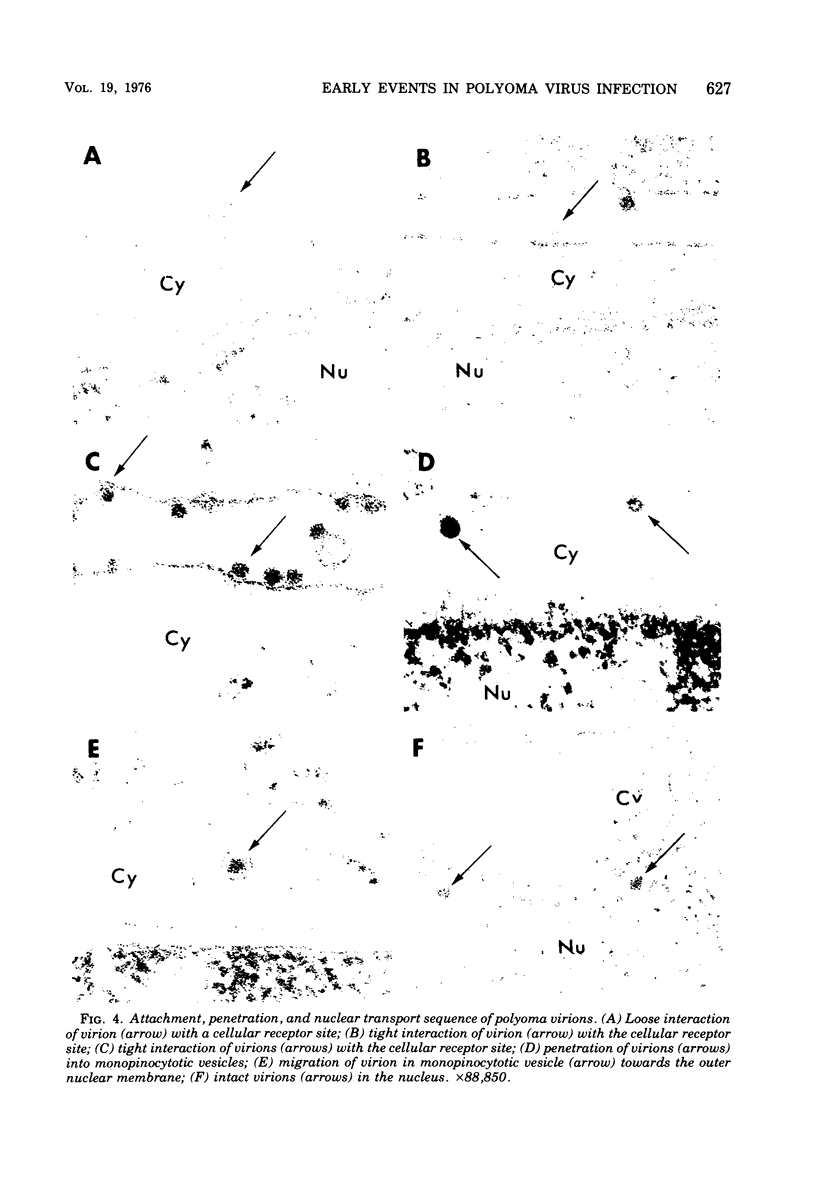
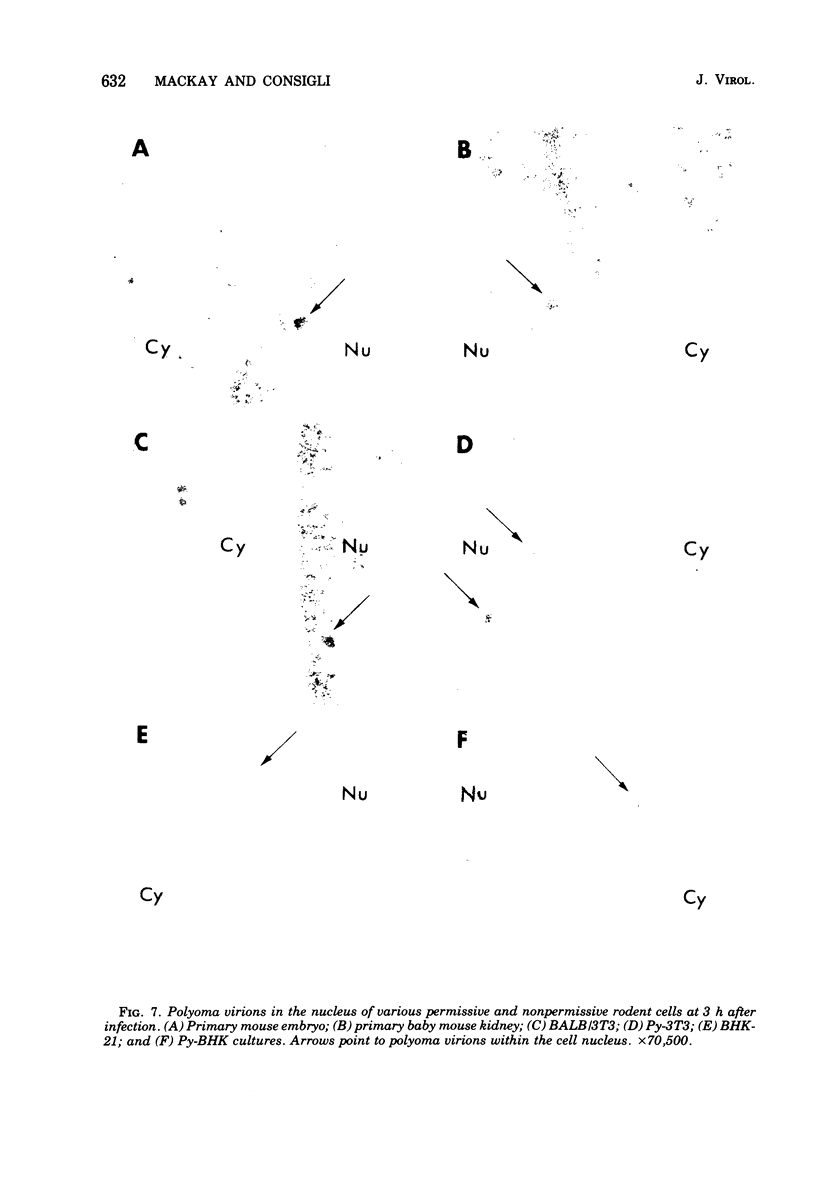
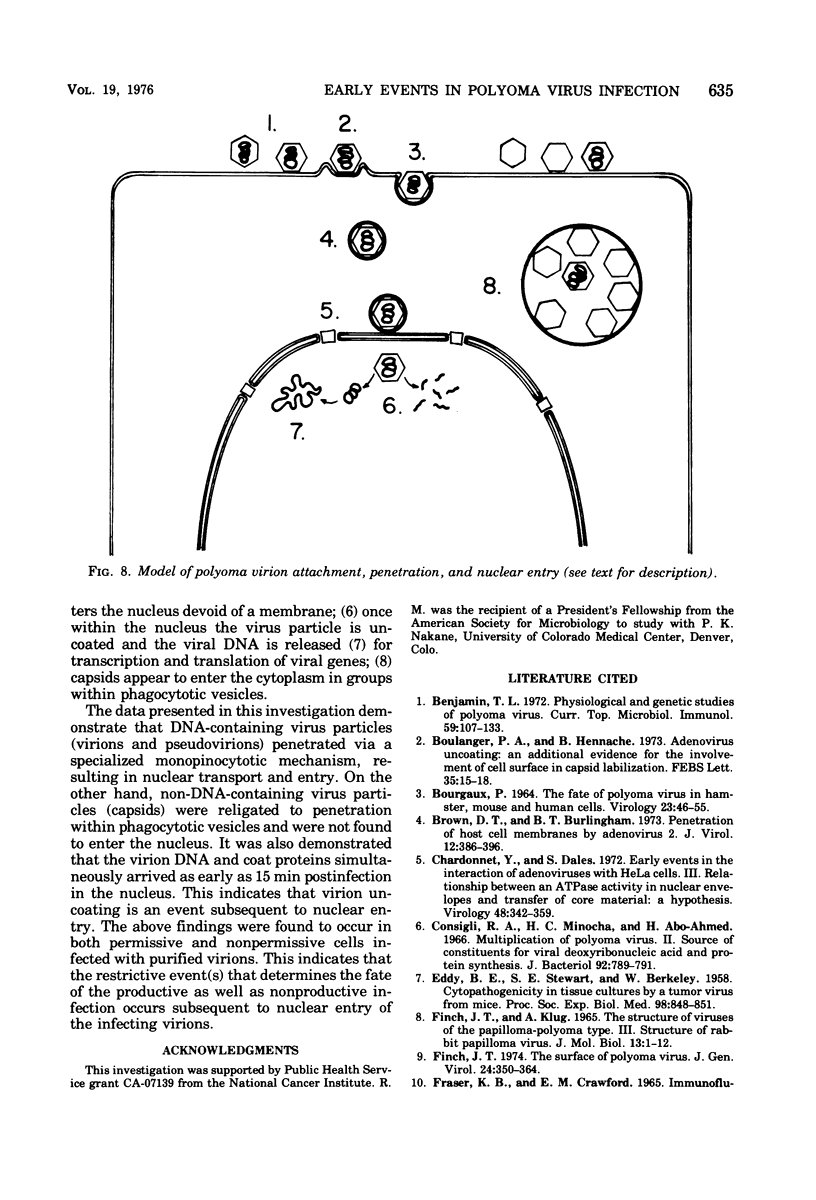
Abstract
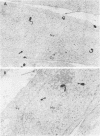
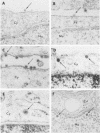
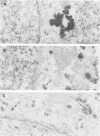
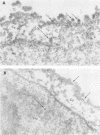
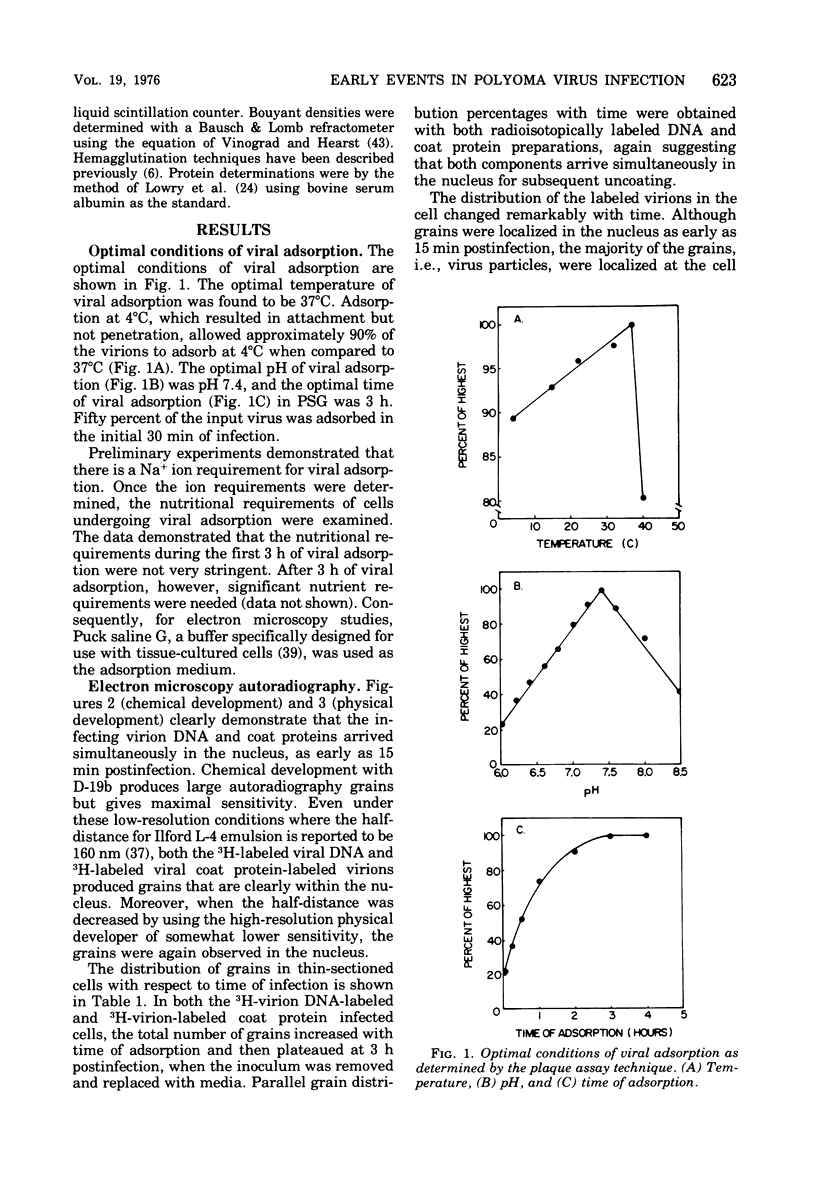
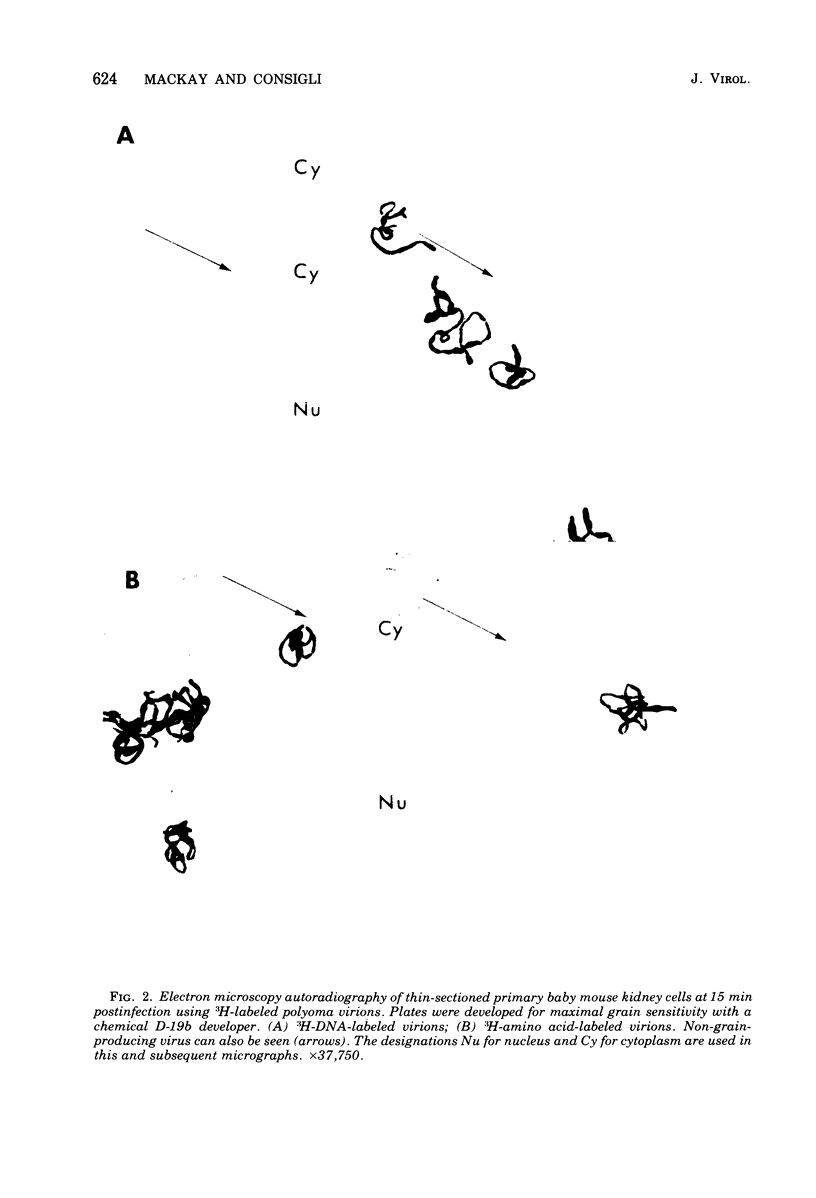
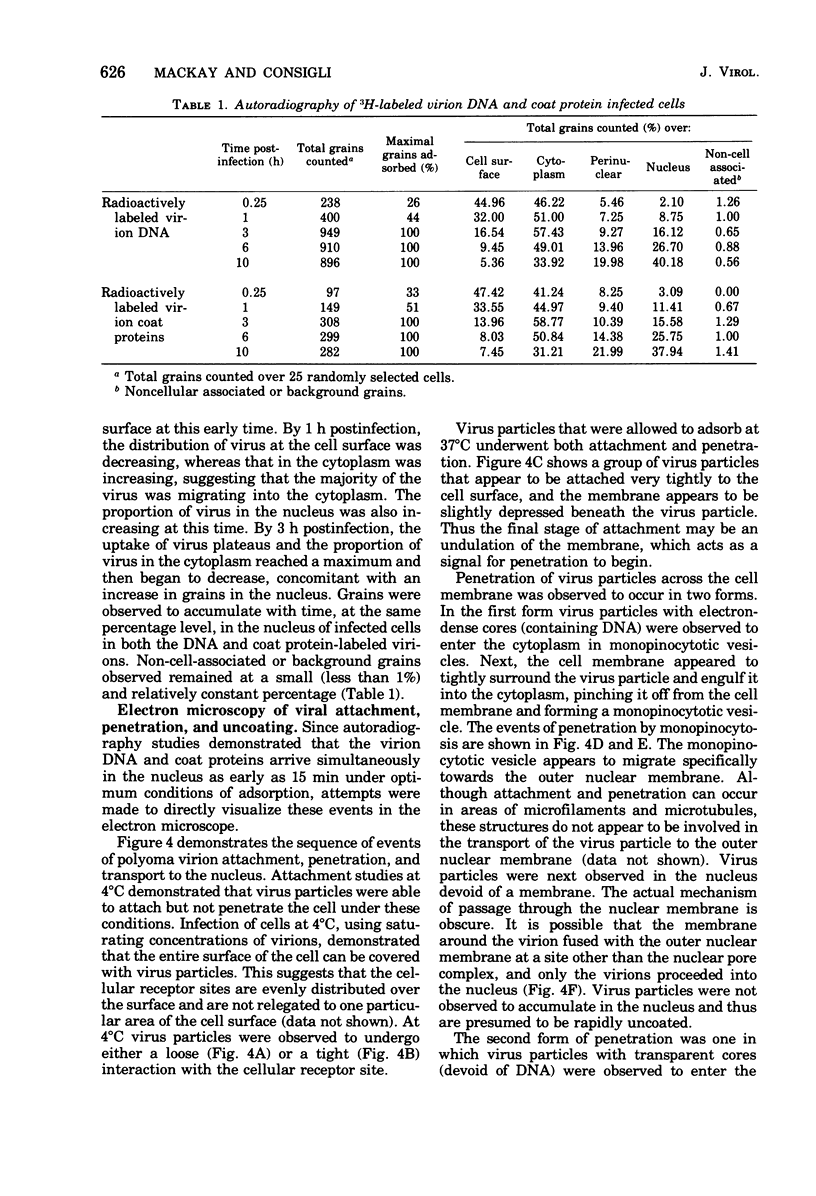
The plaque-assay technique was used as a tool to determine the optimal conditions for adsorption of polyoma virions to host cells. Using these optimal conditions of adsorption, an electron microscopy study of the early events of infection was performed. By electron microscopy and autoradiography, it was demonstrated that both the viral coat proteins and DNA arrive simultaneously in the nucleus as early as 15 min postinfection. When horseradish peroxidase-labeled virions, pseudovirions, and capsids were used to infect cells, only the particles with nucleic acid or a factor(s) associated with the nucleic acid, i.e., histones, appeared to enter the nucleus. Moreover, when virions were used to infect either permissive or nonpermissive