Abstract
Neuroblastoma is uniquely sensitive to single-agent inhibition of the DNA damage checkpoint kinase Chk1, leading us to examine downstream effectors of this pathway and identify mitotic regulator Wee1 as an additional therapeutic target in this disease. Wee1 was overexpressed in both neuroblastoma cell lines and high-risk patient tumors. Genetic or pharmacologic abrogation of Wee1 signaling results in marked cytotoxicity in 10/11 neuroblastoma cell lines with a median IC50 of 300nM for the Wee1-selective small molecule inhibitor MK-1775. Murine tumor lines derived from mice that were either heterozygous or homozygous for MycN were particularly sensitive to single-agent inhibition of Wee1 (IC50s of 160 nM and 62 nM, respectively). Simultaneous pharmacologic inhibition of Chk1 and Wee1 acted in a synergistic fashion to further impede neuroblastoma cell growth in vitro, in a manner greater than the individual inhibitors either alone or combined with chemotherapy. Combination Chk1 and Wee1 inhibition also revealed in vivo efficacy in neuroblastoma xenografts. Taken together, our results demonstrate that neuroblastoma cells depend on Wee1 activity for growth, and that inhibition of this kinase may serve as a therapeutic for neuroblastoma patients.
Keywords: Neuroblastoma, Chk1, Wee1, MK-1775, MK-8776, SCH 900776
INTRODUCTION
Neuroblastoma is a common pediatric tumor derived from the cells of the sympathetic nervous system that manifests with significant clinical heterogeneity (1–3). Patients are typically stratified into risk groups based upon several criteria at diagnosis, including age, tumor ploidy, MYCN amplification status and histological features (1,2). Although low-risk patients are successfully treated with surgery, approximately 50% of all children with neuroblastoma are diagnosed with high-risk disease, requiring myeloablative chemotherapy followed by maintenance with retinoids and anti-GD2-based immunotherapy (3). Despite this intense multi-modal treatment regimen, half of these patients will eventually relapse and succumb to the disease, and those that survive are typically burdened with treatment-related chronic illnesses (4). The intense chemotherapy schedule and poor survival rate characteristic of high-risk and/or relapsed neuroblastoma underscores the need for novel therapies to successfully treat children burdened with this disease. To this end, our group recently identified the DNA damage response protein checkpoint kinase 1 (Chk1), as a bona fide molecular target through a siRNA screen of the neuroblastoma kinome (5). Our findings demonstrated that neuroblastoma possesses disproportionate Chk1 activity, and compared to any other tumor histotype, is uniquely sensitive to single-agent Chk1 inhibition, likely due to MYC and MYCN oncogene induced replicative stress. Due to this reliance upon Chk1 signaling, we hypothesized that neuroblastoma is particularly susceptible to DNA damage response (DDR) pathway interference, leading us to investigate potentially tractable pathway members.
The maintenance of genomic integrity through error-free DNA replication and precisely timed cellular division is essential for the accurate transfer of genetic information to daughter cells. Flawless cell cycle progression is dependent upon the tightly regulated coordination between several cell cycle checkpoint proteins and the cyclin-dependent kinase (CDK) family of proteins. Malignant cells frequently exploit defects in DNA repair and/or cell cycle checkpoint pathways to stimulate aberrant cellular division, thereby gaining a distinct survival advantage (6). Key regulators of DNA damage surveillance pathways, such as the checkpoint kinases ATR, Chk1 and Wee1, recognize DNA aberrations and arrest cellular division until genomic integrity is restored (7,8). Paradoxically however, the aberrant activity of DNA damage response proteins can also serve to dampen the replication stress generated by oncogenic transformation, thereby protecting cancer cells (9).
Both of the serine/threonine kinases Chk1 and Wee1 are overexpressed and/or aberrantly activated in several cancer types, suggesting that they may serve as attractive molecular targets for pharmacologic intervention (7–13). Chk1 exerts indirect checkpoint control through both S-phase and G2-M phases of the cell cycle, and is phosphorylated by ATR in response to DNA damage caused by oncogene induced stress, stalled replication forks, ultraviolet light or genotoxic agents (14,15). Activation of Chk1 results in the sequestration and/or subsequent degradation of the Cdc25A and Cdc25C phosphatases, consequently causing cell cycle arrest through accumulation of inactive cyclin dependent kinases (CDKs) (14). In a parallel pathway, Wee1 signals in concert with Chk1 by directly catalyzing an inhibitory phosphorylation at the Y15 residue of Cdc2 (CDK1), abrogating its activity and inhibiting mitotic progression at the G2 checkpoint (16). Because both Chk1 and Wee1 signaling ultimately result in cell cycle arrest, it seems counterintuitive that constituitive activity of these kinases would contribute to a malignant phenotype. However, many cancer types harbor G1 checkpoint deficiencies, such as TP53 inactivation, necessitating reliance upon G2 checkpoint signaling for continued survival. In particular, cancers with G2 checkpoint hyperactivation may be more efficient at repairing the genetic lesions generated by genotoxic insults (17,18). Therefore, small molecule inhibitors targeting the G2 checkpoint are intriguing as both single-agent and combination therapies aimed at enhancing conventional chemotherapy with the rationale that abrogation of Chk1/Wee1 signaling forces mitotic progression with incompletely replicated or damaged DNA, leading to mitotic catastrophe and subsequent cell death (11, 18, 19).
Therefore, with the goal of identifying additional therapeutic targets of the DNA damage pathway in neuroblastoma, we focused our efforts on Wee1, for which there are inhibitors in clinical trials (NCT00648648) and evidence of synthetic lethality with Chk1 (20). We found that Wee1 is highly expressed and phosphorylated in neuroblastoma, with single-agent sensitivity reminiscent of what we previously observed for Chk1. In addition, we provide evidence that combination therapy with small molecule inhibitors of Chk1 and Wee1 exhibits synergistic efficacy in vitro and in vivo models of neuroblastoma. Therefore, attenuation of Chk1 and Wee1 signaling may be a rational therapeutic approach for the treatment of neuroblastoma.
MATERIALS AND METHODS
Cell culturing
All neuroblastoma cell lines were obtained from the CHOP neuroblastoma cell line bank. They are routinely mycoplasma and identity tested using AmpFLSTR Identifiler (Applied Biosystems), last done in October 2011. The non-neuroblastoma lines were purchased directly from ATCC (where they do STR testing) within 6 months of use in this study.
siRNA Transfection
Transfections were performed in triplicate as previously described (21) using ON-TARGET SMARTpool siRNAs (Thermo Scientific) specific for GAPDH, Plk1, Wee1 and Chk1. Cell viability was quantified at 72h by use of Cell Titer-Glo assay (Promega). Gene knockdown was confirmed to be >90% by quantitative real-time PCR.
Immunohistochemistry
Following standard antigen retrieval protocol, phospho Wee1 antibody (Cell Signaling #4910) was used to stain formalin fixed paraffin embedded sections at a 1:1000 dilution for 1hr at room temperature. Slides were again rinsed then incubated with biotinylated anti-Rabbit IgG (Vector Laboratories BA-1000) at a 1:200 dilution for 30min at room temp, followed by avidin biotin complex (Vector Laboratories PK-6100) for 30 min at room temp. Slides were then rinsed and incubated with DAB (DAKO Cytomation K3468). Counterstaining was performed for 1min in Harris Hematoxylin (Fisher Scientific 6765001). Slides were rinsed and dehydrated through a series of ascending concentrations of ethanol and xylene, then coverslipped. After drying, slides were digitally scanned at 20X magnification on an Aperio OS slide scanner (Aperio Technologies Inc., Vista, CA).
Pharmacological Inhibition
MK-8776 (also known as SCH 900776) and MK-1775 were provided by Merck & Co. Twenty-four hours after plating, cells were treated in triplicate over a four-log dose range (10–10,000 nM) and a DMSO control. Cells were cultured for 72 hours and cell viability was measured using Cell Titer-Glo assays (Promega). IC50 determination was made using a non-linear log inhibitor vs. normalized response curve fit function (Graphpad). Caspase activation assays and the cleaved PARP Westerns were performed at 16h and quantified by use of the Caspase-Glo 3/7 assay (Promega).
Combination Studies
Following single-agent IC50 determination, neuroblastoma cells were plated in duplicate in 96-well plates and treated with two agents at doses ranging in a 2-fold difference above and below each individual IC50 (i.e. 0.25X, 0.5X, 1X, 2X and 4X). Combination indices were determined using CalcuSyn software via the Chou-Talalay method (22). All combination studies were repeated at least once (total of n≥4 for each cell line).
Western Blotting
Cell lysates were prepared as described previously (21, 23). Neuroblastoma cell lines or primary tumor lysates (40 μg) were separated on 4–12% gradient polyacrylamide gels via SDS-PAGE and transferred to PVDF membranes (Millipore). Primary antibody dilutions included 1:1,000 Chk1, Chk1S296, Wee1, Wee1S642, p-H2A.X(S139), Cdc2(Y15), cleaved PARP (Asp214) and 1:3,000 β-actin (Cell Signaling).
In Vivo Studies
CB17SC-M SCID−/− mice were used to propagate subcutaneously implanted neuroblastoma xenografts. Caliper measurements were obtained, and tumor volumes were calculated using the formula, (π/6) × d2, where d represents the mean diameter. Once the tumor was greater than 200 mm3, mice bearing neuroblastoma tumors were randomized to treatment arms of: 1) 30 mg/kg/dose twice daily i.p. MK-8776, 2) 30 mg/kg/dose twice daily p.o. MK-1775, 3) the two compounds combined or 4) vehicle control administered for five consecutive days for two weeks. Tumors were measured twice weekly for a total of 28 days or until tumor volume reached 3cm3. The Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee approved all animal studies.
Statistical Analysis
Group comparisons were determined with a two-tailed t-test. For the xenograft studies, a linear mixed effects model was used to test the difference in the rate of tumor volume changing over time between different the vehicle group and treatment groups.
