Abstract
Mucopolysaccharidosis I (MPS I) is a lysosomal storage disease due to α-L-iduronidase (IDUA) deficiency that results in the accumulation of glycosaminoglycans (GAG). Systemic gene therapy to MPS I mice can reduce lysosomal storage in the brain, but few data are available regarding the effect upon behavioral function. Here, we investigated the effect of gene therapy with a long-terminal repeat (LTR)-intact retroviral vector or a self-inactivating (SIN) vector on behavioral function in MPS I mice. The LTR vector was injected intravenously to 6 week-old MPS I mice, while the SIN vector was given to neonatal or 6 week-old mice. Adult-LTR, Neonatal-SIN, and Adult-SIN-treated mice achieved serum IDUA activity that was 235±20 (84-fold normal), 127±10, and 71±7 units/ml, respectively. All groups had reduction in histochemical evidence of lysosomal storage in the brain, with the Adult-LTR group showing the best response, while Adult-LTR mice had reductions in lysosomal storage in the cristae of the vestibular system. Behavioral evaluation was performed at 8 months. Untreated MPS I mice had a markedly reduced ability to hold onto an inverted screen or climb down a pole. LTR vector-treated mice had marked improvements on both of these tests, while Neonatal-SIN mice had improvements in the pole test. We conclude that both vectors can reduce brain disease in MPS I mice, with the LTR vector achieving higher serum IDUA levels and better correction. Vestibular abnormalities may contribute to mobility problems in patients with MPS I, and gene therapy may reduce symptoms.
Introduction
Mucopolysaccharidosis I (MPS I) is an autosomal recessive lysosomal storage disease with an incidence of 1:100,000 (Baehner et al 2005). It is due to deficient α-L-iduronidase (IDUA; EC 3.2.1.76) activity and results in the accumulation of the glycosaminoglycans (GAGs) heparan and dermatan sulfate in the lysosome (Neufeld and Muenzer 2001). Clinical manifestations include, but are not limited to, disease of the brain, ear, eye, upper airway, bones and joints, and cardiovascular system. Severe behavioral impairment occurs in the severe form of MPS I known as Hurler syndrome (OMIM #607014). Although the attenuated-severity Scheie syndrome is not classically felt to reduce behavioral function, 10% of these patients have mild to moderate impairments in cognitive function (Thomas et al 2010). The etiology of behavioral dysfunction is unclear. Neurons and microglial cells accumulate GAGs, which can inhibit ganglioside-degradative lysosomal enzymes, causing accumulation of the gangliosides GM2 and GM3 (Walkley 2004), although a causal role for these abnormalities has not been demonstrated. Increased oxidation occurs in the cerebellum (Reolon et al 2009).
Available treatments for patients with MPS I include hematopoietic stem cell transplantation (HSCT) (Staba et al 2004) and enzyme replacement therapy (ERT) (Kakkis et al 2001). All approaches rely at least in part upon uptake of mannose 6-phosphate (M6P)-modified IDUA from the extracellular space by cells via the M6P receptor and translocation of enzyme to the lysosome. For HSCT, blood-derived cells migrate into organs including the brain and secrete enzyme locally, although some enzyme can be secreted into blood. HSCT has improved behavioral function in children with Hurler syndrome if performed before 2 years of age (Aldenhoven et al 2008). ERT involves intravenous (IV) injection of M6P-modified IDUA protein, which can be taken up by cells after diffusion from blood. Although it has been assumed that ERT would not improve behavioral function due to the blood-brain barrier, early results from a study of Hurler patients that received ERT suggests that they are developing better-than-expected (Wraith et al 2007), while ERT reduced abnormalities seen on MRI (Wang et al 2009b).
Gene therapy with gamma retroviral vectors (RV), adenovirus-associated (AAV) vectors, adenoviral vectors, lentiviral vectors, and plasmid-based vectors is being evaluated in animal models of MPS and involves transfer of a gene into cells in the body, which can secrete enzyme locally or into the blood (Ponder and Haskins 2007). Gene therapy has resulted in stable expression of IDUA in blood and reduction in lysosomal storage in the brain. However, most studies, including ours with RV, have not evaluated the effect on behavioral function. MPS I mice show multiple behavior defects, which include reduced ambulations and rearings in an open-field test, reduced habituation to a new environment, and/or other abnormalities (Pan et al 2008; Hartung et al 2004; Reolon et al 2006; Wang et al 2009a). Neonatal administration of an AAV vector improved habituation in the open field test (Hartung et al 2004), ex vivo transduction of hematopoietic stem cells with expression in red blood cells improved habituation to a novel environment (Wang et al 2009a), while direct injection of an AAV8 vector into the brain improved function in a modified Morris water maze test (Wolf et al 2011).
The goal of this study was to determine if RV-mediated gene therapy could improve behavioral function in MPS I mice. We have previously injected RV vectors intravenously (IV) into MPS I mice, and demonstrated reduction in lysosomal storage in the brain for some of the vectors and doses that we were used (Liu et al 2005; Ma et al 2007; Chung et al 2007). However, other vectors and doses (Metcalf et al 2010) have not been evaluated for their effect on storage in the brain, and none of these studies have evaluated the effect on behavioral function. In this study, one vector contained a complete long-terminal repeat (LTR; LTR vector), while a second vector contained a deletion in the enhancer region of the 3′ LTR that results in self-inactivation upon transduction of a cell by transferring the deletion at the 3′ end of the LTR to the 5′ end of the LTR to create the self-inactivating (SIN) vector (Metcalf et al 2010). The SIN vector lacks the enhancer of the LTR, and is less likely to activate nearby oncogenes (Trobridge 2011). We demonstrate here marked improvement in behavioral dysfunction in MPS I mice after IV injection of the LTR vector to adult MPS I mice, and partial improvements after IV injection of the SIN vector to neonatal or adult MPS I mice.
Materials and Methods
Reagents and Vectors
All reagents were purchased from Sigma-Aldrich Chemical (St. Louis, MO) unless otherwise stated. hAAT-cIDUA-WPRE (designated as the LTR vector here) is a gamma RV with an intact LTR at both the 5′ and 3′ end, an extended packaging signal, the liver-specific human α1-antitrypsin (hAAT) promoter, the canine IDUA cDNA, and the Woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) (Liu et al 2005). SIN-hAAT-cIDUA-oPRE (designated as the SIN vector here) is a self-inactivating RV that has a deletion of the retroviral enhancer sequences in the U3 region of the 3′-LTR and lacks promoter or enhancer function at 5′- and 3′-ends of the provirus after integration (Metcalf et al 2010); the oPRE is an optimized version of the WPRE, as diagramed in Supplementary Fig. 1.
Animal care
All experiments were approved by our ethics committee and National Institutes of Health (NIH) guidelines for the care and use of animals in research were followed. The specific mice evaluated here are identical to those reported previously (Metcalf et al 2010). For the neonatal treatment, MPS I mice on a C57BL/6 background (Ohmi et al 2003) received intravenous (IV) injection of 1×1010 transducing units (TU)/kg of the SIN vector at 2–3 days of age (designated hereafter as Neonatal-SIN; N=11) as previously described. For adult treatment, 6 week-old MPS I mice were injected intraperitoneally (IP) with hepatocyte growth factor (HGF) as detailed previously, followed by IV injection of the LTR-vector (designated hereafter as Adult-LTR; N=10) or the SIN vector (designated hereafter as Adult-SIN; N=6). The cumulative dose of RV was 1.7×1010 transducing units (TU)/kg for Adult-LTR-treated mice and 1×1010 TU/kg for Adult-SIN-treated mice. Adult LTR-treated MPS I mice were also treated transiently with anti-CD40 ligand antibody (blocks the CD40:CD40 ligand co-stimulatory pathway in lymphocytes) and CTLA4-Ig (blocks the CD80/CD86:CD28 co-stimulatory pathway in lymphocytes) to suppress the immune system and prevent mice from developing a cytotoxic T lymphocyte response to IDUA-expressing cells, while Adult-SIN-treated mice did not receive immunosuppression. Phenotypically normal heterozygous normal and untreated MPS I mice from the same breeding colony were used as controls. Serum was obtained from the tail vein.
IDUA and GUSB activities
Organs were homogenized in lysis buffer at pH 5.5 as described previously (Baldo et al 2011). GUSB and IDUA assays were performed using the fluorogenic substrates 4-methylumbelliferyl-β-L-glucuronide (Sigma-Aldrich, St Louis, MO) for GUSB and 4-methylumbelliferyl-α-L-iduronide (Toronto Research Chemicals, North York, Canada) for IDUA and a Fluoroskan Ascent microplate fluorometer (Thermo Electron, Milford, MA) as previously described. One unit of enzyme activity converts 1 nmole of substrate to product per hour at 37°C.
Histological analysis
Eight-month old mice were perfused intracardially with 20 mL of PBS, and brains were removed and fixed in PBS with 4% paraformaldehyde and 2% glutaraldehyde. Pieces of brain were embedded in plastic, and 1 μm-thick sections of cerebellum, cortex, and hippocampus were stained with toluidine blue as described (Ma et al 2007). Neurons of the cortex and hippocampus were identified by their large nuclei and their large dendrites, microglial cells were identified by their small size and dark nuclei, and Purkinje cells were identified by their large size and their location at the edge of the granular layer of the cerebellum (Fuller and Burger, 2007). Sections were scored without knowledge of the genotype or treatment status of the sample. Purkinje cells in the cerebellum were considered to have storage if they had 2 or more cytoplasmic vacuoles with GAGs when evaluated at 40X magnification. Ten fields were evaluated and the average percentage of positive cells was determined. Neurons and microglial cells of the cortex and hippocampus were considered to have storage if they had at least 3 vacuoles with storage per cell. Mouse temporal bones containing the ears were fixed in the same fashion, decalcified, embedded in paraffin, and 4 μm-thick mid-modiolar sections were stained with toluidine blue.
Behavioral tests
Sensorimotor tests were performed to assess balance, strength, and coordination, as previously described (Wozniak et al 2004). For the screen tests, the mouse was placed on a wire mesh grid with 16 squares per 10 cm. For the vertical screen test, the screen was elevated 47 cm above the floor and inclined vertically. Each mouse was placed in the middle of the screen with its head oriented down, and the time to climb to the top of the screen was determined. For the inverted screen test, mice were placed on top of a screen that was oriented 60° from the horizontal plane, and the screen was gently inverted until it was horizontal with the mouse upside-down, and the time to fall off was recorded. A maximum score of 60 seconds was given if the mouse did not fall. For the screen tests, two trials were performed, and values for the second trial are reported. For the pole test, a mouse was placed head upward on top of a vertical rod (8 mm diameter and 55 cm tall) that had a finely textured surface, and was timed for how long it took to climb down the pole. If a mouse fell from the pole before reaching the floor or refused to try, it was given the maximum score of 120 seconds. Two trials were performed, and the values for the second trial were averaged. Forelimb grip strength was evaluated with a grip strength meter (Stoelting Co., Wooddale, IL) as described (Wozniak et al 2007). The mouse was placed over a Perspex plate in front of a “grasping trapeze”, which functions as the arm of a force transducer connected to a peak amplifier. The mouse was taught to grab the trapeze when pulled by the tail until the pulling force overcame its grip strength, and the peak pull force was measured. Each mouse received 3 days of habituation in which several trials were performed until it performed 5 good pulls. Testing involved two 5-trial sessions, with each session being conducted on consecutive days. The mean of the pull force of the 5 trials performed on the second day are shown here.
Motor coordination and balance were evaluated using the rotorod test (Rotamex-5, Columbus Instruments, Columbus, OH) when it was stationary, rotating at 5 revolutions per minute (rpm) for up to 60 seconds, and accelerating where the speed increased from 5 rpm to 20 rpm over 180 seconds, and the time to fall off the rod was determined (Wozniak et al 2007). The Morris water maze test was performed as described (Wozniak et al 2004). During cued trials, mice were trained to swim to a submerged platform marked with visual cues, and mice received four trials per day for two consecutive days. Place trials, which are shown in the supplement, were also performed to test the ability of a mouse to learn the position of a platform. General locomotor activity was quantified using a computerized, photobeam system (MotorMonitor, Hamilton-Kinder, LLC, Poway, CA), according to previously published methods (Wozniak et al 2004). The number of ambulations (whole body movements) and vertical rearings made over 12 separate 5 minute blocks or for the entire hour were recorded. In addition, walking initiation, ledge, and platform tests were performed as described previously and are shown in the supplement.
Statistics
ANOVA with Tukey post-hoc analysis or the Student’s t test compared values in different groups using Sigma Stat software version 3.1 (Systat Software, Inc., Point Richmond, CA). If the normality and equal variance parameters of ANOVA failed, ANOVA on ranks was used to test for significance as per the recommendation of the program. The Chi-square test determined if the frequency of an event differed between different groups.
Results
Gene transfer and brain enzyme activity
The goal of this study was to evaluate the effect of gene transfer to neonatal or adult (1.5 month-old) MPS I mice on behavioral function at 8 months of age. All mice evaluated here were previously described in terms of their transduction, but the effect on lysosomal storage in the brain and behavioral function was not reported. Adult-LTR mice were treated at 6 weeks of age with transient administration of HGF to induce the hepatocyte replication needed for transduction with this vector in adults, followed by a cumulative dose of 1.7×1010 TU/kg of the LTR vector (Metcalf et al 2010), which was 1.7 fold the dose given in our initial study with this vector in adult mice (Ma et al 2007). Mice achieved stable expression of IDUA activity in serum with a mean average lifetime activity of 235±20 U/ml, as shown in Fig. 1A, which was 84-fold the value of 2.8±0.1 U/ml in homozygous normal mice, and was 1175-fold the value of 0.2±0.01 U/ml in untreated MPS I mice. A different cohort of MPS I mice were treated with the SIN vector (Metcalf et al 2010). Some received neonatal IV injection of 1×1010 TU/kg (Neonatal-SIN), while others received IV injection of HGF at 6 weeks of age to induce hepatocyte replication followed by 1×1010 TU/kg (Adult-SIN). Neonatal-SIN and Adult-SIN mice achieved serum IDUA activity of 127±10 U/mL (45-fold normal) and 71±7 U/mL (25-fold normal), respectively, as shown in Fig. 1A.
