Abstract
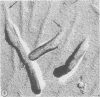
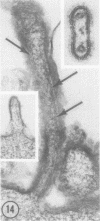
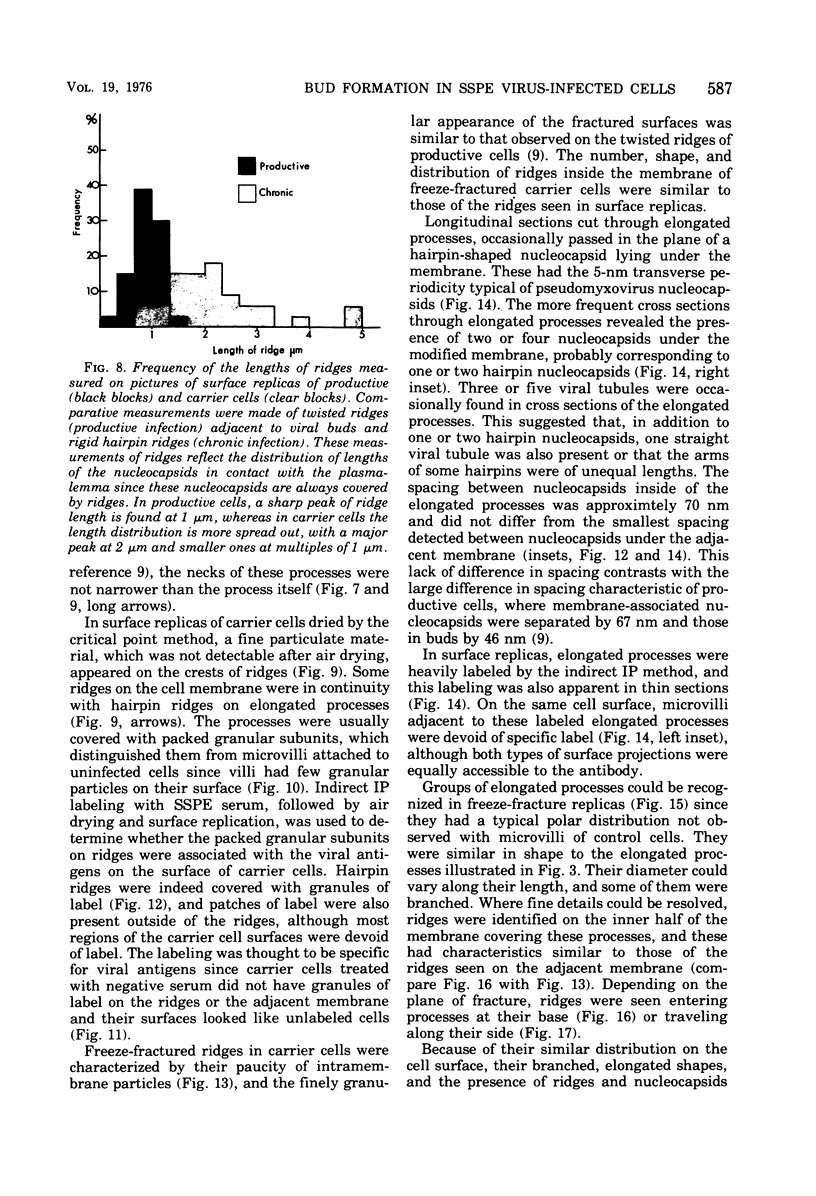
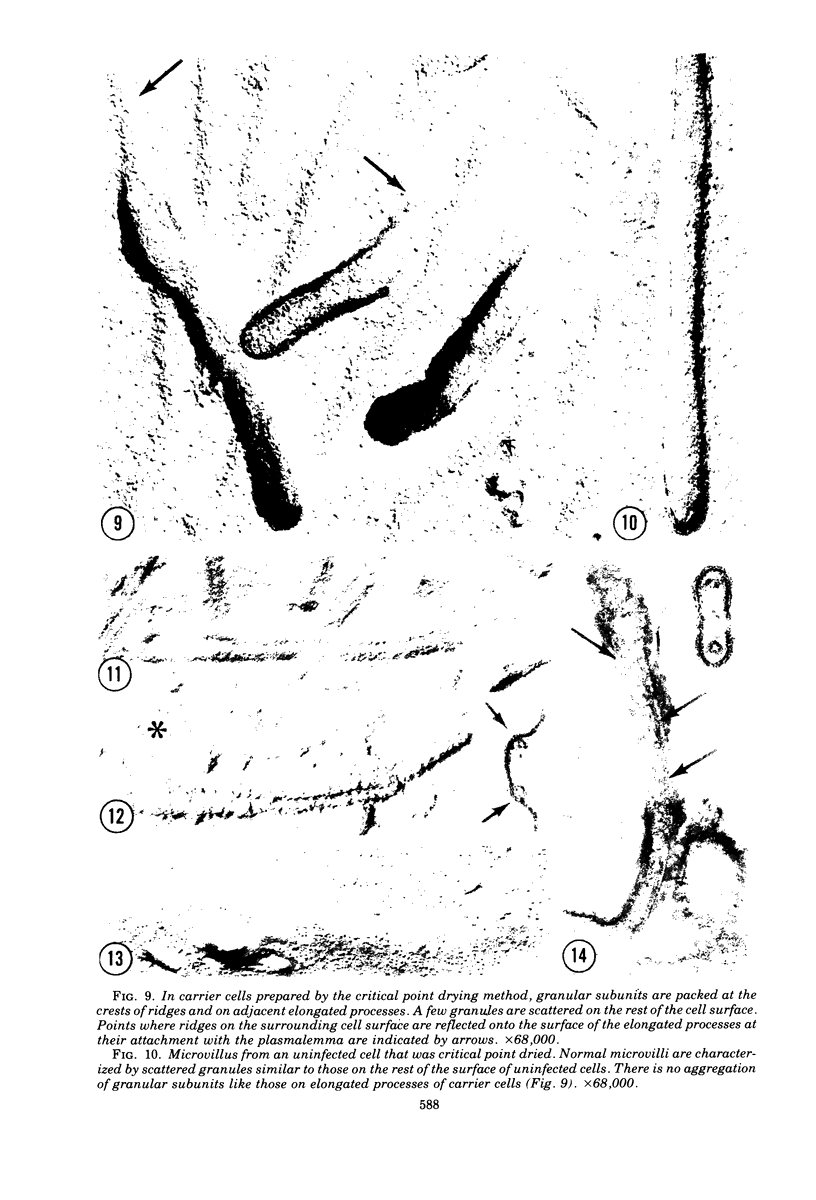
Human prostate cells chronically infected with the Mantooth strain of subacute sclerosing panencephalitis (SSPE) virus multiply normally, fuse only occasionally to form giant cells, and yet have twisted intracytoplasmic nucleocapsids. These cells are able to support replication of vesicular stomatitis virus, although they release only small amounts of SSPE virus. To determine why carrier cells do not produce virus, they were examined with techniques for surface replication, freeze-fracturing, and immunoperoxidase labeling with SSPE antibody. The surface of carrier cells, like that of productive cells, is characterized by ridges crowned with viral antigens and devoid of the intramembrane particles revealed by freeze-fracture techniques. Since surface ridges form where nucleocapsids attach to the membrane, the shape and length of ridges are indicative of the shape and length of the underlying nucleocapsid. Whereas ridges on productive cells are serpentine in shape, those on carrier cells are typically straight or hairpin shaped, and the hairpin ridges are twice as long as serpentine ridges on productive cells. Furthermore, the spacing between ridges on carrier cells is never as small as that in productive infections, so that continuous sheets of viral membrane are never formed. The majority of carrier cells lack the round viral buds observed in productive cells but have, instead, many elongated processes attached to the cell surface. Each of these processes contains one or two hairpin ridges overlying hairpin-shaped nucleocapsids. These "hairpin buds" are restricted to a single region of the carrier cell surface, whereas viral buds are distributed over the entire surface of productive cells. Thus, there are several structural defects in carrier cells that depend on the specific interaction of a certain viral strain with a certain cell type. These defects prevent the deployment of viral antigen in some regions of the cell surface, the formation of nucleocapsids of normal length, the coiling of attached nucleocapsids, and the consolidation of sheets of viral membrane into spherical buds with the nucleocapsids coiled inside. These defects may account for the failure of carrier cells to shed infectious virus.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdwell C. R., Strauss E. G., Strauss J. H. Replication of Sindbis virus. 3. An electron microscopic study of virus maturation using the surface replica technique. Virology. 1973 Dec;56(2):429–438. doi: 10.1016/0042-6822(73)90047-0. [DOI] [PubMed] [Google Scholar]