RESULTS
Neuroblastoma harbors elevated Wee1S642 phosphorylation
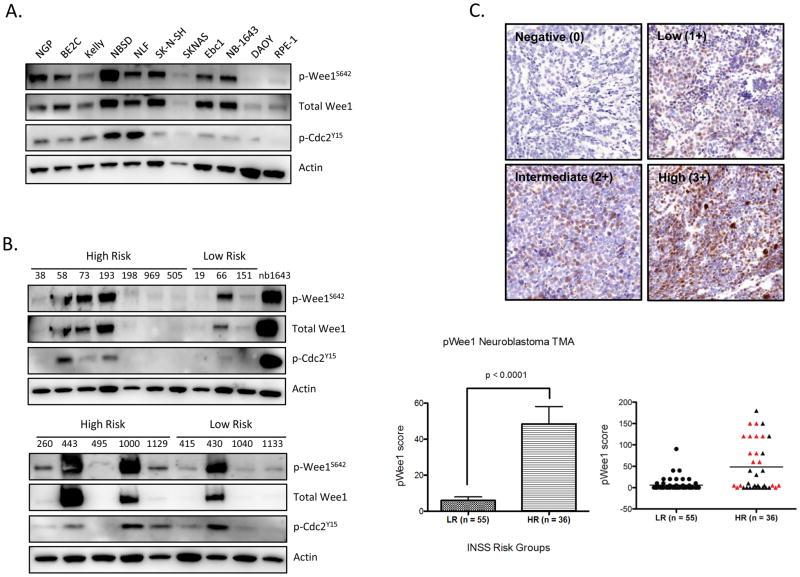
We previously demonstrated that Chk1, a DNA damage response kinase, is highly expressed and aberrantly activated in neuroblastoma, leading to cellular dependence on this pathway. In order to elucidate mechanisms underlying sensitivity to Chk1 inhibition, we interrogated Wee1 – a serine/threonine kinase that signals through a pathway complimentary to Chk1. In a manner similar to Chk1, we observed robust expression of Wee1 protein levels with extensive phosphorylation in a comprehensive panel (n=18) of neuroblastoma lines. In addition, canonical signal transduction through the Wee1 pathway was intact in these neuroblastoma lines, as phosphorylation of Cdc2 at Y15 often phenocopied Wee1 activation (Figures 1A and S1A). In contrast, substantial Wee1 expression or activation was not detected in DAOY medulloblastoma or the h-tert immortalized non-transformed, retinal pigmented epithelial (RPE-1) cells. Similarly, the several adult tumor types we investigated revealed very low basal Wee1 activity in comparison to what was observed for neuroblastoma (Figure S1B).
Figure 1.
Wee1 kinase is highly expressed in neuroblastoma. (A) Western blot analysis of neuroblastoma cell lines demonstrates that Wee1 is highly expressed at the protein level and constitutively activated compared to non-NB lines such as DAOY medulloblastoma cells or non-transformed RPE-1 cells. Phosphorylated Wee1S642 is present in the majority of neuroblastoma lines, and often coincides with downstream phosphorylation of Cdc2Y15 (Cdk1). (B) Wee1 is also highly expressed in diagnostic patient tumor samples, with increased Wee1 activity in 67% (8 of 12) of tumors derived from high-risk patients, compared to 28.5% (2 of 7) of tumors derived from low-risk patients (C) Neuroblastoma tissue microarray (TMA) also stained positively for phospo-Wee1 (S642), confirming higher expression levels in high-risk, including MYCN amplified tumors (red triangles), as compared to low-risk samples. Representative staining for each tumor risk group is shown (top). Abbreviations: HR = high-risk, LR = low-risk, INSS = International Neuroblastoma Staging System. pWee1 score = antibody intensity (0, 1+, 2+, 3+) x the % of cells positive.
To exclude the possibility that robust Wee1 activity was solely an artifact of DNA damage induction during cell culturing, we examined the Wee1 status in patient primary tumor samples obtained at diagnosis. As shown in Figure 1B, 7 of 12 high risk samples (58.3%) had appreciable p-Wee1 expression compared to 2 of 7 low risk patient samples (28.5%). These findings were supported by immunohistochemical evidence of Wee1 phosphorylation (Ser642) obtained by staining neuroblastoma tissue microarray representing tumors from 91 patients. We again found that high-risk neuroblastoma tumors had increased levels of Wee1 phosphorylation compared to low risk tumors (p < 0.0001) (Figure 1C). Lastly, quantification of Wee1 mRNA expression levels in a large number of neuroblastoma tumor samples (n=251) confirmed, like Chk1, there was significantly higher expression in high-risk, MYCN-amplified tumors (Figure S2). Taken together, these data demonstrate that Wee1 expression and activity appears significantly elevated in neuroblastoma - particularly in MYCN-amplified, high-risk disease.