cells, identical early events of viral infection, i.e., adsorption, penetration, and nuclear transport, were observed, suggesting that these early events of infection are a property of the virion and not the host cell.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOURGAUX P. THE FATE OF POLYOMA VIRUS IN HAMSTER, MOUSE, AND HUMAN CELLS. Virology. 1964 May;23:46–55. doi: 10.1016/s0042-6822(64)80006-4. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Physiological and genetic studies of polyoma virus. Curr Top Microbiol Immunol. 1972;59:107–133. doi: 10.1007/978-3-642-65444-2_4. [DOI] [PubMed] [Google Scholar]
- Boulanger P. A., Hennache B. Adenovirus uncoating: an additional evidence for the involvement of cell surface in capsid labilization. FEBS Lett. 1973 Sep 1;35(1):15–18. doi: 10.1016/0014-5793(73)80567-8. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Burlingham B. T. Penetration of host cell membranes by adenovirus 2. J Virol. 1973 Aug;12(2):386–396. doi: 10.1128/jvi.12.2.386-396.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. 3. Relationship between an ATPase activity in nuclear envelopes and transfer of core material: a hypothesis. Virology. 1972 May;48(2):342–359. doi: 10.1016/0042-6822(72)90045-1. [DOI] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. E., STEWART S. E., BERKELEY W. Cytopathogenicity in tissue culture by a tumor virus from mice. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):848–851. doi: 10.3181/00379727-98-24205. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. The structure of viruses of the papilloma-polyoma type 3. Structure of rabbit papilloma virus, with an appendix on the topography of contrast in negative-staining for electron-microscopy. J Mol Biol. 1965 Aug;13(1):1–12. doi: 10.1016/s0022-2836(65)80075-4. [DOI] [PubMed] [Google Scholar]
- Finch J. T. The surface structure of polyoma virus. J Gen Virol. 1974 Aug;24(2):359–364. doi: 10.1099/0022-1317-24-2-359. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Haas M. Rapid concentration and purification of polyoma virus and SV40 with polyethylene glycol. Virology. 1970 Sep;42(1):248–250. doi: 10.1016/0042-6822(70)90263-1. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J. The use of fluorocarbon for "unmasking" polyoma virus hemagglutinin. Virology. 