Abstract
Pathogens use a variety of strategies to evade host immune defenses. A powerful way to suppress immune function is to increase intracellular concentrations of cAMP in host immune cells, which dampens inflammatory responses and prevents microbial killing. We found that the yeast cell wall extract, zymosan, is capable of increasing intracellular cAMP and activates the protein kinase A pathway in bone marrow derived macrophage (BMDM) cells from mice. This response is dependent on adenylyl cyclase type VII (AC7) and heterotrimeric G proteins, primarily G12/13. Consequently, zymosan induced production of the inflammatory cytokine, TNFα, was much stronger in BMDMs from AC7 deficient mice compared to the response in wild type cells. In a model of zymosan induced peritonitis, mice deficient of AC7 in the myeloid lineage displayed prolonged inflammation. We propose that zymosan induced increases in cAMP and activation of PKA serve as a mechanism to dampen inflammatory responses in host cells, which consequently favors the survival of microbes. This would also help explain a well documented phenomenon, that the ability of zymosan to stimulate inflammatory cytokine responses via TLR2 appears to be weaker than other stimuli of TLR2.
Keywords: cAMP, Adenylyl cyclase, G13, Zymosan, PKA, BRET
1. Introduction
Bacterial, viral, and eukaryotic parasitic pathogens exploit a variety of strategies to disable innate immune responses and evade host defenses (Finlay and McFadden, 2006). They do so by neutralizing, hijacking, or paralyzing signal transduction pathways in host cells, such as the complement pathway, the Toll-like receptor (TLR) signaling pathway, and the apoptosis pathway (Bowie and Unterholzner, 2008; Hajishengallis and Lambris, 2011; Lambris et al., 2008). One of the powerful ways to disrupt host cell functions is through increasing intracellular cAMP because this ubiquitous second messenger has pleiotropic effects in many host cell types and is essential for many cellular functions. A well-known example for this effect is secretion of cholera toxin by Vibrio cholera; upon entry into host cells, this toxin directly activates the heterotrimeric G protein, Gs, and leads to uncontrolled synthesis of cAMP (Cassel and Pfeuffer, 1978). Other bacterial effectors have been reported to increase host cell cAMP concentration by binding to adenylyl cyclases (ACs) to potentiate Gs-dependent cAMP production or by inhibiting degradation of cAMP through attenuation of phosphodiesterase activity (Hosono and Suzuki, 1985; Pulliainen et al., 2012).
Cyclic-AMP has been shown to regulate many aspects of immune responses over the past decades. In general, cAMP suppresses inflammatory immune responses and changes their response profiles in both innate and adaptive immune responses (Castro et al., 2005; Mosenden and Tasken, 2011; Peters-Golden, 2009; Serezani et al., 2008). By elevating the concentration of cAMP in innate immune cells, pathogens can evade the initial strong inflammatory responses and potentially cause chronic damage to the host cells. In macrophage cells, cAMP inhibits the activation of the NF-κB pathway induced by TLR stimuli and leads to reduced production of proinflammatory cytokines (Natarajan et al., 2006; Wall et al., 2009), such as TNFα, which play an important role in orchestrating immune responses that result in effective bacterial killing.
Zymosan is the insoluble cell wall extract prepared from Saccharomyces cerevisiae through boiling and trypsin digestion (Di Carlo and Fiore, 1958). It contains several defined polysaccharides such as β-glucan, mannan and chitin. Since zymosan can be phagocytosed by innate immune cells and stimulate inflammatory responses in those cells, it has been used as a model to study the mechanisms of innate immune recognition of microbes, phagocytosis, and regulation of cytokine production. Phagocytosis of zymosan particles is mediated by a repertoire of receptors, including Dectin-1, TLR2, and integrin Mac-1, which recognize various components of the particles (Underhill, 2003; Underhill and Ozinsky, 2002). On the other hand, stimulation of inflammatory cytokine production depends mainly on the heterodimer Toll-like receptors, TLR2 and TLR6 (Underhill et al., 1999). The TLR2/TLR6 and MyD88 signal transduction pathway has been shown to be required for zymosan induced inflammatory cytokine responses, such as production of TNFα (Takeda and Akira, 2004). However, the ability of zymosan to stimulate inflammatory cytokine responses appears to be weaker than other soluble stimuli of the TLR2 receptors. One reason is that zymosan requires direct contact with innate immune cells to activate the TLR2 pathway as elegantly demonstrated by Dr. Underhill (Underhill, 2003).
