Abstract
Background
Cannabinoids inhibit intestinal motility via presynaptic cannabinoid receptor type I(CB1) in enteric neurons while cannabinoid receptor type II (CB2) receptors are located mainly in immune cells. The recently deorphanized G-protein-coupled receptor, GPR55, has been proposed to be the “third” cannabinoid receptor. Although gene expression of GPR55 is evident in the gut, functional evidence for GPR55 in the gut is unknown. In this study, we tested the hypothesis that GPR55 activation inhibits neurogenic contractions in the gut.
Methods
We assessed the inhibitory effect of the atypical cannabinoid O-1602, a GPR55 agonist, in mouse colon. Isometric tension recordings in colonic tissue strips were used from either wild type, GPR55−/− or CB1−/−/CB2−/−knock-out mice.
Results
O-1602 inhibited the electrical field-induced contractions in the colon strips from wild type and CB1−/−/CB2−/− in a concentration–dependent manner, suggesting a non-CB1/CB2-receptor mediated prejunctional effect. The concentration–dependent response of O-1602 was significantly inhibited in GPR55−/− mice. O-1602 did not relax colonic strips pre-contracted with high K+ (80 mmol/l), indicating no involvement of Ca2+ channel blockade in O-1602–induced relaxation. However, 10 μmol/l O-1602 partially inhibited the exogenous acetylcholine (10 μmol/l) –induced contractions. Moreover, we also assessed the inhibitory effects of JWH 015, a CB2/GPR55 agonist on neurogenic contractions of mouse ileum. Surprisingly, the effects of JWH015 were independent of the known cannabinoid receptors.
Conclusion
These findings taken together suggest that activation of GPR55 leads to inhibition of neurogenic contractions in the gut, and are predominantly prejunctional.
Keywords: GPR55, cannabinoid receptors, JWH015, colon contractility, O-1602, ileum
Introduction
The biologically active constituents of Cannabis sativa (marijuana), cannabinoids, have been used or abused for decades for their psychoactive properties. Among over 70 distinct cannabinoid substances in marijuana, Δ9-tetrahydrocannabinol (THC),the main psychoactive ingredient[1], and cannabidiol are the most prevalent and best investigated[2]. Two seven-transmembrane G-protein coupled receptors: type 1 (CB1) cannabinoid receptors mediate most of the psychoactive effects and type 2 (CB2) receptors mediate immunological effects of these phytocannabinoids. Since the studies of Wagner and coworkers on endothelial anandamide receptor-mediated mesenteric vasodilation[3], it has been postulated that a non-CB1/ non-CB2 cannabinoid receptor or “abnormal cannabidiol (ABN-CBD)” receptor exists[4] and that this receptor could mediate effects of phytocannabinoids and endocannabinoids. Following the identification and cloning of novel human G-protein-coupled receptor 55 (GPR55)[5], several cannabinoid ligands were shown to bind to GPR55[6], suggesting that it could be a novel cannabinoid receptor. Based on [35S]GTPγS assays performed in transfected hGPR55-HEK293 cells, ABN-CBD and its analog O-1602, in which the pentyl side chain was shortened to a methyl group[4], were reported as GPR55 agonists[7-9]. In addition, JWH015 (1-propyl-2-methyl-3-( -naphthoyl)indole) is also considered to be a CB2-/GPR55 – receptor agonist[10] These compounds represent potentially useful pharmacological tools to investigate GPR55 receptors in different physiological systems.
The effects of cannabinoid receptor activation and the role of endocannabinoids in the gastrointestinal tract are now being realized as fairly extensive. CB1 receptor activation reduces intestinal motility, alleviates pain, and affects transient lower esophageal sphincter relaxations and emesis [11, 12]. Although the functional role of CB1 receptors has been extensively studied in the gastrointestinal tract, much less is known about the effects of the recently identified GPR55. Utilizing northern blot analysis or quantitative polymerase chain reaction methods that detect the receptor at the message level, high levels of human GPR55 mRNA transcripts have been found in brain regions and in several peripheral tissues, such as the ileum[5, 13]. Rat and mouse GPR55 homologs have also been detected in peripheral tissues including jejunum, ileum, and colon [8, 13, 14]. Recently Lin and co-workers have reported that GPR55 ligands could normalize lipopolysaccharide –induced motility disturbances in the gut [15]. However, the mechanisms by which gut motility is influenced by GPR55 receptors have yet to be verified in functional assays. Since cannabinoids are known to inhibit neurogenic contractions in the gut, we studied the effect of the reported GPR55 agonists, O-1602, an atypical cannabinoid, and JWH015 on the neurogenic (electrical field stimulated) contractions in mouse colon strips and also intended to determine the mechanisms of action of O-1602. In the present study, we used pharmacological and genetic manipulation approaches to test the hypothesis that activation of GPR55 receptors will result in inhibition of the neurogenic contractions in the mouse intestine.
Materials and Methods
Animals
Male Swiss-Webster mice (Harlan, Indianapolis, IN) as well as male GPR55 (−/−), CB1(−/−)/CB2(−/−) double knock -out and (+/+)mice (Transgenic colony facility, Dept, of Pharmacology& Toxicology, Virginia Commonwealth University, Richmond, VA) backcrossed onto a C57BL/6J background served as subjects. These mice weighed from 25 to 30 g and were housed 5-6 per cage in a vivarium maintained at 22 ± 2°C on a 12-h light/dark cycle. Food and water were available ad libitum. The mice were brought to a test room (22 ± 2°C, 12-h light/dark cycle), marked for identification, and allowed 18 h to recover from transport and handling. Protocols and procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University Medical Center and comply with the recommendations of the International Association for the Study of Pain.
Isometric Tension Recording
One-centimeter segments of distal colon (approximately 1 cm proximal to the anus) were dissected, flushed off their contents, and trimmed of mesentery. Preparations were suspended in the axis of longitudinal muscle tied to a glass hook under 1g of passive tension in 15 ml of siliconized organ baths containing Krebs solution (in mmol/l: 118 NaCl, 4.6 KCl, 1.3 NaH2PO4, 1.2 MgSO4, 25 NaHCO3, 11 glucose, and 2.5 CaCl2) maintained at 37°C and bubbled with 95% O2 and 5% CO2, and tissues were allowed to equilibrate for 60 min before start of experiments, with Krebs solution changed every 15 min. Isometric contractions were recorded by a force transducer (GR-FT03; Radnoti, Monrovia, CA) connected to a personal computer using Acqknowledge382 software (BIOPAC Systems, Inc., Santa Barbara, CA).
Neurogenic Contractions by Transmural Electrical Field Stimulation
The gastrointestinal smooth muscle strips can be contracted by inducing acetylcholine (Ach) release by electrical field stimulation of the enteric nervous system. Hence, electrical field stimulation (EFS; 50 V, 7.5 Hz, unless stated otherwise) was applied through concentric electrodes over longitudinal muscles to produce neurogenic contractions. Cumulative doses of the atypical cannabinoid, O-1602 [100 nmol/l to 30 μmol/l] or JWH 015 [10 nmol/l to 10 μmol/l] at 0.5 log unit increments were added over the EFS contractions to determine their inhibitory effects on the neurogenic responses. The amplitude of initial contractions stimulated by electrical field before adding any drug was considered 100% contraction and related to the subsequent inhibitory effects of either O-1602 or JWH 015.
Experimental Protocol for determination of mechanisms of action of GPR55 agonists
To determine whether the site of action of the agonists is neuronal or postjunctional, colonic tissue strips were contracted by cumulative administration of exogenous ACh [10 nmo/l to 30 μmol/l], instead of field stimulation, and the concentration –response curves of ACh were analyzed in the presence and absence of either O-1602 (10 μmol/l) or JWH 015 (3 μmol/l).To assess the involvement of inhibition of Ca2+ influx via voltage-gated Ca2+ channels as possible mechanism in the relaxant effect of O-1602, we also assessed the concentration–dependent relaxation induced by O-1602 on colonic tissue strips precontracted by depolarizing solution of high extracellular K+ (80 mM), which activates voltage-gated Ca2+ channels and also excludes the driving force of the K+ currents. The osmolarity of the high K+ physiological saline solution was maintained by replacing equivalent moles of KCl for NaCl in Krebs solution. Moreover, the cannabinoid receptor subtype involved in the effects of O-1602 and JWH015 was elucidated by performing the electrical field stimulation experiments in intestinal strips from mice that were genetically deleted of CB1, CB2, both CB1 and CB2 or GPR55 receptors.
Drugs Used
Stock solutions of O-1602 from Organix Inc. (Worburn, MA) dissolved in ethanol (35-70%), while rimonabant and JWH 015 were from NIDA (Rockville, MD) and Tocris (Minneapolis, MN), respectively. Lysophosphatidylinositol (LPI), acetylcholine chloride and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis
Data are presented as mean ± S.E.M. O-1602- or JWH 015– induced relaxation is expressed as 100 minus the percentage of the initial precontraction to either electrical field or exogenous ACh. The data were analyzed by Prism (GraphPad Software Inc., San Diego,CA) employing appropriate statistical tools. Means of different groups were analyzed by Student’s unpaired t test, one-way ANOVA or two-way ANOVA with Bonferroni post hoc test. Student’s paired t test or two-way repeated measures ANOVA with Bonferroni post hoc test was used when comparisons were made between control and drug treatments in the same preparation. p<0.05 was considered statistically significant. Individual concentration-response curves of O-1602 or JWH 015 were subjected to nonlinear regression analysis to determine EC50, and data are expressed as pD2 (negative logarithm of the molar concentration of the agonist required to produce half-maximal response).
Results
Inhibitory effect of the atypical cannabinoid O-1602 on neurogenic contractions in mouse colon
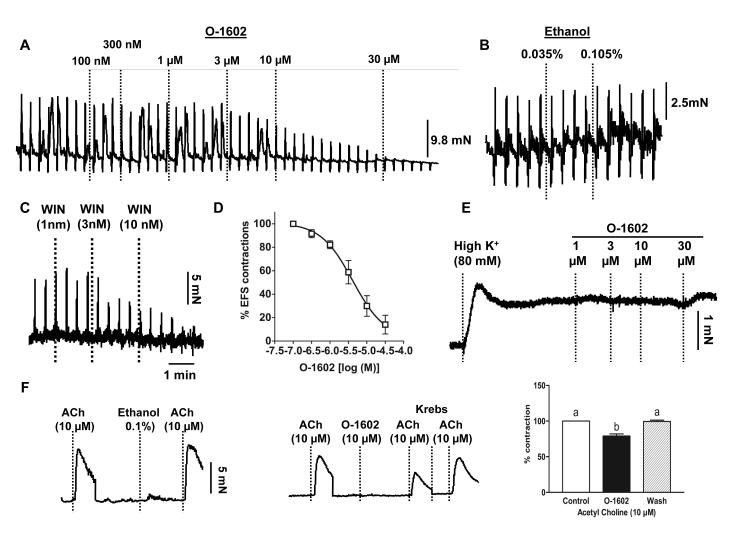
In spite of the fact that the gut expresses GPR55 receptors [13, 16], there has been no evidence of its functional relevance. Since endocannabinoids and other cannabinomimetics are known to decrease neurogenic contractions in the gut by inhibiting ACh release from enteric neurons[17, 18], we determined whether the atypical cannabinoid, O-1602, considered to be a GPR55 agonist[7-9], can also inhibit the neurogenic contractions in mouse colon. As demonstrated in the representative tracing in Fig. 1A, electrical field stimulation –induced depolarization of enteric neurons produced muscle contractions, due to ACh release from these neurons. The atypical cannabinoid O-1602 inhibited these neurogenic contractions concentration – dependently (Fig. 1A&D).The pD2 and Emax values of O-1602 for its inhibitory effect were 5.3 ± 0.08 and 89 ± 4%, n=4, respectively. The vehicle, ethanol (even at maximal final concentration of 0.1%), did not affect the electrical field –stimulated contractions (Figure 1B). As a positive control, we confirmed neurogenic contractions by the inhibitory effect of the selective CB1 agonist, WIN 55212-2 on the EFS-evoked contractions (Fig. 1C). O-1602 did not relax the colonic tissues precontracted by high K+ (80 mM) (Fig. 1E), confirming the lack of effect of O-1602 on Ca2+ influx –induced contractions. However, O-1602 (10 μM) but not ethanol has a significant but small (21 ± 3%) inhibitory effect on the exogenous acetylcholine – induced contractions (Figure 1F). Therefore, the site of action of the atypical cannabinoid O-1602 appears to be predominantly presynaptic and partially postjuctional (only at high concentrations).
Figure 1. Effect of the atypical cannabinoid O-1602 on the longitudinal tissue preparations of mouse colon.
A) Representative tracing showing that O-1602 (100nM to 30μM) concentration dependently inhibited the neurogenic contractions (Electrical field –induced) in the distal colon and B) Ethanol alone, even at the maximum final concentration of 0.105%, did not affect the electrical field –induced contractions; C) Representative tracing demonstrates that the EFS- induced contractions were neurogenic as a selective CB1 agonist, WIN 55212-2 completely inhibited the contractions potently at 10 nmol/l concentration; D) Concentration –response (inhibition of EFS-evoked contractions) curve of O-1602; E) Colonic tissue -precontracted by high K+ (80 mM) was not relaxed by O-1602 suggesting no direct effect on depolarization – induced contraction of smooth muscle; F) Effect of ethanol (0.1%) alone (left), O-1602 (10 μM) (middle) on exogenous acetylcholine –induced contractions and the corresponding bar graph (right), data analyzed by repeated measures one way – ANOVA, n=3.
Inhibitory effect of the atypical cannabinoid O-1602 is independent of CB1- or CB2– but dependent on GPR55 -receptors
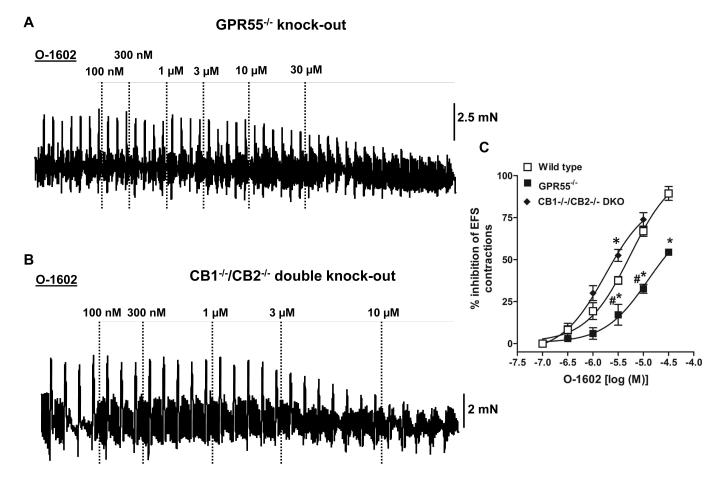
We next evaluated whether O-1602-induced functional effects in the mouse colon are mediated through GPR55 receptors. Although the so-called abnormal-cannabidiol receptor antagonist O-1918 [19] possesses GPR55 receptor antagonist properties[20], this compound lacks selectivity as it also antagonizes GPR18[21] and BK(Ca) channels[22]. Therefore, we tested O-1602 over electrical field stimulated –neurogenic contractions in colon tissue strips from GPR55−/− knock-out mice. O-1602-induced inhibition of neurogenic contractions was significantly reduced in the absence of GPR55 receptors (Fig. 2A). The concentration – response curve of O-1602 in the GPR55−/− tissue showed a significant rightward shift compared with colon from wild type mice. The pD2 and Emax values of O-1602 for its inhibitory effect were 5.3 ± 0.08 and 89 ± 4% in wild type; 4.9 ± 0.2 and 53 ± 2% in GPR55−/−, respectively (n=3-4 in each group). Since some O-1602 effects at higher concentrations appeared to be GPR55-independent, we evaluated the involvement of CB1 and CB2 cannabinoid receptors as well. Therefore, we tested the inhibitory effect of O-1602 over electrical field stimulation – induced neurogenic contractions in colon tissue strips from CB1−/−/CB2−/− double knock-out mice. As demonstrated in Fig. 2B, O-1602 maintained its inhibitory actions on neurogenic contractions in spite of the simultaneous absence of both CB1 and CB2 receptors. The concentration –response curves of O-1602 were not significantly different between wild type and the CB1−/−/CB2−/− double knock-out mice except at the concentration of 5 μmol/l(Fig. 2C). The pD2 and Emax values of O-1602 for its inhibitory effect were 5.9 ± 0.1 and 73 ± 4% in CB1−/−/CB2−/− double knock-out mice, respectively (n=3-4 in each group). Notably, the CB1−/−/CB2−/− double knock-out group shows some enhanced response in relation to wild-type mice at least at the concentration of 5 μmol/l, which could be due to some compensatory effects (enhanced responsiveness of GPR55 receptors) to the lack of both cannabinoid receptors.
Figure 2. Effect of O-1602 on neurogenic contractions in colon from knock-out mice.
Representative tracings that demonstrate the inhibitory effect over neurogenic contractions in mouse colon from (A) GPR55−/− and (B) CB1−/−/CB2−/−double knock-out mice: Right panel displays the corresponding graphs showing concentration –response (inhibition of contraction) curves of O-1602 in GPR55−/− and CB1−/−/CB −/−2double knock-out mice. The potency and efficacy of O-1602 was significantly reduced with the deletion of GPR55 but not with the deletion of both CB1/CB2 receptors vs wild type. The concentration –response curves were subjected to non –linear regression for calculation of pD2 values. Data are mean ±S.E. n= 3-4. Data were analyzed by two-way ANOVA followed by Bonferroni’s posttest.*P<0.05 vs wild type; #P<0.05 vs CB1−/−/CB2−/− double knock-out
Inhibitory effect of JWH 015, a CB2/GPR55 agonist on neurogenic contractions in mouse ileum
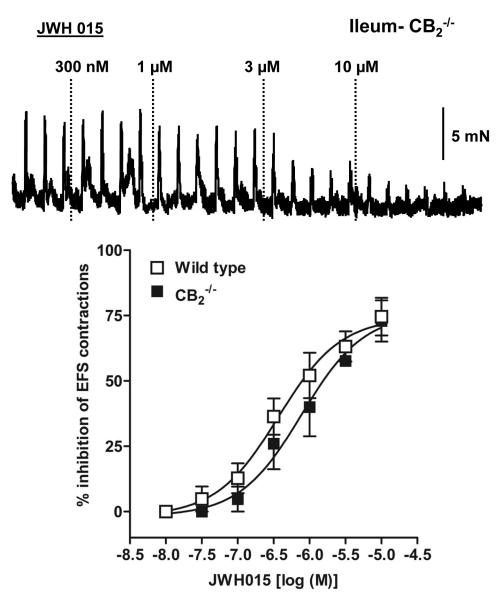
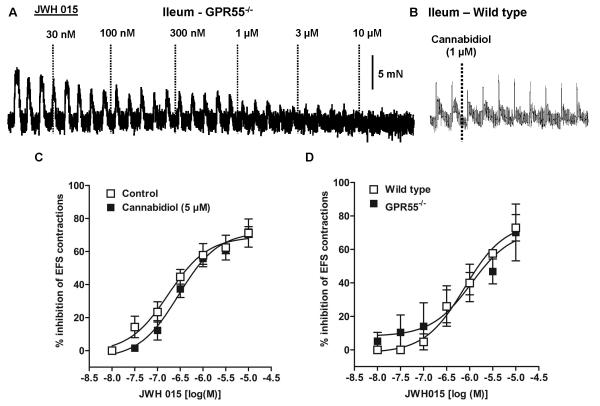
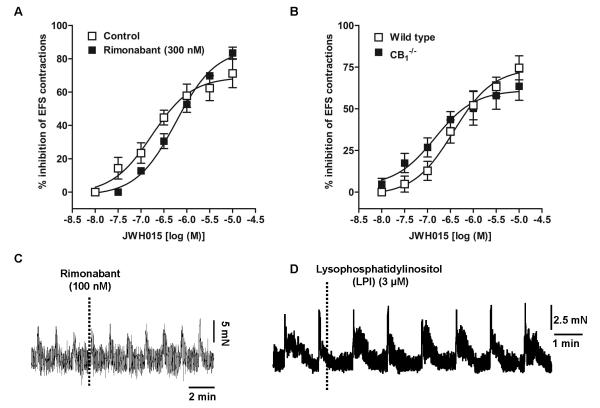
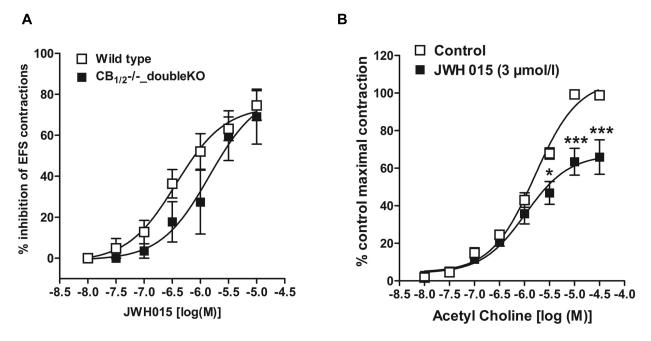
CB2 receptors have been found predominantly in the peripheral immune system and DRG cells while their role in intestinal contractility is not known in normal mice. Since JWH 015 has inhibitory effects on neurogenic contractions in guinea pig ileum[23], we determined whether GPR55 receptors contribute to the effects of JWH015 in mouse ileum. Compared to the wild type mice, the inhibitory effect of JWH 015 on the neurogenic contractions was not significantly affected by the absence of either CB2 (Fig. 3), GPR55 (Fig. 4A & D), CB1 (Fig. 5B) or CB1/2 (Fig 6A) receptors. Moreover, pharmacological experiments using antagonists of CB1, rimonabant (300 nmol/l) (Fig 5A) or GPR55, cannabidiol (5 μmol/l) (Fig 4C) showed no significant difference compared to the corresponding controls. The respective pD2 and Emax values of JWH 015 for its