1959 Nov;9:488–489. doi: 10.1016/0042-6822(59)90141-2. [DOI] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- KLUG A. STRUCTURE OF VIRUSES OF THE PAPILLOMA-POLYOMA TYPE. II. COMMENTS ON OTHER WORK. J Mol Biol. 1965 Feb;11:424–431. doi: 10.1016/s0022-2836(65)80067-5. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare G. P., Consigli R. A. Cytologic studies in polyoma virus-infected mouse embryo cells. Am J Vet Res. 1967 Sep;28(126):1527–1536. [PubMed] [Google Scholar]
- Khare G. P., Consigli R. A. Multiplication of Polyoma Virus I. Use of Selectively Labeled (H) Virus to Follow the Course of Infection. J Bacteriol. 1965 Sep;90(3):819–821. doi: 10.1128/jb.90.3.819-821.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriwa B. M. A reliable, standardized method for ultrastructural electron microscopic radioautography. Histochemie. 1973 Oct 3;37(1):1–17. doi: 10.1007/BF00306855. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Weihing R. R. Adenovirus binds to rat brain microtubules in vitro. J Virol. 1975 Sep;16(3):696–706. doi: 10.1128/jvi.16.3.696-706.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Takemoto K. K., Daniel W. A. Replication of polyoma virus in mouse embryo cells: electron microscopic observations. Virology. 1966 Oct;30(2):242–256. doi: 10.1016/0042-6822(66)90099-7. [DOI] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Characterization of polyoma DNA-protein complexes. I. Electrophoretic identification of the proteins in a nucleoprotein complex isolated from polyoma-infected cells. J Virol. 1974 Dec;14(6):1326–1336. doi: 10.1128/jvi.14.6.1326-1336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. In vitro radioisotopic labeling of proteins associated with purified polyoma virions. J Virol. 1974 Dec;14(6):1627–1629. doi: 10.1128/jvi.14.6.1627-1629.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Recent progress in the peroxidase-labeled antibody method. Ann N Y Acad Sci. 1975 Jun 30;254:203–211. doi: 10.1111/j.1749-6632.1975.tb29170.x. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., ROBINSON A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. 1958 Dec 1;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACHS L., FOGEL M., WINOCOUR E. In vitro analysis of a mammalian tumour virus. Nature. 1959 Mar 7;183(4662):663–664. doi: 10.1038/183663a0. [DOI] [PubMed] [Google Scholar]
- SACHS L., MEDINA D. In vitro transformation of normal cells by polyoma virus. Nature. 1961 Feb 11;189:457–458. doi: 10.1038/189457a0. [DOI] [PubMed] [Google Scholar]
- STOKER M., ABEL P. Conditions affecting transformation by polyoma virus. Cold Spring Harb Symp Quant Biol. 1962;27:375–386. doi: 10.1101/sqb.1962.027.001.035. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Bachmann L., Salpeter E. E. Resolution in electron microscope radioautography. J Cell Biol. 1969 Apr;41(1):1–32. doi: 10.1083/jcb.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969 Jan;130(1):305–310. doi: 10.3181/00379727-130-33543. [DOI] [PubMed] [Google Scholar]
- Steiner L. A., Lowey S. Optical rotatory dispersion studies of rabbit gamma-G-immunoglobulin and its papain fragments. J Biol Chem. 1966 Jan 10;241(1):231–240. [PubMed] [Google Scholar]
- Thorne H. V., House W., Kisch A. L. Electrophoretic properties and purification of large and small plaque-forming strains of polyoma virus. Virology. 1965 Sep;27(1):37–43. doi: 10.1016/0042-6822(65)90141-8. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]