In this study, we report that zymosan is also capable of stimulating the production of intracellular cAMP and activation of protein kinase A (PKA) in bone marrow derived primary macrophages (BMDMs). We show that this response is dependent on a specific isoform of adenylyl cyclase, AC7, as AC7 deficient BMDMs fail to produce this zymosan-induced increase in cAMP and PKA activity. The physiological consequence of this is that AC7 deficient BMDMs produce a much higher level of TNFα than wild type cells upon exposure to zymosan. In the zymosan induced peritonitis model, mice deficient of AC7 in the myeloid lineage displayed prolonged inflammation when compared to wild type littermates. We propose that zymosan induced cAMP responses could be a mechanism for microbial evasion of host immune responses. Initial characterization of the zymosan associated factor suggests that it triggers cAMP responses via G-protein coupled receptors in macrophage cells.
2. Materials and Methods
2.1 Reagents
Zymosan was purchased from Sigma (Lot# 092K1240, Lot# BCBG6429V). LPS (R595Re) and pertussis toxin were purchased from List biological. LBP (LPS binding protein) was purchased from R&D systems. Complement factor C5a (Sigma), cytochalasin D (Calbiochem), PAM3Cys-SKKKKx3HCl (P3C, EMC Microcollections), FITC-zymosan (Life Technology), sphingosine-1-phosphate (Avanti Polar Lipids), and JTE 013 (Tocris bioscience) were purchased from the sources indicated. The ELISA kit for TNF-α (88-7324) was purchased from eBioscience. Protease inhibitors and phosphatase inhibitor cocktails I and II were purchased from Roche and Sigma, respectively. Antibodies for detecting phosphorylation of PKA substrates with the RRXS*/T* motif (CST 9624) were obtained from Cell Signaling Technology.
2.2 Construction and testing of a BRET sensor to measure PKA activity
A BRET sensor for PKA activity was constructed following the design of the FRET sensor of PKA, AKAR (Zhang et al., 2005). The core of the sensor, a PKA phosphorylation substrate and its binding domain, was flanked by the BRET acceptor and donor pair, citrine and Renilla luciferase (RL) (Supplementary Figure S1A). We name this sensor AKARB (A kinase activity reported by BRET). When the intracellular concentration of cAMP is increased and PKA activated, the BRET signal from AKARB increases similarly to the response from the corresponding FRET sensor, AKAR2. However, the maximal change in signal of the BRET sensor is less than 10%, making it difficult to reliably measure changes in PKA activity in live cells. When the AKARB sensor was localized to the plasma membrane using a myristoylation and palmitoylation sequence, the pm-AKARB sensor displayed a 45% change in signal upon stimulation of PKA activity with 8-bromo-cAMP (Supplementary Figure S1B). The reversibility of the sensor was tested as shown in Supplementary Figure S1C. When the cellular PKA activity was inhibited by H-89, the pm-AKARB sensor reported no change in BRET signal to stimulation with prostaglandin E2 (PGE2) (Supplementary Figure S1D). Thus the pm-AKARB sensor can reliably measure the transient changes of PKA activity in cells and is used for all experiments in this study.
2.3 Animal handling
The AC7 conditional knockout mouse was generated with the help of the transgenic core laboratory at UT Southwestern Medical Center (Supplementary Figure S3). The strain was crossed to the LysMcre strain (Ferron and Vacher, 2005) kindly provided by Dr. Edward Wakeland (UT Southwestern Medical Center) and backcrossed to C57BL/6J mice for at least 5 generations. The LysMCre; G12−/−; G13fl/fl mouse strain was a gift from Dr. Nina Wettschureck (Moers et al., 2003). All experimental procedures involving animals in this study were reviewed and approved by the Institutional Animal Care and Research Advisory Committee at University of Texas Southwestern Medical Center.
2.4 Generation of the AC7 conditional knockout mouse strain
Mice deficient of the Adcy7 gene in the germline display a severe embryonic lethal phenotype (Duan et al., 2010; Jiang et al., 2008), making it difficult to study the functions of AC7 in the immune system. We generated a conditional knockout strain so that the Adcy7 gene can be deleted in specific cell lineages for targeted functional studies. Exon 6 of the Adcy7 gene was chosen to be the targeted deletion site because deletion of any of the first five exons will only result in an in-frame deletion of part of the protein sequence. Details of the targeting and selection strategy are shown in Supplementary Figure S2. Two conditional knockout strains were established. They were crossed with C57BL/6J-LysMcre line to generate the conditional knockout of AC7 in the myeloid lineage. The mice were further backcrossed to C57BL/6J for at least 3 additional generations.
2.5 Genotyping
Tissue samples were digested in lysis buffer (25 mM NaOH, 0.2 mM EDTA) at 100 °C for 1 hour and then neutralized with an equal volume of 40 mM Tris·HCl, pH5.5. