suppression of host mRNA translation.
Full text
PDF

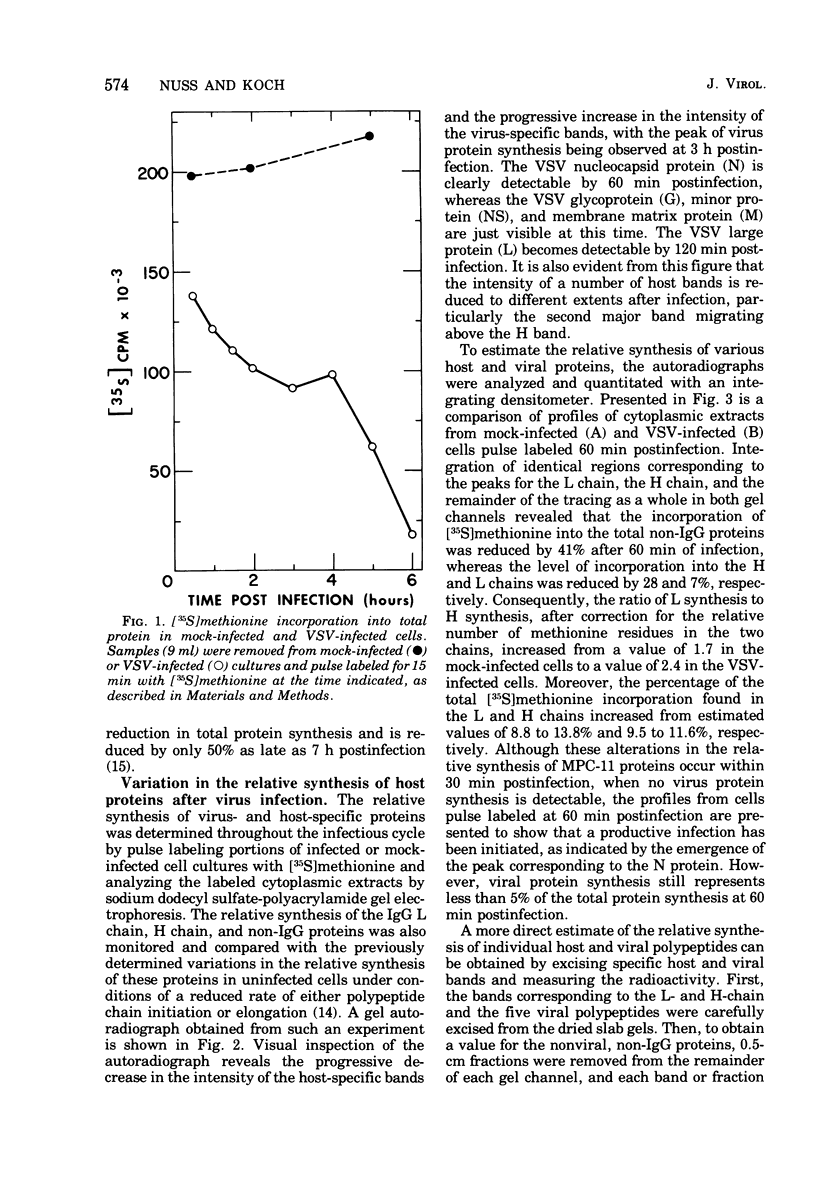
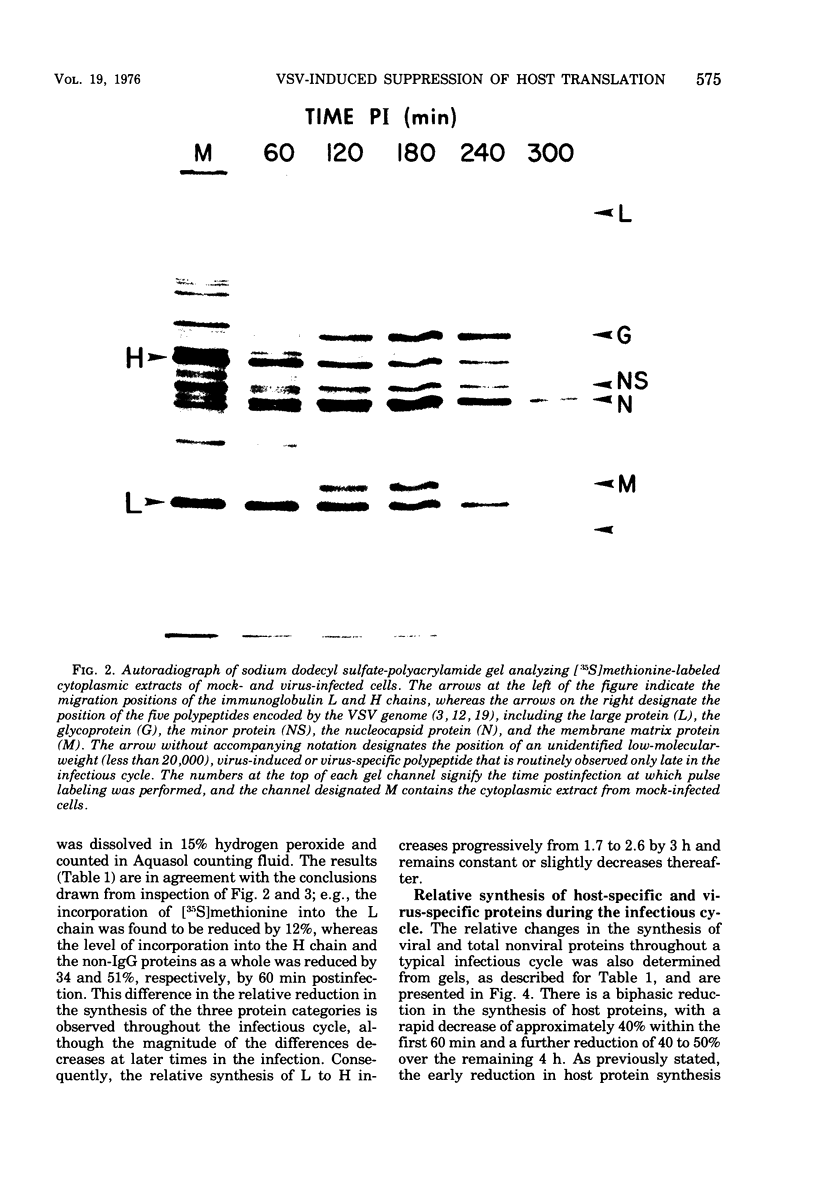
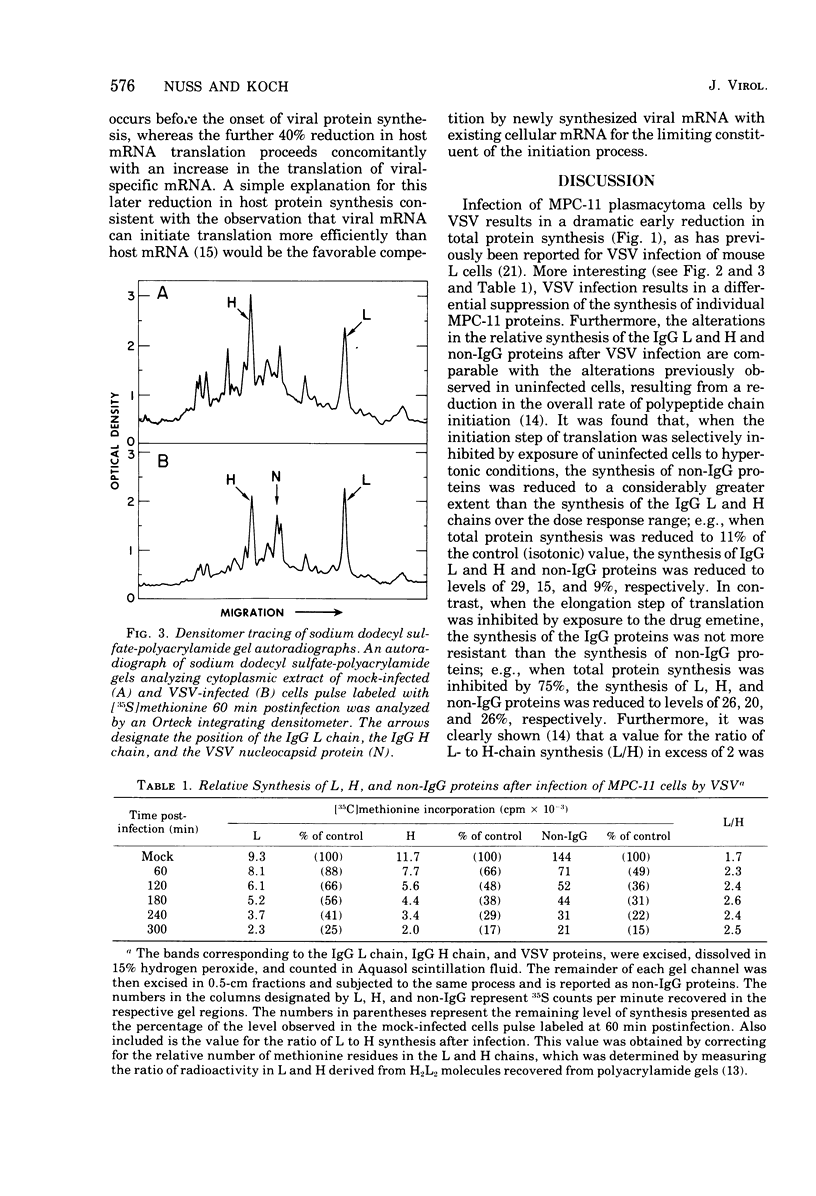

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature. 1974 Oct 4;251(5474):385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Selective suppression of cellular protein synthesis in BHK-21 cells infected with rabies virus. J Virol. 1975 Nov;16(5):1351–1354. doi: 10.1128/jvi.16.5.1351-1354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Differential inhibition of vesicular stomatitis virus polypeptide synthesis by hypertonic initiation block. J Virol. 1975 Jan;17(1):283–286. doi: 10.1128/jvi.17.1.283-286.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Variation in the relative synthesis of immunoglobulin G and non-immunoglobulin G proteins in cultured MPC-11 cells with changes in the overall rate of polypeptide chain initiation and elongation. J Mol Biol. 1976 Apr 15;102(3):601–612. doi: 10.1016/0022-2836(76)90337-5. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Translational control of immunoglobulin synthesis. I. Repression of heavy chain synthesis. J Mol Biol. 1973 Aug 15;78(3):505–516. doi: 10.1016/0022-2836(73)90471-3. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J Virol. 1970 Oct;6(4):476–484. doi: 10.1128/jvi.6.4.476-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Wagner R. R. Action of interferon: kinetics and differential effects on viral functions. J Virol. 1970 Oct;6(4):421–429. doi: 10.1128/jvi.6.4.421-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]