inhibitory effect are given in table 1. Since the inhibitory effects of JWH 015 were independent of cannabinoid- and GPR55- receptors, we assessed whether JWH015 directly affects muscarinic –contractions by determining its effects on exogenous acetylcholine-induced concentration–dependent contractions. Preincubation of JWH 015 (3 μmol/l) significantly reduced the Emax of acetyl choline–induced contractions from 107 ± 3% to 67 ± 4%, without affecting pD2 values (5.8 ±0.1 vs 6.0±0.1; Fig 6B) suggesting an antagonistic effect on muscarinic receptors. However, it is not clear whether the site of action is at receptor level or at some downstream signaling molecule, which needs further investigations.
Figure 3. Effect of JWH015, a CB2/GPR55 agonist, on neurogenic contractions in mouse ileum.
Representative tracings that demonstrate the inhibitory effect over neurogenic contractions in mouse colon from CB2−/− knock-out mice. Bottom panel represents comparison of concentration –response curves of JWH015 between wild type (open squares) and CB2−/− (filled squares) mice. The pD2 values were calculated by subjecting the dose –response curves to non-linear regression analysis. Each data point represents mean ± SE, analyzed by two-way ANOVA followed by Bonferroni’s posttest, n=3-4.
Figure 4. Effect of JWH015, a CB2/GPR55 agonist, on neurogenic contractions in ileum from knock-out mice.
Representative tracings that demonstrate the inhibitory effect over neurogenic contractions in mouse colon from GPR55−/− knock-out mice(A) and lack of effect of cannabidiol (1 μM), considered to be a GPR55 antagonist, on EFS-contractions of ileum from wild type mice(B). Bottom panel represents comparison of concentration – response curves of JWH015 in C) wild type control (open squares) vs wild type-in the presence of cannabidiol(filled circles) and D) wild type (open squares) vs GPR55−/− (filled circles) mice. The concentration –response curves were subjected to non –linear regression for calculation of pD2 values. Each data point represents mean ± SE, analyzed by two-way ANOVA followed by Bonferroni’s posttest, n=3-5.
Figure 5. Concentration –dependent response curves of JWH015, a CB2/GPR55 agonist, on neurogenic contraction inhibition in mouse ileum.
A) control (open squares) vs in the presence of rimonabant (300 nM), a CB1 receptor antagonist (filled squares) in ileum strips from wild type mice and B) comparison between wild type (open squares) and CB1−/− (filled squares). The concentration –response curves were subjected to non – linear regression for calculation of pD2 values. Each data point represents mean ± SE, analyzed by two-way ANOVA followed by Bonferroni’s posttest, n=3-4. Bottom panel displays representative tracing showing lack of effect of rimonabant (100 nM) (C) or lysophosphatidylinositol (3 μM) (D), considered to be an endogenous ligand for GPR55 receptors, on neurogenic contractions of wild –type mouse ileum.
Figure 6.
A) Concentration –dependent response curves of JWH015, a CB2/GPR55 agonist, on neurogenic contraction inhibition in ileum from wild type (open squares) vs CB1−/−/CB2−/− double knock out (filled squares) mice. B) Effect of JWH015 on exogenous acetylcholine –induced contractions of ileum from wild type mice: control (open squares) vs in the presence of JWH015 (3 μmol/l) (filled squares) . Each data point represents mean ± SE, analyzed by two-way ANOVA followed by Bonferroni’s posttest, n=3. *P<0.05, ***P<0.001 considered significant vs control (in the absence of JWH015).