- Burnstein T., Jacobsen L. B., Zeman W., Chen T. T. Persistent infection of BSC-1 cells by defective measles virus derived from subacute sclerosing panencephalitis. Infect Immun. 1974 Dec;10(6):1378–1382. doi: 10.1128/iai.10.6.1378-1382.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y., Sanpe T., Nakajima M., Okawa S., Koto T. Properties of a cytopathic agent isolated from a patient with subacute sclerosing panencephalitis in Japan. Jpn J Med Sci Biol. 1972 Oct;25(5):321–333. doi: 10.7883/yoken1952.25.321. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Barbosa L. H., Hamilton R., Sever J. L. Comparison between productive and latent subacute sclerosing panencephalitis viral infection in vitro. An electron microscopic and immunoperoxidase study. Lab Invest. 1974 Mar;30(3):241–250. [PubMed] [Google Scholar]
- Dubois-Dalcq M., Barbosa L. H. Immunoperoxidase stain of measles antigen in tissue culture. J Virol. 1973 Oct;12(4):909–918. doi: 10.1128/jvi.12.4.909-918.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Coblentz J. M., Pleet A. B. Subacute sclerosing panencephalitis. Unusual nuclear inclusions and lengthy clinical course. Arch Neurol. 1974 Dec;31(6):355–363. doi: 10.1001/archneur.1974.00490420021001. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Reese T. S. Structural changes in the membrane of vero cells infected with a paramyxovirus. J Cell Biol. 1975 Dec;67(3):551–565. doi: 10.1083/jcb.67.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Worthington K., Gutenson O., Barbosa L. H. Immunoperoxidase labeling of subacute sclerosing panencephalitis virus in hamster acute encephalitis. Lab Invest. 1975 Apr;32(4):518–526. [PubMed] [Google Scholar]
- Ehrnst A., Weiner L., Norrby E. Fluctuations and distribution of measles virus antigens in chronically infected cells. Nature. 1974 Apr 19;248(5450):691–693. doi: 10.1038/248691a0. [DOI] [PubMed] [Google Scholar]