Fig. 1. Serum α-L-iduronidase (IDUA) activity.

A. Serum IDUA activity after treatment in MPS I mice. Mice were injected with 1.7×1010 TU/kg of the RV designated hAAT-cIDUA-WPRE at 1.5 months of age (Adult-LTR), with 1×1010 TU/kg of the self-inactivating version of the RV designated SIN-hAAT-cIDUA-oPRE at 2 to 3 days after birth (Neonatal-SIN), or with 1×1010 TU/kg of the SIN vector at 1.5 months of age (Adult-SIN) and serum IDUA ± standard deviation (SD) was measured using a fluorogenic substrate as described in the methods section from 1 week until 8 months of age. Results represent the average of the lifetime average for the indicated number of mice in each group. B. Forebrain and cerebellar IDUA activity. Animals were sacrificed at 8 months and IDUA levels were measured in the forebrain and cerebellum for 5 normal, 6 untreated MPS I, 4 Neonatal-SIN, and 3 Adult-SIN mice, and from forebrain of 6 Adult-LTR mice; some treated mice were not evaluated due to lack of sample availability. The number of mice of each gender was similar. C. β-glucuronidase (GUSB) activity. The X over the position of the cerebellum data for the Adult-LTR-treated mice indicates that those samples were not collected and thus could not be assayed. * represents a p value of 0.01 to 0.05 and ** indicates a p value <0.01 for the indicated groups compared with MPS I mice using ANOVA with Tukey post hoc analysis for samples for which the normality and equal variance tests passed, and with ANOVA on ranks if these tests did not pass.
Brain IDUA activity is shown in Fig. 1B, which was performed on samples that were collected after perfusion of mice with saline to remove enzyme from blood. Adult-LTR mice achieved 5±6 U/mg of IDUA activity in the front half of the brain, which was 73% normal (7±2 U/mg) and 96-fold the level in untreated MPS I mice (p=0.05 for Adult-LTR vs. MPS I), although there was marked variation in individual mice, and it is possible that some treated animals had poor perfusion prior to the collective of tissues and were contaminated with IDUA from blood. Average IDUA levels in the forebrain from Neonatal-SIN and Adult-SIN were 0.3±0.2 U/mg (4% normal) and 1.4±1.1 U/mg (20% normal), respectively, which were 5-fold and 19-fold, respectively, the values for untreated mice (0.05±0.03 U/mg), although none of these differences were significant. Cerebellum IDUA activity in Neonatal-SIN and Adult-SIN-treated mice were 1.8 and 3.1-fold the values in untreated MPS I mice, respectively, (2% and 4% normal), and did not reach significance. IDUA levels were not analyzed in cerebellum of Adult-LTR mice due to the failure to collect samples.
Activity of the lysosomal enzyme, β-glucuronidase (GUSB), was also evaluated, as other lysosomal enzymes are usually elevated in MPS I, and normalization of activity is a good biochemical indicator of correction of disease (Baldo et al 2011). Adult-LTR-treated mice had 55±19 U/mg of GUSB activity in their forebrain, which was significantly lower than the value of 127±62 U/mg in untreated MPS I mice, but appeared to be slightly higher than the value of 31±3 U/mg in normal mice, while Neonatal-SIN- and Adult-SIN-treated MPS I mice did not have significant reductions in forebrain GUSB activity compared with untreated MPS I mice. Neonatal-SIN- and Adult-SIN-treated MPS I mice did have a significant reduction in cerebellar GUSB activity with values of 56±13 and 47±14, respectively, while untreated MPS I mice had 98±31 U/mg, and normal mice had 40±6 U/mg. GAG levels were not evaluated in treated mice, as our prior studies failed to identify elevations in untreated MPS I mice.
Histopathological analysis of brain
Histopathological analysis was performed to determine if neurons and microglial cells had a reduction in accumulation of lysosomal storage material, as shown for representative examples in Supplemental Fig. 2, and as quantified in Fig. 2. In untreated MPS I mice, 44±13% of Purkinje cells had 2 or more vacuoles, which was higher than the value of 4±3% of cells in normal mice (p<0.01). Adult-LTR-treated mice had storage in 7±7% of cells (p<0.01 vs. MPS I), while Neonatal-SIN and adult-SIN mice were partially corrected with storage in 23±12% and 18±6% of cells, respectively (p<0.01 vs. MPS I mice; p=0.02 for Neonatal SIN vs. normal). In addition, the number of vacuoles per positive cell and the size of the vacuoles appeared to be greater in the untreated MPS I mice than in the treated mice, although this was not evaluated quantitatively.
Fig. 2. Histochemical analysis for lysosomal storage in the brain.
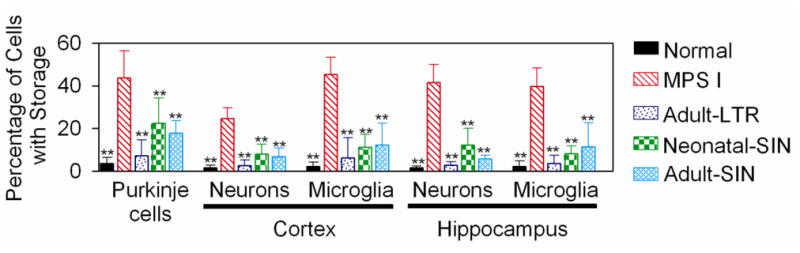
Normal mice, untreated MPS I mice, or MPS I mice that were treated as detailed in Fig. 1 were sacrificed at 8 months of age. Brains were fixed, embedded in plastic, and 1-μm thick sections were stained with toluidine blue, as shown for representative samples in Supplementary Fig. 2. The percentage of Purkinje cells in the cerebellum with 2 or more vacuoles thought to be lysosamal storage was determined as detailed in the methods. For the neurons and microglial cells of the cortex and the hippocampus, the percentage of the cells with 3 or more granules thought to represent lysosomal storage was determined. Histology was evaluated for most parts of the brain from 4 normal, 5 untreated MPS I, 4 Adult-LTR-treated, 4 Neonatal-SIN-treated, and 4 Adult-SIN-treated MPS I mice, and the gender distribution was similar. For the hippocampus, the number evaluated was 3, 3, 4, 2, and 2, respectively, as some sections did not have adequate regions of this somewhat difficult-to-obtain region. ** indicates that values were statistically significant for a particular group compared with those in untreated MPS I mice with a p< 0.01 using ANOVA and Tukey post hoc analysis.
In the cortex, MPS I mice had 3 or more vacuoles in 25±5% of neurons and 45±8% of microglial cells, which were higher than the values of 2±1% and 2±2% in normal mice, respectively (p<0.01 in both cases). Adult-LTR treated mice had marked reductions in the percentage of cells with 3 or more vacuoles for both cell types (p<0.01 vs. MPS I), while Neonatal-SIN and Adult-SIN-treated mice had partial reductions (p<0.01 vs. MPS I). A similar pattern was seen in the hippocampus, where the percentage of cells with 3 or more vacuoles was elevated in untreated MPS I mice, and treated mice had statistically significant reductions.
Effect of MPS I on behavioral function
A battery of tests were performed at 8 months of age to evaluate MPS I mice for behavioral abnormalities. Since Adult-LTR-treated MPS I mice were evaluated at a different time from the Neonatal-SIN- and the Adult-SIN-treated mice, behavioral tests are shown separately for these cohorts of mice. MPS I mice performed similarly to normal controls in the time that it took to climb to the top of a vertically-oriented screen, suggesting that their general coordination and strength were relatively intact (Fig. 3A). However, MPS I mice could not hold onto an inverted screen for the entire 60-second trial, as 100% fell off the screen when it was inverted so that the mouse was hanging upside-down, although only 13% of normal mice fell off (p<0.001 with Chi-square test). For Adult-LTR-treated MPS I mice, only 20% of the mice fell off the inverted screen, which was significantly lower than the value in MPS I mice (p<0.001), but was not different from the value in normal mice (Fig. 3B). In addition, the average time that MPS I mice held onto the inverted screen was very short at 23±17 seconds (Fig. 3C). In contrast, normal mice held onto the screen for 57±7 seconds (p<0.01 vs. MPS I), which was very close to 60 seconds, the maximum duration of the trial. Adult-LTR-treated mice stayed on the screen for 56±9 seconds, which was significantly longer (p<0.01) than for untreated MPS I mice, but was not different from the value in normal mice.
Fig. 3. Behavioral tests in Adult-LTR mice.
Some MPS I mice with treated with IV injection of the LTR-intact RV at 1.5 months of age as described in Fig. 1 (Adult-LTR), while other MPS I and phenotypically normal heterozygous littermates were not treated. Behavioral tests were performed for the total number of mice indicated inside the bar in panel A as detailed in the methods seconds at 8 months of age for normal mice (4 females and 6 males), untreated MPS I mice (5 females and 5 males), and Adult-LTR MPS I mice (5 females and 5 males). A. Vertical screen. The average time in seconds ± SD to climb to the top of a vertical screen was determined. B–C. Inverted screen test. The percentage of mice that fell off an inverted screen (panel B) and the average time that mice held onto the inverted screen (panel C) was determined. For the latter panel, the trial was terminated after 60 seconds. Analysis for the significance of the frequency of events between two groups was determined with Chi-squared test, while ANOVA with Tukey post-hoc analysis was performed to compare values in panel C. DE. Vertical pole test. The percentage of mice that fell of a vertical pole and the time to climb down the vertical pole were determined as described for panels B–C. F. Forelimb grip strength. The mean of the pull force at which mice in each group released a trapeze was determined. G. Swim speed. The average swim speed in the cued trials of the Morris water maze test was determined for 8 trials over 2 days. H. Rearing. The number of rearings per hour when placed into a novel environment was determined. I. Ambulations. The number of ambulations over 5 minutes was determined for 12 consecutive intervals over an hour. For panels A to H, * and ** indicate that there were significant differences between the indicated group and untreated MPS I mice with p=0.01 to 0.05 and p<0.01, respectively. Statistical analysis of panel I is discussed in the text.
Mice were also evaluated for their ability to climb down a pole (Fig. 3D and 3E), which is a task that requires strength, coordination, and vestibular function. For MPS I mice, 70% fell off the pole, and the average time to climb down was long at 96±41 seconds, where any animal that fell or refused to try was considered to take 120 seconds to climb down. For phenotypically- normal heterozygous normal mice, 13% fell off (p=0.004 vs. MPS I), and they took only 33±36 seconds to climb down the pole (p=0.005 vs. MPS I). For Adult-LTR-treated mice, only 10% fell off (p=0.02 vs. MPS I; not significant vs. normal), and they took 38±37 seconds to climb down (p=0.006 vs. MPS I, not significant vs. normal). It is unlikely that the MPS I mice were weak, as their forelimb grip strength was normal (Fig. 3F). MPS I mice did have slightly reduced swimming speeds in the Morris water maze test (Fig. 3G), although spatial learning and memory capabilities were not found to be impaired (Supplemental Fig. 4). The swim speed improved with Adult-LTR treatment.
Evaluation of Activity
During the 1 hour-locomotion test, untreated MPS I mice exhibited 168±81 rearings, which represented a 51% reduction relative to normal mice (341±106; p=0.007), as shown in Fig. 3H. The number of rearings in Adult-LTR-treated MPS I mice was at an intermediate level (232±134; 68% normal), although values were not significantly different from those in either normal or untreated MPS I mice. The MPS I mice also showed a greatly attenuated ambulatory response to initially being placed in a novel environment. Specifically, untreated MPS I mice showed reduced activity during the first 5 minutes (126±64 ambulations), which was 56% of normal (p=0.005). In contrast, RV-treated MPS I mice exhibited normal levels of ambulatory activity during the first 5 minutes of the session (232±112; 102% normal; p=0.002 vs. MPS I, not significant vs. normal). In addition, the number of ambulations did not change much over time over the 1-hour test session in untreated MPS I mice, as the value in the last 5 minute block was 81% of that found in the first 5 minute block (not significant for comparison of the initial and late values with the Students t test), while ambulations at the end of the hour in normal mice fell to 51% of the initial value (p=0.001), and those in RV-treated mice fell to 50% of the initial value (p<0.001). Thus, the MPS I mice showed a blunted ambulatory response to novelty and did not show the typical decrease in activity across the test session indicative of habituation, and Adult-LTR-treated mice were normalized in both of these parameters. Values did not differ between normal, untreated MPS I, and Adult-LTR-treated MPS I mice for the Rotarod test (Supplemental Fig. 4), for time to initiate walking, the time spent on a ledge, and the time spent on a platform (Supplemental Fig. 5).
Behavior studies in Neonatal-SIN- and Adult-SIN-treated MPS I mice
Behavioral studies were also performed in Neonatal-SIN and Adult-SIN-treated MPS I mice along with a separate cohort of heterozygous normal and untreated MPS I mice, as shown in Fig. 4. For this cohort of untreated MPS I mice, reduced ability to hold onto an inverted screen, reduced ability to climb down a pole, reduced swim speed, reduced rearing, reduced initial activity in a novel environment, and reduced habituation to the novel environment were similar to that depicted in Fig. 3. The only test where performance differed from that shown in Fig. 3 was the grip strength test, where the MPS I mice had a grip strength of 34±6 grams, which was lower than the value of 42±6 grams in normal mice (71% of normal, p<0.001 for MPS I vs. normal). The reason for the latter discrepancy is unclear, as the age and gender composition of the normal and untreated MPS I mice in the two studies were similar. Ambulatory activity during the first 5 minutes of the 1-hour locomotor activity test in MPS I mice was 50% of normal levels (p=0.007 for MPS I vs. normal), which resembles the result found in the MPS I mice in Fig. 3. MPS I mice did not exhibit habituation of ambulatory activity, since levels during the last 5 minutes were not significantly different from those observed in the first 5 minutes [105 ambulations in the first 5 minutes and 73 ambulations in the last 5 minutes, for a 30% reduction with time (p=0.10)]. In contrast, the normal mice exhibited robust habituation of ambulatory activity across the test session (209 vs. 121 ambulations during the first and last 5 minute blocks), for a 42% reduction (p=0.002).
Fig. 4. Behavior tests in Neonatal-SIN and Adult-SIN mice.
MPS I mice were injected with the SIN vector as newborns (Neo-SIN) or as adults (Adult-SIN), as detailed in Fig. 1, while phenotypically normal heterozygous and untreated MPS I littermates served as controls. Behavioral tests were performed for the total number of mice indicated inside the bar in panel A as detailed in the methods seconds at 8 months of age for normal mice (6 females and 7 males), untreated MPS I mice (11 females and 10 males), Neonatal-SIN MPS I mice (5 females and 6 males), and Adult-LTR MPS I mice (3 females and 3 males), as detailed in Fig. 3.
The Neonatal-SIN-treated mice demonstrated a significant reduction in the percent that fell off the vertical pole (p=0.003 vs. MPS I), a reduction in the time to crawl down the vertical pole (p=0.002 vs. MPS I), increase in forelimb grip strength (p<0.001 vs. MPS I), and increased numbers of ambulations in the first 5-minute block in the 1 hour-locomotor activity test (p=0.05) relative to untreated MPS I mice, but did not show improvements in their ability to hold onto an inverted pole, swim speeds, or the number of rearings relative to MPS I mice. In addition, the number of ambulations in the last 5-minute time block of the 1 hour-locomotion test were only reduced by 37%, which was not a significant difference from the number of ambulations in the first 5-minute block (p=0.15). Adult-SIN-treated mice had greater numbers of ambulations in the first 5 minute block of the activity test compared to untreated MPS I mice (p=0.007 vs. MPS I) and showed habituation, with a 48% reduction in movement from the first to the last 5-minute blocks compared with the first interval (p=0.009), but did not show improvements in other tests.
Histopathological evaluation of the middle and inner ear
Since the reduced ability of MPS I mice to climb down a pole and to hold onto an inverted screen could reflect reduced vestibular function, ears were evaluated for histopathological abnormalities of the vestibular system. Another reason for evaluating the ear is that we previously demonstrated that hearing was abnormal in MPS I mice and was partially improved with Adult-LTR therapy (Metcalf et al 2010), and we wanted to determine the histopathological explanation for this improvement. The stapes is one of the three ossicles of the middle ear, and its footplate attaches to the wall of the vestibule via the annular ligament to form the oval window which separates the middle from the inner ear. Sound waves cause the footplate of the stapes to move, which results in a fluid wave in the inner ear that is detected by sensory cells in the cochlea. Fig. 5 shows histopathology of the stapes where it attaches via the annular ligament to the bony wall of the vestibule of the inner ear. In the MPS I mouse, the ligament shows a great deal of lysosomal storage material that appears as white vacuoles (Fig. 5F), which may be a factor in the reduced hearing. The ligaments of two Adult-LTR-treated mice looked normal. All animals had some degree of exudate surrounding the stapes, which is abnormal and is consistent with a middle ear infection.
Fig. 5. Histopathology of the stapes of the middle ear.
Ears were collected at 8 months of age from normal, untreated MPS I, or MPS I mice that were treated with the LTR vector at 1.5 months of age (Adult-LTR), and processed as detailed in the methods. Sections from mice of the indicated groups were stained with toluidine blue. For the Adult-LTR mice, the average serum IDUA activity for the specific animals shown is indicated. A–D. Stapes. Low power view of the stapes where it articulates with the cochlear capsule via the annular ligament at the oval window. Arrows indicate the margins of the stapes footplate. E–H. Annular ligament. The annular ligament has large amounts of lysosomal storage that appear as white bubbles (white arrow) in untreated MPS I mice, while there is also storage in the chondrocytes of the articular cartilage. No storage is visible in normal or treated mice. The size markers are indicated in the panels. The results shown are representative for 6 normal mice, 4 untreated MPS I mice, and 4 Adult-LTR treated mice.
The round window membrane is the second region where the boundary between the inner and the middle ear is not bone. Proper compliance of the round window membrane is necessary for the stapes to generate a fluid wave within the inner ear. Fig. 6A, 6E, and 6I show that the round window membrane is thin in a normal mouse, and that the surrounding space (the antrum) is clear. In contrast, the round window membrane of an MPS I mouse is markedly thickened and contains white vacuoles with lysosomal storage material (Fig. 6J), while the round window antrum appears full of exudative material (Fig. 6B and 6F). We do not believe differences in the plane of sectioning can account for apparent differences in the thickness of the round window membrane, as Fig. 6A–6D show that the planes of sectioning are similar for the same ears, as demonstrated by the fact that all samples have 4 organs of Corti within the cochlea at similar locations. Both the thickness of the round window membrane and the exudate in the adjacent antrum may reduce the compliance of the round window membrane and contribute to hearing loss. The round window membrane of two Adult-LTR-treated mice was less thickened than for the MPS I mouse, but the middle ear still contained substantial exudate, as shown in Fig. 6C–6D and Fig. 6G–6K.
Fig. 6. Histopathological analysis of the cochlea, round window, and cristae.
Mice were treated as detailed in Fig. 3, and ears were collected at 8 months of age, processed, sectioned, and stained with toluidine blue. A–D. Low power cochlear images. The asterisk indicates the inner ear and the arrows indicates a crista of one semicircular canal. The box indicates the region shown at higher power in panels E–H with some rotation. E–H. Intermediate power views of the inner ear, round window membrane, and round window antrum. The asterisk indicates the inner ear, and the box indicates the region with the round window membrane shown at higher power in panels I–L. The middle ear is located to the right of the round window membrane in all panels. The antrum is clear in the normal mouse (panel E) but is filled with exudate in the other panels. The arrow indicates crista of one semicircular canal. I–L. Round window membrane. The asterisk indicates the inner ear, and the arrow indicates lysosomal storage material in the round window membrane in the untreated MPS I mouse. M–P. High power view of crista. The black arrow in the untreated MPS I mouse shown in panel N identify lysosomal storage material, which is present in hair and accessory cells of the crista. The Adult-LTR-treated mouse shown in panel O also has lysosomal storage, although the Adult-LTR-treated mouse in panel P does not have detectable storage. Size markers are indicated in the panels. The results shown are representative for 6 normal mice, 4 untreated MPS I mice, and 4 Adult-LTR treated mice.