Table 1.
Potency and efficacy parameters of JWH015 in mouse ileum
| JWH 015 |
||
|---|---|---|
| Group | pD2 | Emax (%) |
| Wild type | 6.4 [6.7 - 6.1] | 74 ± 5 |
| CB2−/− | 6.1 [6.5 - 5.7] | 76 ± 7 |
| GPR55−/− | 6.0 [6.7 - 5.2] | 71 ± 13 |
| Wild type + Cannabidiol | 6.5 [6.7 - 6.3] | 72 ± 4 |
| CB1−/− | 6.8 [7.3 - 6.3] | 61 ± 5 |
| Wild type + Rimonabant | 6.2 [6.4 - 6.0] | 86 ± 3 |
| CB1−/−/CB2−/− Double KO | 5.8 [6.4 - 5.3] | 81 ± 13 |
| CB1−/− + SR144528 | 6.0 [6.4 - 5.7] | 61 ± 6 |
Discussion
The salient finding from this study is that we have shown for the first time functional evidence for GPR55- mediated neurogenic effect on intestinal contractility. This conclusion is based on our observation that the atypical cannabinoid, O-1602, a GPR55 agonist, inhibited the neurogenic contractions in the gut and its effects were mediated through GPR55, but neither CB1 nor CB2 receptors were necessary, as evident from corresponding gene knockout mice. The inhibitory effects of O-1602 are predominantly pre-junctional and partially post –junctional (only at high concentrations). Surprisingly, JWH015, considered to be a CB2/GPR55 agonist, inhibits intestinal contractility independently of these receptors.
Two types of cannabinoid receptors: CB1 and CB2[24, 25], which are part of the endocannabinoid system are well studied in several physiological systems. CB1 receptor activation can alleviate pain, reduce GI motility, and affect transient lower esophageal sphincter relaxations and emesis [11, 12]. CB2 receptors are located mainly in immune cells and their activation reduces inflammation. However, there have been several lines of pharmacological evidence supporting the existence of additional putative cannabinoid receptors [4, 26], such as the recently de-orphanized G protein-coupled receptor (GPCR) GPR55 [13, 27, 28]. The functional role of CB1 receptors has been extensively studied in the gastrointestinal tract, however, much less is known about the effects of either CB2 receptors or the newly identified GPR55. Previous reports showed expression of GPR55 receptor in the gut at gene level [13, 16], although contradictory sequences have been reported for hGPR55[5, 29, 30], which could be due to errors in sequencing as alternative splicing is not possible in the intronless GPR55 gene. Therefore, expression of GPR55 at protein level in the system under study becomes critical for further characterization of these receptors. However, the gene expression of GPR55 in the gut suggests a potential role for these orphan GPCRs in gut physiology. Nevertheless, the exact location of GPR55 receptor expression with respect to enteric nervous system, smooth muscle cells, mucosa or other gut cell type remains to be characterized.
The objective of the present study was to elucidate the function of GPR55 receptors in mouse colon using the atypical cannabinoid O-1602, generally considered and used as a pharmacological GPR55 agonist [7-9]. As endocannabinoids, phytocannabinoids and their analogs are known to reduce neurogenic contractions in ileum by suppression of ACh release from enteric neurons [17, 18], we determined whether activation of the GPR55 receptor by O-1602 would also inhibit neurogenic contractions in mouse colon. GPR55 appears to mediate the concentration–dependent inhibitory effect of O-1602 over the electrical field-stimulated contractions because the Emax value of this compound was significantly reduced in colon strips from GPR55−/− compared to tissue from GPR55+/+ mice. We also evaluated the involvement of the two accepted cannabinoid receptor subtypes: CB1 and CB2, by testing the O-1602-induced inhibition of neurogenic contractions in colon tissue strips from CB1−/−/CB2−/− mice. The finding that O-1602 inhibited the contractions with similar pD2 and Emax values between double knock-out and wild type mice rules out the involvement of these traditional cannabinoid receptors. The affinity of O-1602 for GPR55 receptors is not clear because EC50 values range from nanomolar to micromolar concentrations and depend on the functional endpoint and cellular systems [6-9]. The data in the present study are consistent with the affinity of O-1602 being in the micromolar range. Although the relatively high concentrations of O-1602 to inhibit contractions raise the likelihood of activating multiple targets, the finding that deletion of GPR55 reduced these effects supports the involvement of these receptors. The use of other GPR55 agonists that possess greater potency and selectivity than O-1602 may help to discern further the physiological function and mechanisms of this receptor. In any event, the results of the present study clearly indicate the actions of O-1602 on the colon are predominantly mediated through GPR55. Although other compounds elicit agonistic effects on GPR55 in vitro such as rimonabant (SR141716A) or AM251 [31], these compounds are not selective. Nonetheless, rimonabant and the reported endogenous ligand for GPR55 lysophosphatidylinositol[32] did not have any effect on the neurogenic contractions in the mouse gut (Fig.5C &D). As of now, further functional characterization of GPR55 receptor is limited by the lack of specific and potent pharmacological modulators. GPR55 is also reported [33] to be activated by the acylethanolamides: palmitoylethanolamide, anandamide and oleoylethanolamide which could also be used for further studies of GPR55 –mediated effects on intestinal motility.