Neuroblastoma is sensitive to targeted Chk1/Wee1 inhibition
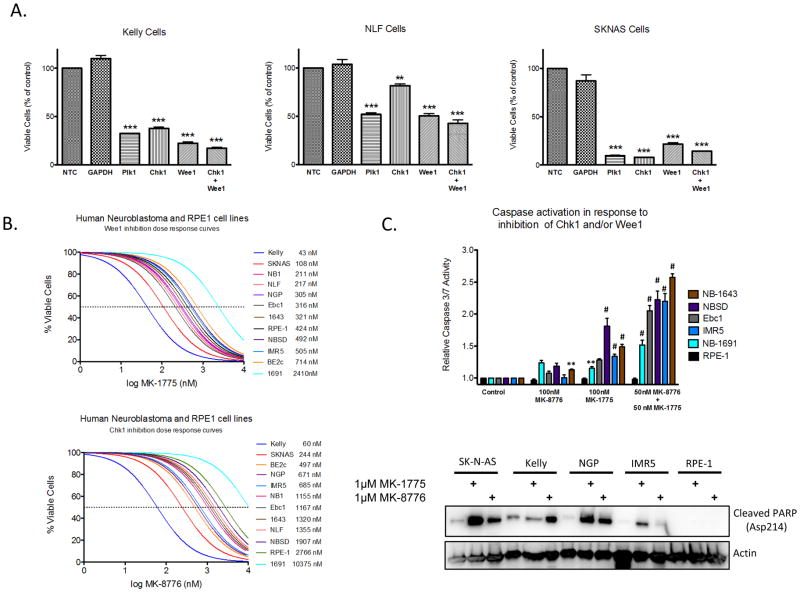
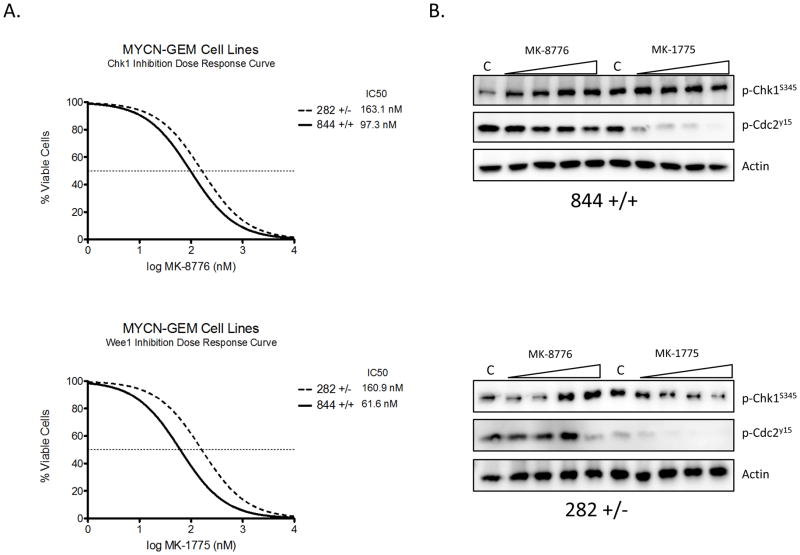
To ascertain the contribution of Wee1 signaling in neuroblastoma, we depleted Wee1 in three neuroblastoma lines using siRNA. Depletion of Wee1 substantially inhibited the growth and viability of neuroblastoma cell lines (Figure 2A). In order to assess the effect of pharmacologic kinase inhibition, we tested small molecular inhibitors of Chk1 (MK-8776) and Wee1 (MK-1775) on neuroblastoma growth and viability. The majority of neuroblastoma lines were sensitive to single-agent inhibition of Chk1 (82%) or Wee1 (91%), with median IC50s of roughly 900nM and 300nM, respectively (Figure 2B). We subsequently confirmed that the observed decrease in cell viability was due primarily to apoptosis, rather than cell cycle arrest as we observed activation of caspase 3/7 or cleavage of poly ADP ribosome polymerase (PARP) in eight neuroblastoma lines (Figure 2C). We also tested two murine tumor lines derived from the MYCN transgenic mouse model to examine the consequence of MYCN expression on the potency of these inhibitors (24). Both single-agent Wee1 and Chk1 inhibition were cytotoxic in these MYCN-derived lines, with the MYCN- homozygous line roughly twice as sensitive to both Chk1 and Wee1 inhibition than the heterozygous line, although this was not statistically significant (Figure 3A). We confirmed target engagement in these murine cells, with the expected ATR compensatory Chk1S345 phosphorylation in response to MK-8776 administration (25), and a decrease in Cdc2Y15 phosphorylation following exposure to MK-1775 (Figure 3B).
Figure 2.
Abrogation of either Wee1 or Chk1 signaling is cytotoxic to neuroblastoma cells. (A) siRNA-mediated depletion of Chk1 or Wee1 resulted in a significant reduction in cell viability in several neuroblastoma cell lines. (B) The majority of neuroblastoma cells were sensitive to single-agent inhibition of Wee1 (MK-1775) or Chk1 (MK-8776) activity, with median IC50s of 300nM and 900nM, respectively (curve shifted <0.05 on x-axis to allow visualization where overlapped). (C) Neuroblastoma cell lines underwent apoptosis in response to Chk1 and/or Wee1 inhibition as evidenced by caspase 3/7 activation (top panel) or PARP cleavage (bottom panel) (** = p ≤0.01, *** = p ≤0.001, # = p < 0.0001).
Figure 3.
Murine neuroblastoma lines derived from MYCN transgenic mice are sensitive to Chk1/Wee1 inhibition. (A) Cells homozygous (844) or heterozygous (282) for the MYCN oncogene were derived from MYCN-transgenic murine tumors, and were found to be sensitive to single-agent MK-1775 (bottom) and MK-8776 (top), (n=9 for each cell type). (B) Target engagement was confirmed via Western blot 6h after treatment with increasing concentrations of MK-1775 or MK-8776. Increasing phosphorylation of Chk1S345 has been shown to be a biomarker of Chk1 inhibition (15).