- Gibson P. E., Bell T. M. Persistent infection of measles virus in mouse brain cell cultures infected in vivo. Arch Gesamte Virusforsch. 1972;37(1):45–53. doi: 10.1007/BF01241149. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J., Gould E. Defective interfering particles produced during the replication of measles virus. Med Microbiol Immunol. 1974;160(2-3):155–164. doi: 10.1007/BF02121722. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Hamilton R., Barbosa L., Dubois M. Subacute sclerosing panencephalitis measles virus: study of biological markers. J Virol. 1973 Sep;12(3):632–642. doi: 10.1128/jvi.12.3.632-642.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Koprowski H., Müller D., ter Meulen V., Käckell Y. M. Morphogenesis and structure of a virus in cells cultured from brain tissue from two cases of multiple sclerosis. Lab Invest. 1973 Apr;28(4):494–500. [PubMed] [Google Scholar]
- Johnson K. P., Norrby E. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. 3. Induction of defective measles infection in hamster brain. Exp Mol Pathol. 1974 Oct;21(2):166–178. doi: 10.1016/0014-4800(74)90087-2. [DOI] [PubMed] [Google Scholar]
- Klein G., Clifford P., Klein E., Smith R. T., Minowada J., Kourilsky F. M., Burchenal J. H. Membrane immunofluorescence reactions of Burkitt lymphoma cells from biopsy specimens and tissue cultures. J Natl Cancer Inst. 1967 Nov;39(5):1027–1044. [PubMed] [Google Scholar]
- Knight P., Duff R., Rapp F. Latency of human measles virus in hamster cells. J Virol. 1972 Nov;10(5):995–1001. doi: 10.1128/jvi.10.5.995-1001.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Joseph B. S., Oldstone M. B. Antibody-induced capping of measles virus antigens on plasma membrane studied by electron microscopy. J Virol. 1975 May;15(5):1248–1255. doi: 10.1128/jvi.15.5.1248-1255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menna J. H., Collins A. R., Flanagan T. D. Characterization of an in vitro persistent-state measles virus infection: establishment and virological characterization of the BGM/MV cell line. Infect Immun. 1975 Jan;11(1):152–158. doi: 10.1128/iai.11.1.152-158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T. Studies on the persistent infection with measles virus in HeLa cells. I. Clonal analysis of cells of carrier cultures. Jpn J Microbiol. 1971 Jul;15(4):325–331. doi: 10.1111/j.1348-0421.1971.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Minagawa T. Studies on the persistent infection with measles virus in HeLa cells. II. The properties of carried virus. Jpn J Microbiol. 