We further examined the mechanisms of the inhibitory action of O-1602 in mouse colon. Even though O-1602 inhibited the neurogenic contractions, the site of action seems to be predominantly prejuctional and partially post-junctional because it also inhibited significantly (at high concentration) the contractions induced by exogenous ACh, suggesting antimuscarinic effects or effects on downstream signaling molecules involved in smooth muscle contractility as well. Depolarization induced by high extracellular K+ opens the plasma membrane voltage-gated L-type Ca2+ channels, thereby leading to Ca2+ influx. O-1602 did not affect the high K+-induced contraction, ruling out an inhibitory effect on Ca2+ influx.
The role of CB2 receptors in the GI tract is uncertain. In an earlier study, the increased GI transit induced by the Gram-negative bacterial endotoxin, lipopolysaccharide, was reduced to normal transit by CB2 agonist. Hence, activation of CB2 receptor in response to lipopolysaccharide is suggested as a mechanism for the re-establishment of normal GI transit after an inflammatory stimulus [34]. Also, JWH015 reduces motility in the croton oil model of intestinal inflammation [35]. Interestingly, hitherto, there is no evidence for any functional role of CB2 receptors in the neurogenic contractions of the normal gut. However, in inflamed gut (ie. LPS –treated), another CB2 agonist, JWH133 reduced the enhanced contractile response in a concentration-dependent manner [36]). Unlike the intestinal preparations, in mouse gastric preparations, JWH015 is demonstrated to inhibit the EFS-evoked cholinergic contractions, which was reduced by AM630, a CB2 antagonist, suggesting a CB2-mediated inhibitory effect [37]. Moreover, CB2 receptors have been found predominantly in the peripheral immune system and DRG cells suggesting no role for CB2 receptors in the regulation of gut motility by enteric neurons. We used JWH 015, considered to be a dual CB2/GPR55 agonist with the twin idea of elucidating the role of each of these receptors in the contractility of intestine. Surprisingly, the inhibitory effects of JWH015 were not at all affected by genetic deletion of either CB2 or GPR55 receptors. Of note, JWH015 has also been shown to mediate its inhibitory effects in guinea pig ileum via CB1 receptors that are sensitive to SR141716A. But, in the present study with the mouse ileum, either pharmacological inhibition of CB1 receptors by SR141716A (rimonabant) or genetic deletion of CB1 receptors, did not affect the JWH015 effects. It has to be noted that the EC50 value of JWH015 is about one order in magnitude higher in mouse ileum (i.e., micromolar range in the current study) than in the guinea pig ileum (i.e., sub-micromolar range)[23]. Interestingly, the same group also found that JWH015 was highly potent in the mouse vas deferentia with an EC50 value in the subnanomolar range; however, these effects were non-CB1 receptor mediated [23]. Thus, the effects of JWH015 appear to be dependent on the system and expression/distribution profile of different cannabinoid receptor subtypes. Since the pD2 values are much lower and JWH015 effects in the mouse ileum are still present in the various knockout mice used in the present study, its underlying mechanism of action is most likely independent of CB1, CB2 and GPR55 receptors in the mouse gut.
In conclusion, the atypical cannabinoid O-1602, a GPR55 agonist produces its inhibitory effect in the colon via a GPR55 dependent mechanism of action and independent of CB1- or CB2- receptors. Similar to the site of action by established cannabinoids, which mostly occur presynaptically in the gut, the atypical cannabinoid O-1602 also acts predominantly prejunctional and partially postjuctional (at high concentration). The effects of JWH015 in the gut are independent of GPR55, CB1, and CB2 receptors. This study reveals novel functional effects and mechanisms of the atypical cannabinoid O-1602 and GPR55 receptors in the gut, which could have therapeutic benefit in management of intestinal dysmotility in various pathological conditions.
Acknowledgement
The authors are grateful to NIH and VCU as this work was supported by grants from National Institutes of Health (to H.Akbarali, W.Dewey, A.Lichtman): DK046367, DA024009, P01DA009789, P50DA005274 and Virginia Commonwealth University - AD Williams grant #648729 to Gracious Ross.