Chk1 and Wee1 inhibition acts synergistically with chemotherapy in neuroblastoma cells
Current development strategies for Chk1/Wee1 inhibitors have been focused on their chemosensitizing properties, leading us to assess the ability of these compounds to potentiate both a topoisomerase inhibitor (irinotecan) and a nucleoside analog (gemcitabine) in our neuroblastoma cell line panel. Prior to combination experiments, we calculated single-agent IC50s for both SN-38 (the active metabolite of irinotecan) and gemcitabine for our neuroblastoma cell line panel (n=10) using a 4-log dose-response cell viability assay with a 72 hour time point. We subsequently combined either MK-1775 (Wee1), or MK-8776 (Chk1), with these chemotherapeutic agents as well as each other, to generate a combination index (CI) value denoting the level of observed synergy (Table 1, S1). Nearly all of our neuroblastoma lines had a pronounced synergistic effect when combining these inhibitors with either SN-38 (7/10 cell lines for both MK-1775 and MK-8776) or gemcitabine (8/10 lines for MK-1775, 10/10 cell lines for MK-8776), (Table S1). In addition, there is potent cytotoxicity in almost all neuroblastoma lines when combining MK-1775 and MK-8776 (9/10), suggesting that dual inhibition of Chk1 and Wee1 was a robust synergistic combination.
Table 1.
Chk1 and Wee1 inhibitors combine synergistically with chemotherapy, and each other, in neuroblastoma cells. Neuroblastoma cell lines treated with a combination of inhibitor and chemotherapy, or a combination of Chk1 and Wee1 inhibitor together demonstrated cytotoxic synergy.
| Cell Line | MK-8776 + SN38 | MK-1775 + SN38 | MK-8776 + MK1775 | MK-8776 + Gemcitabine | MK-1775 + Gemcitabine |
|---|---|---|---|---|---|
| IMR-5 | ± | + | +++++ | +++ | +++++ |
| BE2C | ± | +++ | ++ | ++++ | +++ |
| NB1 | ++ | −− | −− | +++ | −−− |
| Ebc1 | +++++ | ± | +++++ | +++++ | ++++ |
| NBSD | +++ | ++++ | +++ | +++++ | ± |
| Kelly | +++++ | +++++ | ++ | ++++ | +++++ |
| NGP | ++++ | +++ | ++++ | +++++ | +++++ |
| SKNAS | ++++ | +++++ | +++ | +++++ | +++++ |
| NLF | ++++ | +++ | ++++ | +++++ | − |
| NB-1643 | +++ | +++ | +++++ | +++++ | +++++ |
| CI Value | Symbol | Observed Synergy |
|---|---|---|
| <0.1 | +++++ | very strong |
| 0.1–0.3 | ++++ | strong |
| 0.3–0.7 | +++ | synergy |
| 0.7–0.85 | ++ | moderate synergism |
| 0.85–0.9 | + | slight synergism |
| 0.9–1.1 | ± | additive |
| >1.1 | − | antagonistic |
Simultaneous inhibition of Chk1 and Wee1 results in substantial DNA double strand breakage
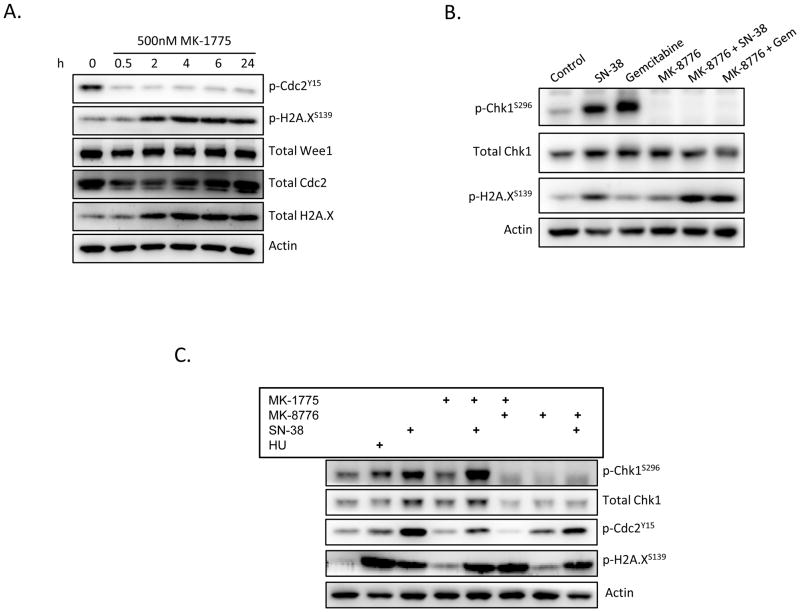
In an effort to elucidate downstream events responsible for the synergistic cytotoxicity in the MK-8776/MK-1775 combination group, we examined pathway signaling following pharmacologic disruption. As expected, there was a substantial inhibition of Cdc2 phosphorylation downstream of Wee1 following administration of MK-1775 and decreased CHK1 phosphorylation following administration of MK-8776, at doses at or below the respective IC50s in sensitive lines (Figure 4 and Figure S3). In addition, inhibition of Wee1 resulted in a modest accumulation of DNA double-strand breaks as evidenced by sustained phosphorylation of histone H2A.X (Figure 4A). Similarly, although inhibition of Chk1 alone did result in activation of histone H2A.X, MK-8776 in combination with chemotherapy potentiated the DNA damaging aspect of both SN-38 and gemcitabine (Figure 4B). The dual combination of MK-1775 and MK-8776 resulted in greater than additive histone H2A.X phosphorylation (lane 6) than either inhibitor alone (lanes 4 and 7) and caused DNA damage equivalent to that induced by the cytotoxic chemotherapeutic agents. (Figure 4C). Furthermore, despite the presence of DNA damage, cells were unable to inhibit the cell cycle as evidenced by Cdc2 phosphorylation. This suggests that mitotic progression is occurring in the presence of damaged DNA, and this has been shown to be indicative of mitotic catastrophe (18).