The semicircular canals contain ampular cristae with hair cells, which are mechanoreceptor cells that sense rotation. The cristae appear as cone-shaped structures identified with arrows in the low and middle power views in Fig. 6A–6D, and Fig. 6E–6H, respectively. In the high power image shown in Fig. 6N of the MPS I mouse, both hair cells and surrounding support cells contain large amounts of lysosomal storage material that is absent in the normal mouse in Fig. 6M. These defects are consistent with the hypothesis that abnormal vestibular function could contribute to sensorimotor abnormalities. The Adult-LTR-treated mouse with the lowest expression of the group had a partial reduction in lysosomal storage material in the cristae (Fig. 6O), while an Adult-LTR-treated mouse with higher expression had complete resolution of lysosomal storage (Fig. 6P).
Discussion
Behavioral impairment is an important feature of MPS I in human patients. We had previously demonstrated that Neonatal-LTR-treated (Chung et al 2007) and Adult-LTR-treated (Ma et al 2007) MPS I mice that received 1×1010 TU/kg of vector had reduced lysosomal storage in neurons and microglial cells of the brain, but had not previously evaluated brains for storage in Adult-LTR mice that received a higher dose of 1.7×1010 TU/kg of the LTR vector, or in Neonatal-SIN or Adult-SIN mice that received 1×1010 TU/kg of the SIN vector. In addition, although hearing and visual tests were performed previously for all of these groups of mice, we had not previously tested behavioral function. These studies evaluated the latter 3 groups of treated mice mentioned above, but did not evaluate the former 2 groups, as the behavioral tests were not set up when they were sacrificed.
All mice that were evaluated here had stable serum IDUA activity from 1 week after transduction until the end of the study at 8 months, as shown previously for the identical mice studied here that received the SIN vector (Metcalf et al 2010), and as shown for a different cohort of Adult-LTR mice that received a slightly lower dose of vector (Ma et al 2007). There were a few mice that lost expression over time, presumably due to an immune response, and all such mice were excluded from this study. Adult-LTR-, Neonatal-SIN, and Adult-SIN-treated MPS I mice achieved an average lifetime serum IDUA activity of 235±20 (84-fold normal), 127±10 (45-fold normal), and 71±7 U/mL (25-fold normal), respectively, although serum enzyme does not necessarily mean that enzyme will diffuse to all tissues. In general, Adult-LTR mice had higher brain IDUA activity, lower levels of another lysosomal enzyme (GUSB; such a reduction is a biochemical marker of disease correction), and a lower percentage of cells in the brain with lysosomal storage material than did Neonatal-SIN and Adult-SIN-treated mice, which correlated with greater improvements in behavioral tests in the Adult-LTR group, as will be expanded on below. The finding that Adult-SIN mice had slightly higher forebrain IDUA activity than did Neonatal-SIN mice is discrepant with the fact that Adult-SIN mice had slightly lower serum IDUA, similar brain GUSB activity, and slightly worse performance on some behavioral tests than did Neonatal-SIN mice. We believe that the brain IDUA assay is the least reliable test due to difficulties in consistently removing all blood from brain with perfusion, while behavioral tests sometimes require larger numbers of animals to achieve statistical significance if differences are modest. Although it has been reported than the blood:brain barrier is more permissive to enzyme in the neonatal period (Vogler et al, 2005), the greater efficacy of Adult-LTR-treatment than Neonatal-SIN treatment likely reflects the fact that reductions in lysosomal storage observed at 8 months of age are due to enzyme than reached the brain during adulthood rather than during the newborn period. Treated mice were not evaluated for anti-canine IDUA antibodies in this study, but such antibodies were absent in a previous study after adult gene transfer (Ma et al, 2007).
Vestibular function
In this study, MPS I mice had many behavioral abnormalities. This is the first report that MPS I mice have a profoundly reduced ability to hold onto an inverted screen and to climb down a pole. Since these mice behaved normally in both the constant speed and accelerating rotorod test (Supplemental Fig. 4), could readily climb to the top of a vertical screen (Fig. 3A and 4A), and had a normal grip strength in one of the two studies that were performed (Fig. 3F), we infer that their cerebellar and motor functions were relatively well-maintained. Nevertheless, since the grip strength was abnormal in the second study (Fig. 4F), the swim speed was consistently reduced, the rearings were consistently reduced, and MPS I mice have abnormal joint histology (GB and RG, unpublished data), we cannot rule out the possibility that musculoskeletal abnormalities contributed to reduced function in these tests. We favor the hypothesis that abnormal vestibular function contributes to the reduced ability to hold onto an inverted screen and climb down a pole, as we demonstrate here that MPS I mice have enormous amounts of lysosomal storage material in the hair cells and accessory cells of the cristae ampullaris of the semicircular canals, which are critical for sensing the position in space. Similar histochemical abnormalities were observed in the cristae of MPS VII mice (Ohlemiller et al 2002) and of MPS IIIB mice (Heldermon et al 2007), although no functional abnormality was associated with this finding. It is unclear if humans have abnormalities in tests that might correlate with those observed here in mice.
Adult-LTR-treated mice showed an improved ability to hold onto an inverted screen and climb down a pole compared with untreated MPS I mice. The reduction in lysosomal storage in the cristae of the semicircular canals of the inner ear in Adult-LTR-treated compared with untreated MPS I mice may have contributed to their improved function on these tests, although improvements in bone and joint disease or in muscle strength could play a role. The Neonatal-SIN and Adult-SIN-treated mice did not have statistically significant improvements in their performance on the inverted screen, although the Neonatal-SIN-treated mice (but not Adult-SIN-treated mice) had improvements in their ability to climb down a pole and in their grip strength compared with untreated MPS I mice. The failure to correct the inverted screen performance may reflect the lower serum IDUA activity achieved for both SIN groups compared with the Adult-LTR-treated mice. Ears were not evaluated histopathologically in either of the SIN groups due to the time involved in sectioning through an entire ear.
Locomotor activity
This paper confirms the consistent report of others that the number of rearings is reduced, the initial activity in a novel environment is lower, and activity fails to decline with habituation to an environment in MPS I compared with normal mice (Reolon et al 2006; Pan et al 2008). This study failed to identify significant differences in the time and path length to reach a platform in both cued and place trials between normal and MPS I mice in the Morris water maze test for the first cohort of mice that were evaluated (Supplemental Fig. 3), although the swimming speed was reduced to ~80% of that in normal mice, as noted above. Some (Wolf et al. 2011) but not others (Pan et al. 2008) have reported differences at older ages in the Morris water maze test, although in these studies, the time to reach the platform was reported and the swim speed was not commented upon, raising the possibility that slow swimming was a factor. This study also failed to identify abnormalities in the rotarod test (Supplemental Fig. 4) and in other sensorimotor and locomotor tests (Supplemental Fig. 5).