References
- 1.Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38:151–178. [PubMed] [Google Scholar]
- 2.Mechoulam R, Gaoni Y. Hashish. Iv. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron. 1965;21:1223–1229. doi: 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- 4.Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from cb1 or cb2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawzdargo M, Nguyen T, Lee D, Lynch K, Cheng R, Heng H, George S, O’Dowd B. Identification and cloning of three novel human g protein-coupled receptor genes gpr52,[psi] gpr53 and gpr55: Gpr55 is extensively expressed in human brain1. Molecular Brain Research. 1999;64:193–198. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 6.Moriconi A, Cerbara I, Maccarrone M, Topai A. Gpr55: Current knowledge and future perspectives of a purported “Type-3” Cannabinoid receptor. Curr Med Chem. 2010;17:1411–1429. doi: 10.2174/092986710790980069. [DOI] [PubMed] [Google Scholar]
- 7.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor gpr55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson N, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley P. The orphan receptor gpr55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, Ross RA, Rogers MJ. The putative cannabinoid receptor gpr55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009;106:16511–16516. doi: 10.1073/pnas.0902743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauckner J, Jensen J, Chen H, Lu H, Hille B, Mackie K. Gpr55 is a cannabinoid receptor that increases intracellular calcium and inhibits m current. Proceedings of the National Academy of Sciences. 2008;105:2699. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin BR, Wiley JL. Mechanism of action of cannabinoids: How it may lead to treatment of cachexia, emesis, and pain. J Support Oncol. 2004;2:305–314. discussion 314-306. [PubMed] [Google Scholar]
- 12.Izzo AA, Sharkey KA. Cannabinoids and the gut: New developments and emerging concepts. Pharmacol Ther. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito J, Ito M, Nambu H, Fujikawa T, Tanaka K, Iwaasa H, Tokita S. Anatomical and histological profiling of orphan g-protein-coupled receptor expression in gastrointestinal tract of c57bl/6j mice. Cell Tissue Res. 2009;338:257–269. doi: 10.1007/s00441-009-0859-x. [DOI] [PubMed] [Google Scholar]
- 15.Lin XH, Yuece B, Li YY, Feng YJ, Feng JY, Yu LY, Li K, Li YN, Storr M. A novel cb receptor gpr55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol Motil. 2011;23:862–e342. doi: 10.1111/j.1365-2982.2011.01742.x. [DOI] [PubMed] [Google Scholar]
- 16.Schicho R, Bashashati M, Bawa M, McHugh D, Saur D, Hu HM, Zimmer A, Lutz B, Mackie K, Bradshaw HB, McCafferty DM, Sharkey KA, Storr M. The atypical cannabinoid o-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis. 2011;17:1651–1664. doi: 10.1002/ibd.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinemann A, Shahbazian A, Holzer P. Cannabinoid inhibition of guinea-pig intestinal peristalsis via inhibition of excitatory and activation of inhibitory neural pathways. Neuropharmacology. 1999;38:1289–1297. doi: 10.1016/s0028-3908(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 18.Roth SH. Stereospecific presynaptic inhibitory effect of delta9-tetrahydrocannabinol on cholinergic transmission in the myenteric plexus of the guinea pig. Can J Physiol Pharmacol. 1978;56:968–975. doi: 10.1139/y78-154. [DOI] [PubMed] [Google Scholar]
- 19.Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a g protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 20.Schuelert N, McDougall JJ. The abnormal cannabidiol analogue o-1602 reduces nociception in a rat model of acute arthritis via the putative cannabinoid receptor gpr55. Neurosci Lett. 2011;500:72–76. doi: 10.1016/j.neulet.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, Bradshaw HB. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through gpr18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44. doi: 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godlewski G, Offertaler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J, Davis MI, Zhang L, Razdan RK, Milman G, Pacher P, Mukhopadhyay P, Lovinger DM, Kunos G. The endogenous brain constituent n-arachidonoyl l-serine is an activator of large conductance ca2+-activated k+ channels. J Pharmacol Exp Ther. 2009;328:351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, Huffman JW, Yu S, Lainton JA, Pertwee RG. Evidence for the presence of cb2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- 24.Munro S, Thomas K, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. 1993. [DOI] [PubMed]
- 25.Matsuda L, Lolait S, Brownstein M, Young A, Bonner T. Structure of a cannabinoid receptor and functional expression of the cloned cdna. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 26.Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Mo F, Liu J, Kunos G. Evidence for novel cannabinoid receptors. Pharmacology & therapeutics. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Ross RA. The enigmatic pharmacology of gpr55. Trends Pharmacol Sci. 2009;30:156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Hiley C, Kaup S. Gpr55 and the vascular receptors for cannabinoids. Br J Pharmacol. 2007;152:559. doi: 10.1038/sj.bjp.0707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O’Dowd BF. A cluster of four novel human g protein-coupled receptor genes occurring in close proximity to cd22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997;239:543–547. doi: 10.1006/bbrc.1997.7513. [DOI] [PubMed] [Google Scholar]
- 30.Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA. Generation and initial analysis of more than 15,000 full-length human and mouse cdna sequences. Proc Natl Acad Sci U S A. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME. Atypical responsiveness of the orphan receptor gpr55 to cannabinoid ligands. J Biol Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corda D, Iurisci C, Berrie C. Biological activities and metabolism of the lysophosphoinositides and glycerophosphoinositols. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2002;1582:52–69. doi: 10.1016/s1388-1981(02)00137-3. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli F, Izzo AA. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Pract Res Clin Endocrinol Metab. 2009;23:33–49. doi: 10.1016/j.beem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, Marini P, Romano B, Di Marzo V, Capasso F, Izzo AA. Inhibitory effect of salvinorin a, from salvia divinorum, on ileitis-induced hypermotility: Cross-talk between kappa-opioid and cannabinoid cb(1) receptors. Br J Pharmacol. 2008;155:681–689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, Patel KD, Pittman QJ, Sharkey KA. Cannabinoid cb2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mule F, Amato A, Baldassano S, Serio R. Involvement of cb1 and cb2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res. 2007;56:185–192. doi: 10.1016/j.phrs.2007.06.002. [DOI] [PubMed] [Google Scholar]