Figure 4.
Dual Chk1 and Wee1 inhibition results in accumulation of DNA double-strand breaks and mitotic catastrophe. (A) Be2C cells treated with MK-1775 demonstrated an abrogation of Cdc2 activity with a concomitant increase in H2A.X phosphorylation, indicative of DNA damage. (B) Inhibition of Chk1, on the other hand, results in only marginal increases in γH2A.X, however in combination with chemotherapy MK-8776 rapidly (2h) induces double strand breaks (shown in NB-1643 cells). (C) Simultaneous inhibition of Chk1 and Wee1 (16h) resulted in robust H2A.x activation and complete abolishment of Cdc2 signaling, suggesting cells were progressing through mitosis with DNA damage (shown in NGP cells). (HU = 1mM hydroxyurea, [MK-1775] = 250nM, [MK-8776] = 500nM, [SN-38] = 100nM, Gem = 100nM gemcitabine)
Dual Chk1/Wee1 inhibition is efficacious in vivo
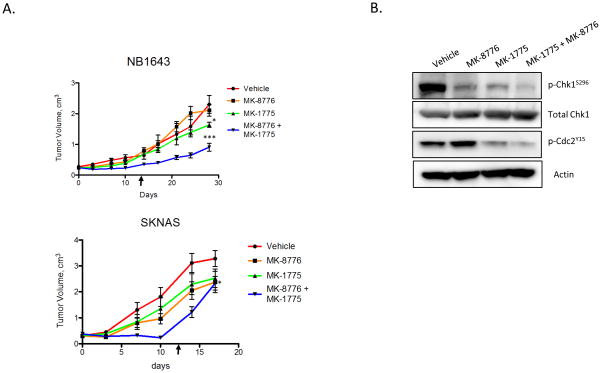
Based on the dual efficacy of MK-8776 and MK-1775 in our in vitro models, we administered Wee1 and Chk1 combination therapy to mice bearing neuroblastoma tumors. Mice harboring xenografts from NB-1643 or SKNAS cell lines were treated with vehicle control, MK-1775 or MK-8776 (each dosed at approximately half the single agent MTD) or the combination of MK-1775 and MK-8776, dosed daily x 5 for 2 weeks, with one week off. The regimen was well tolerated. Mice receiving both the Wee1 and Chk1 inhibitors had a reduction in tumor growth rate as compared to control mice receiving vehicle alone (p < 0.0001 for NB-1643, p < 0.05 for SKNAS) (Figure 5A, S4). Mice with NB-1643 tumors demonstrated growth inhibition with single-agent MK-1775 (p = 0.042), whereas mice harboring SKNAS tumors responded to single-agent MK-8776 (p = 0.023), which with Bonferroni correction does not meet the significant p-value of p ≤ 0.017, presumably due to the lower single agent doses chosen. Tumor resection following 48 hours of treatment (4 doses) with these inhibitors showed the expected reduction of the Chk1 (S296) autophosphorylation site in the MK-8776 group, as well as abrogation of Cdc2 (Y15) phosphorylation in the MK-1775 treated mice bearing NB-1643 tumors (Figure S4) and EBC1 tumors (Figure 5B). These in vivo markers of pathway inhibition mimic those seen in our in vitro experiments and provide a rationale for the biological effect of tumor growth inhibition seen in these experiments.
Figure 5.
Growth of neuroblastoma xenografts is significantly impaired in response to Chk1/Wee1 combinatorial therapy. (A) Mice subcutaneously implanted with neuroblastoma xenografts were treated twice daily with 30 mg/kg/dose MK-1775, MK-8776 or both for 5d x 2 weeks. Mixed linear analysis indicated a significant reduction in tumor burden in the combination treatment group in both neuroblastoma cell lines. For graphical purposes, mice removed from study from excessive tumor burden had their last measurements carried forward. Arrows indicate end of treatment. (* = p ≤ 0.05, *** = p ≤ 0.0001) (B) Target engagement was verified by resection of Ebc1 xenografts, where both Chk1 and Cdc2 activity was substantially reduced following four doses of MK-8776 or MK-1775, respectively.
DISCUSSION
Children with high-risk neuroblastoma have a poor prognosis despite toxicity-limiting chemotherapy, emphasizing the need to develop novel targeted therapies for the treatment of this disease (1–3). Because an unbiased siRNA screen previously identified unique sensitivity to DDR pathway interference in neuroblastoma, and because Wee1 ultimately impacts Cdc2 via pathways complementary to Chk1 signaling, we focused our current research efforts on combination therapies targeting this pathway. Elevated expression and phosphorylation of Wee1 was observed in neuroblastoma cell lines, particularly in contrast to other cancer cell types. In addition, both Chk1 and Wee1 expression were significantly elevated in samples obtained from high-risk, MYCN-amplified tumors, suggesting that these tumors may be particularly sensitive to pathway perturbation (Figure 1, S1, S2). We previously demonstrated that induction of MYCN activity resulted in Chk1S296 phosphorylation, which we reasoned to be a DNA damage response due to replicative stress (6). In support of this theory, two lines derived from MYCN transgenic mice were both highly sensitive to single-agent Chk1 or Wee1 inhibition, with the MYCN homozygous line twice as sensitive as the heterozygous line (Figure 3). These results support the hypothesis that MYCN-driven replicative stress may underlie aberrant activation of the DDR pathway in neuroblastoma, resulting in a dependence on Chk1/Wee1 signaling to maintain cell viability. Indeed, siRNA-mediated depletion of Wee1 significantly impairs cell viability, and the majority of neuroblastoma cells demonstrate single-agent sensitivity to Wee1 or Chk1 inhibition, with IC50s in physiologically attainable ranges (Figure 2). Consequently, sustained inhibition of Chk1 or Wee1 results in apoptosis as evidenced by PARP cleavage and activation of Caspase 3/7.