In this study, the Adult-LTR-treated mice did not have statistically significant improvement in the number of rearings, although there appeared to be partial improvement. Adult-LTR-treated mice did have significant improvements in their initial activity in a novel environment and their habituation over time, suggesting that treatment normalized their ambulatory response to a novel environment and subsequent habituation in activity. Neonatal-SIN and Adult-SIN-treated mice did not have improvement in the number of rearings, although both had increased activity in the first 5-minute block in a novel environment compared with untreated MPS I mice. The Adult-SIN-treated mice showed habituation over time, while the Neonatal-SIN mice did not show significant habituation, although the activity for this latter group in the last 5 minute block of an hour-long test session was reduced by 37% relative to the activity in the first 5 minutes, which was marginally non-significant (p=0.15). Altogether, these data suggest that Neonatal-SIN and Adult-SIN gene therapy was less effective than Adult-LTR gene therapy, which likely reflects the lower serum IDUA activity achieved with the SIN vector compared with the LTR vector. However, the SIN vector did result in some behavioral improvements.
Histochemical evaluation of the ear
Schachern et al (2007) previously reported that reduced hearing in MPS I mice was associated with a middle ear exudate at 2 months or older, that modest amounts of lysosomal storage were present in the fibrocytes of the spiral ligament and mesothelial cells of the basilar and Reissner’s membranes, and that there was a reduction in the number of hair cells of the cochlea at 1 year or older. We previously demonstrated that hearing was improved, but not normalized, in the same Adult-LTR-treated MPS I mice that were evaluated here relative to untreated MPS I mice, as MPS I mice require 89±2 decibels (db) of sound at 10 kHz to evoke a brainstem response, and the same Adult-LTR-treated mice whose ears were evaluated here only required 59±2 db, although this remained higher than the value in normal mice of 39±6 db (Metcalf et al 2010). Here, we report that lysosomal storage was markedly reduced in the annular ligament, and was reduced, but not eliminated, in the round window membrane, which could contribute to improved hearing. However, a middle ear exudate was still consistently present in Adult-LTR-treated mice, and we hypothesize that the failure to prevent an exudate with gene therapy may be a factor in the hearing deficit that persists in the Adult-LTR-treated MPS I mice. A middle ear infiltrate has also been reported in MPS VII mice (Ohlemiller et al 2002) and MPS IIIB mice (Heldermon et al 2007), while modest amounts of lysosomal storage were found in the chondrocytes in the articular cartilage of the stapes in MPS IIIB mice.
Mechanism of improvement in behavioral function
These data suggest a beneficial effect of Adult-LTR gene therapy on biochemical, histopathological, and functional abnormalities in the brain of MPS I mice, and partial improvement in some parameters in Neonatal-SIN and Adult-SIN-treated mice. Since we were previously unable to detect RNA in the brain (Metcalf et al 2010) of the same mice that were evaluated here, we believe that improvements in behavioral function likely reflect diffusion of enzyme from blood into the brain. This contradicts the dogma that enzyme cannot cross the blood:brain barrier, although the barrier is not absolute (Banks 2004), and there are some data that it is disrupted in MPS IIIB (Garbuzova-Davis et al 2011) as well as other data that GUSB in serum can traverse the barrier and reach the brain (Vogler et al 2005, Grubb et al 2008). The SIN vector was less effective in this study than the LTR vector, which likely reflects the lower expression observed in vivo, as discussed previously (Metcalf et al 2010). Thus, although SIN vectors are generally safer in vivo than LTR-intact vectors due to their inability to enhance expression of a nearby oncogene (Trobridge 2011), their reduced efficacy in our hands poses a problem, and a more detailed risk:benefit analysis will need to be done in the future to decide which vector to use. It remains possible that a better effect could be observed with the SIN vector if higher doses were used.
Other vectors have exhibited a beneficial effect on behavioral tests or would be predicted to do so. Administration of a sleeping beauty transposon resulted in 1300 U/ml (Aronovich EL et al, 2009), while a mini-circle DNA resulted in 1000 U/ml (Osborn et al 2011), both of which would be predicted to result in a marked behavioral improvement. Achieving IDUA levels of ~100 U/ml in serum from red blood cells after transduction of hematopoietic stem cells with a lentiviral vector (Wang et al 2009a) improved habituation to a novel environment and increased grooming, which is a level where we also found some level of habituation to a novel environment, although the test that we performed was different. The fact that neonatal AAV2 injection that resulted in ~10 U/ml in plasma at late times improved habituation (Hartung et al 2004) was surprising, but expression might have been substantially higher in the newborn period as copies of episomal AAV vectors generally decline with animal growth, and the test they performed was different from ours. Systemic administration of a lentiviral vector resulted in relatively low organ enzyme activity and behavioral testing was not done (Di Domenico et al 2006). Ex vivo hematopoietic stem cell-directed gene therapy resulted in migration of blood cells into the brain and improved habituation to a novel environment (Visigalli et al 2010). Further studies will need to evaluate the risk of insertional mutagenesis or other adverse events against the efficacy of different vectors for gene therapy.
Although transduction of brain cells after gene therapy does not appear to contribute to the beneficial effect in this study, it has contributed to improvements in other studies. Lentiviral vector administration to newborns resulted in transduction of neurons in the brain, although the effect on behavioral function was not reported (Kobayashi et al 2005). Direct injection of AAV8 resulted in expression in brain and improved function in the Morris water maze test (Wolf et al 2011), while direct injection of AAV into MPS I dogs has resulted in expression (Ellinwood et al 2011; Ciron et al 2006), although behavioral function has not been assessed. Similarly, although intrathecal injection of AAV resulted in expression in the brain, behavioral function was not evaluated (Watson et al. 2006). Thus, there are a variety of approaches that have been successfully used to achieve production of IDUA in brain, and some of these studies have also documented behavioral improvement.
Implications for future gene therapy studies
One of the major concerns of any therapy for MPS I or related diseases is the question of whether or not it will improve behavioral function. Although it has been presumed that enzyme in blood will not cross the blood brain barrier, a variety of data suggest that some enzyme can reach the brain from blood, albeit the process is somewhat inefficient. This study demonstrating that a gene therapy approach that does not result in expression in the brain can indeed improve behavioral function is encouraging that either ERT or systemic gene therapy can improve this important parameter, although the caveat is that very high levels of enzyme in blood, over 200 U/ml (71-fold normal), are required to achieve an optimal effect in mice.
Supplementary Material
Synopsis.
Retroviral vectors can improve behavioral and inner ear abnormalities in MPS I mice.
Acknowledgments
We thank Elizabeth Neufeld for sending us the MPS I mice, and Sara Conyers for performing behavioral tests. This work was supported by the Ryan Foundation, the National MPS Society, and the National Institutes of Health (DK66448 awarded to KPP). Histology was supported by P30 DC004665 awarded to R. Chole, and behavioral studies were supported by NIH Neuroscience Blueprint Interdisciplinary Center Core Grant P30 NS057105 awarded to Washington University (DFW). GB received a scholarship from the Conselho Nacional de Desenvolvimento Cientifico (CNPq) of Brazil (200584/2010–3).