1971 Jul;15(4):333–340. doi: 10.1111/j.1348-0421.1971.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Lackland H., Choppin P. W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J Virol. 1974 Nov;14(5):1253–1261. doi: 10.1128/jvi.14.5.1253-1261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E. C., Magnusson P. Some morphological characteristics of the internal component of measles virus. Arch Gesamte Virusforsch. 1965;17(3):443–447. doi: 10.1007/BF01241199. [DOI] [PubMed] [Google Scholar]
- Norrby E. A carrier cell line of measles virus in Lu 106 cells. Arch Gesamte Virusforsch. 1967;20(2):215–224. doi: 10.1007/BF01241275. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Weinstein I. B. Filamentous forms of enveloped A particles in cell cultures from chemically induced rat hepatomas. Cancer Res. 1973 Sep;33(9):1998–2004. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Barbosa L. H., Bornstein M. B. Subacute sclerosing panencephalitis virus. Observations on a neuroadapted and non-neuroadapted strain in organotypic central nervous system cultures. Lab Invest. 1974 Jul;31(1):42–53. [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Subacute sclerosing panencephalitis virus in cultures of organized central nervous tissue. Lab Invest. 1973 May;28(5):627–640. [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. II. Effect of measles antibody on persistently infected HeLa sublines and recovery of a HeLa clonal line persistently infected with incomplete virus. J Bacteriol. 1966 Dec;92(6):1805–1811. doi: 10.1128/jb.92.6.1805-1811.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. W., Fuccillo D. A., Hensen S. A., Vincent M. M., Bellanti J. A. Specific inhibitory factors of cellular immunity in children with subacute sclerosing panencephalitis. J Pediatr. 1976 Jan;88(1):56–62. doi: 10.1016/s0022-3476(76)80727-5. [DOI] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Isolation and comparative study of the nucleocapsids of measles and canine distemper viruses from infected cells. Virology. 1974 Sep;61(1):74–79. [PubMed] [Google Scholar]
- Waters D. J., Hersh R. T., Bussell R. H. Isolation and characterization of measles nucleocapsid from infected cells. Virology. 1972 Apr;48(1):278–281. doi: 10.1016/0042-6822(72)90138-9. [DOI] [PubMed] [Google Scholar]