Rationale for the development of Chk1 and Wee1 inhibitors as chemosensitizers is based on their role as integrative regulators of the cell cycle and DNA damage response pathways. To assess chemopotentiation in neuroblastoma, combination strategies utilizing gemcitabine and SN-38 (the active metabolite of irinotecan) were tested across a panel of neuroblastoma cell lines. Gemcitabine, although not used for the treatment of neuroblastoma (26), has been previously shown to be potently chemosensitized by Chk1 and Wee1 inhibition (9,20), whereas irinotecan is used clinically for treatment of relapsed neuroblastoma (27). Nearly all neuroblastoma cell lines were found to demonstrate synergy in a variety of combinations (Table 1). Dual inhibition of Chk1 and Wee1 was highly synergistic in nearly every line - equivalent to, or exceeding many of the chemotherapy combinations. In support of these findings, recent data from Davies et al. demonstrated that dual Chk1/Wee1 inhibition was synergistic in several different cancer lines, and suggested that further studies be done to identify sensitive cancer cell types or if p53 status dependency were necessary (10). Here we have shown that Chk1 and Wee1 inhibition in combination with chemotherapy is synergistic in virtually all neuroblastomas tested, and is likely due to the sensitivity to DDR pathway interference in this disease.
Investigating the cellular signaling events following pathway perturbation yielded some insights to explain the potency observed in the Chk1/Wee1 combination (Figure 4). Exposure to single-agent MK-1775 results in a continuous abrogation of Cdc2Y15 signaling concurrent with sustained H2A.X phosphorylation indicative of double-stranded DNA breakage (28). Although MK-8776-mediated inhibition of Chk1 resulted in DNA damage, exposure to chemotherapy induced activation of Chk1, and therefore concomitant inhibition of Chk1 resulted in increased DNA damage compared to chemotherapy alone. Lastly, simultaneous inhibition of Chk1 and Wee1 signaling resulted in robust accumulation of DNA damage as evidenced by H2A.X phosphorylation. Therefore, it appears that dual Chk1/Wee1 inhibition induces DNA strand breakage similar to that observed in combinations with chemotherapy, accounting for the comparable synergy we observed in many of our neuroblastoma lines. Furthermore, as we also observed a complete absence of Cdc2 phosphorylation following Chk1/Wee1 inhibition, it is likely that mitotic progression is proceeding with damaged DNA, leading to mitotic catastrophe and resultant apoptosis. In support of this hypothesis, data from Potapova et al. recently showed that simultaneous inhibition of Wee1 and the Cdc25 family members results in irreversible mitotic progression (29).
Based on the cumulative results of these in vitro experiments, we chose a highly sensitive (SKNAS), and moderately sensitive (NB-1643) neuroblastoma line to investigate the in vivo efficacy of targeted Chk1/Wee1 combination therapy, noting that the SKNAS xenograft is highly chemo-resistant in vivo. We chose a dosing strategy intended to recapitulate a clinically relevant schedule of two weeks on and one week off, and our mixed linear effects analysis accounted for data through the entire cycle, and not just during dosing. For NB-1643, there remained a statistically significant reduction in tumor burden with dual Chk1/Wee1 inhibition, even after treatment had stopped. For SKNAS, however, there was significant reduction while being dosed, but regrowth after treatment was stopped, suggesting that for some tumors it may be necessary to continually dose, or combine with chemotherapy in the manner with which these inhibitors were designed and are being developed. Nonetheless, these results argue strongly for further optimization of a Chk1/Wee inhibition strategy in neuroblastoma.
CONCLUSION
In conclusion, these results strengthen previous findings that neuroblastoma is particularly susceptible to therapies targeting the DNA damage response pathway. In addition, because many relapsed neuroblastomas are refractory to conventional chemotherapy, targeted inhibition of Chk1 and Wee1 also serves to sensitize these tumors to currently used agents. The finding that Wee1 is highly expressed in neuroblastoma, particularly in high-risk, MYCN-amplified tumors, provides rationale for targeting this kinase. We have demonstrated that neuroblastoma is sensitive to single-agent inhibition of Chk1 and Wee1 through the use of two small molecule inhibitors, MK-8776 and MK-1775. These compounds act synergistically when combined with gemcitabine and irinotecan (SN-38), or with each other, by inducing DNA double strand breaks and forcing cells to undergo mitotic catastrophe and subsequently, apoptosis. Furthermore, dual inhibition therapy of Chk1 and Wee1 is able to substantially inhibit the growth of neuroblastoma xenografts in vivo. Taken together, these data demonstrate the effectiveness of dual inhibition therapies aimed at targeting the DNA damage repair pathways in pediatric neuroblastoma.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Children’s Oncology Group (U10---CA98543) for providing blood and tumor specimens from neuroblastoma patients.