References
- Aldenhoven M, Boelens JJ, de Koning TJ. The Clinical Outcome of Hurler Syndrome after Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Aronovich EL, Bell JB, Khan SA, et al. Systemic correction of storage disease in MPS I NOD/SCID mice using the sleeping beauty transposon system. Mol Ther. 2009;17:1136–44. doi: 10.1038/mt.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner F, Schmiedeskamp C, Krummenauer F, et al. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- Baldo G, Wu S, Howe R, et al. Pathogenesis of aortic dilatation in MPS VII mice may involve complement activation. Mol Genet Metab. 2011;104:608–19. doi: 10.1016/j.ymgme.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. Are the extracellular pathways a conduit for the delivery of therapeutics to the brain. Curr Pharm Des. 2004;10:1365–70. doi: 10.2174/1381612043384862. [DOI] [PubMed] [Google Scholar]
- Chung S, Ma X, Liu Y, Lee D, Tittiger M, Ponder KP. Effect of neonatal administration of a retroviral vector expressing alpha-L-iduronidase upon lysosomal storage in brain and other organs in mucopolysaccharidosis I mice. Mol Genet Metab. 2007;90:181–92. doi: 10.1016/j.ymgme.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ciron C, Desmaris N, Colle MA, et al. Gene therapy of the brain in the dog model of Hurler’s syndrome. Ann Neurol. 2006;60:204–13. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- Di Domenico C, Di Napoli D, Gonzalez Y, et al. Limited transgene immune response and long-term expression of human alpha-L-iduronidase in young adult mice with mucopolysaccharidosis type I by liver-directed gene therapy. Hum Gene Ther. 2006;17:1112–21. doi: 10.1089/hum.2006.17.1112. [DOI] [PubMed] [Google Scholar]
- Ellinwood NM, Ausseil J, Desmaris N, et al. Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol Ther. 2011;19:251–9. doi: 10.1038/mt.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller GN, Burger PC. Central nervous system, in Histology for Pathologists. In: Mills S, Williams Lippincott, Wilkins, editors. 3. Philadelphia USA: 2007. pp. 273–319. [Google Scholar]
- Garbuzova-Davis S, Louis MK, Haller EM, Derasari HM, Rawls AE, Sanberg PR. Blood-brain barrier impairment in an animal model of MPS III B. PLoS One. 2011;6:e16601. doi: 10.1371/journal.pone.0016601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb JH, Vogler C, Levy B, Galvin N, Tan Y, Sly WS. Chemically modified beta-glucuronidase crosses blood-brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2008;105:2616–21. doi: 10.1073/pnas.0712147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung SD, Frandsen JL, Pan D, et al. Correction of metabolic, craniofacial, and neurologic abnormalities in MPS I mice treated at birth with adeno-associated virus vector transducing the human alpha-L-iduronidase gene. Mol Ther. 2004;9:866–875. doi: 10.1016/j.ymthe.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Heldermon CD, Hennig AK, Ohlemiller KK, et al. Development of sensory, motor and behavioral deficits in the murine model of Sanfilippo syndrome type B. PLoS One. 2007;2:e772. doi: 10.1371/journal.pone.0000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–8. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Carbonaro D, Pepper K, et al. Neonatal gene therapy of MPS I mice by intravenous injection of a lentiviral vector. Mol Ther. 2005;11:776–89. doi: 10.1016/j.ymthe.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu L, Hennig AK, et al. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Ma X, Liu Y, Tittiger M, et al. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15:889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]
- Metcalf J, Ma X, Linders B, Wu S, et al. A self-inactivating gamma-retroviral vector reduces manifestations of mucopolysaccharidosis I in mice. Molecular Therapy. 2010;18:334–42. doi: 10.1038/mt.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill; 2001. pp. 3421–3452. [Google Scholar]
- Ohlemiller KK, Hennig AK, Lett JM, Heidbreder AF, Sands MS. Inner ear pathology in the mucopolysaccharidosis VII mouse. Hear Res. 2002;16:969–84. doi: 10.1016/s0378-5955(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MJ, McElmurry RT, Lees CJ, et al. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of α-L-iduronidase in mice with mucopolysaccharidosis type I. Mol Ther. 2011;19:450–60. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Sciascia A, Vorhees C, Williams MT. Progression of multiple behavior deficits with various age of onsets in a murine model of Hurler syndrome. Brain Research. 2008;1188:241–253. doi: 10.1016/j.brainres.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder KP, Haskins ME. Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther. 2007;7:1333–45. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reolon GK, Braga LM, Camassola M, et al. Long-term memory for aversive training is impaired in IDUA−/− mice, a genetic model of mucopolysaccharidosis type I. Brain Research. 2006;1076:225–230. doi: 10.1016/j.brainres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Reolon GK, Reinke A, de Oliveira MR, et al. Alterations in oxidative markers in the cerebellum and peripheral organs in MPS I mice. Cell Mol Neurobiol. 2009;29:443–8. doi: 10.1007/s10571-008-9335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachern PA, Cureoglu S, Tsuprun V, Paparella MM, Whitley CB. Age-related functional and histopathological changes of the ear in the MPS I mouse. Int J Pediatr Otorhinolaryngol. 2007;71:197–203. doi: 10.1016/j.ijporl.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–9. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Beck M, Clarke JT, Cox GF. Childhood onset of Scheie syndrome, the attenuated form of mucopolysaccharidosis I. J Inherit Metab Dis. 2010;33:421–7. doi: 10.1007/s10545-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD. Genotoxicity of retroviral hematopoietic stem cell gene therapy. Expert Opin Biol Ther. 2011;11:581–93. doi: 10.1517/14712598.2011.562496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visigalli I, Delai S, Politi LS, et al. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood. 2010;116:5130–9. doi: 10.1182/blood-2010-04-278234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler C, Levy B, Grubb JH, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102:14777–82. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley SU. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin Cell Dev Biol. 2004;15:433–444. doi: 10.1016/j.semcdb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhang W, Kalfa TA, et al. Reprogramming erythroid cells for lysosomal enzyme production leads to visceral and CNS cross-correction in mice with Hurler syndrome. Proc Natl Acad Sci U S A. 2009a;106:19958–63. doi: 10.1073/pnas.0908528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Cambray-Forker EJ, Ohanian K, et al. Treatment reduces or stabilizes brain imaging abnormalities in patients with MPS I and II. Mol Genet Metab. 2009b;98:406–11. doi: 10.1016/j.ymgme.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Watson G, Bastacky J, Belichenko P, et al. Intrathecal administration of AAV vectors for the treatment of lysosomal storage in the brains of MPS I mice. Gene Ther. 2006;13:917–25. doi: 10.1038/sj.gt.3302735. [DOI] [PubMed] [Google Scholar]
- Wolf DA, Lenander AW, Nan Z, et al. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol Dis. 2011;43:123–33. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Xiao M, Xu L, Yamada KA, Ornitz DM. Impaired spatial learning and defective theta burst induced LTP in mice lacking fibroblast growth factor 14. Neurobiol Dis. 2007;26:4–26. doi: 10.1016/j.nbd.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wraith JE, Beck M, Lane R, et al. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: results of a multinational study of recombinant human alpha-L-iduronidase (laronidase) Pediatrics. 2007;120:e37–46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