GRANT SUPPORT
Bear Necessities and Rally Foundation Fellowship in Pediatric Cancer Research (MRR), The Solving Kids Cancer Foundation (KAC), NIH grant K08 CA136979-01 (KAC)
Footnotes
Author Conflicts of Interest: none
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nature Rev Cancer. 2003;3:203–16. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM. Recent advances in neuroblastoma. NEJM. 2010;362:2202–11. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, et al. Health Status of Adult Long-term A Report From the Childhood Cancer Survivor Study. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 5.Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. PNAS. 2011;108:3336. doi: 10.1073/pnas.1012351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Rev Cancer. 2009;9:153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 7.Sørensen CS, Syljuåsen RG. Safeguarding genome integrity: the checkpoint kinases ATR, CHK1 and WEE1 restrain CDK activity during normal DNA replication. Nucleic Acids Research. 2011:1–10. doi: 10.1093/nar/gkr697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Current Opinion in Cell Biology. 2009;21:245–55. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Cheung IY, Wei XX, Tran H, Gao X, et al. Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G(1) checkpoint-defective neuroblastoma. International Journal of Cancer. 2011;129(8):1953–62. doi: 10.1002/ijc.25842. [DOI] [PubMed] [Google Scholar]
- 10.Davies KD, Cable PL, Garrus JE, Sullivan FX, von Carlowitz I, et al. Chk1 inhibition and Wee1 inhibition combine synergistically to impede cellular proliferation. Cancer Biology & Therapy. 2011;12:788–96. doi: 10.4161/cbt.12.9.17673. [DOI] [PubMed] [Google Scholar]
- 11.Mir SE, De Witt Hamer PC, Kraqczyk PM, Balaj L, Claes A, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. 2010;18:244–57. doi: 10.1016/j.ccr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Decker SJ, Sebolt-Leopold J. Knockdown of Chk1, Wee1 and Myt1 by RNA interference abrogates G2 checkpoint and induces apoptosis. Cancer Biology & Therapy. 2004;3:305. doi: 10.4161/cbt.3.3.697. [DOI] [PubMed] [Google Scholar]
- 13.Hattori H, Skoulidis F, Russell P, Venkitaraman AR. Context dependence of Checkpoint kinase 1 as a therapeutic target for pancreatic cancers deficient in the BRCA2 tumour suppressor. Mol Cancer Therapeutics. 2011 doi: 10.1158/1535-7163.MCT-10-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev. 2001;15:2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 15.Smith J, Tho LM, Xu N, Gillespie DA. Adv in Cancer Res. Vol. 108. Elsevier Inc; 2010. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer; pp. 73–112. [DOI] [PubMed] [Google Scholar]
- 16.Squire CJ, Dickson JM, Ivanovic I, Baker EN. Structure and inhibition of the human cell cycle checkpoint kinase, Wee1A kinase: an atypical tyrosine kinase with a key role in CDK1 regulation. Structure. 2005;13:541–50. doi: 10.1016/j.str.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Li J, Booher RN, Kraker A, Lawrence T, et al. Radiosensitization of p53 Mutant Cells by PD0166285, a Novel G 2 Checkpoint Abrogator. Cancer Res. 2001:8211–8217. [PubMed] [Google Scholar]
- 18.Castedo M, Perfettini J-L, Roumier T, Andreau, Medema R, et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004;23:2825–37. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 19.Montano R, Chung I, Garner K, Parry D, Eastman A. Preclinical Development of the Novel Chk1 Inhibitor SCH900776 in Combination with DNA Damaging Agents and Antimetabolites. Mol Cancer Therapeutics. 2011 doi: 10.1158/1535-7163.MCT-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Molecular cancer therapeutics. 2009;8:2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 21.Mossé YP, Laudenslager M, Longo L, Cole KA, Wood A, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 23.Russell MR, Liu Q, Lei H, Kazlauskas A, Fatatis A. The alpha-receptor for platelet-derived growth factor confers bone-metastatic potential to prostate cancer cells by ligand- and dimerization-independent mechanisms. Cancer research. 2010;70:4195–203. doi: 10.1158/0008-5472.CAN-09-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. The EMBO journal. 1997;16:2985–95. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR is antagonized by a Chk1-regulated protein phosphatase 2A circuit. Molecular and cellular biology. 2006;26:7529–38. doi: 10.1128/MCB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner-Bohn A, Paulussen M, Pinheiro J, Gerss J, Stoffregen C, et al. Phase II study of gemcitabine in children with solid tumors of mesenchymal and embryonic origin. Anti-cancer drugs. 2006;17:859–64. doi: 10.1097/01.cad.0000217426.82702.74. [DOI] [PubMed] [Google Scholar]
- 27.Bagatell R, London WB, Wagner LM, Voss SD, Stewart CF, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children’s Oncology Group study. Journal of clinical oncology. 2011;29:208–13. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. The Journal of biological chemistry. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 29.Potapova TA, Sivakumar S, Flynn JN, Li R, Gorbsky GJ. Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited. Molecular biology of the cell. 2011;22:1191–206. doi: 10.1091/mbc.E10-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.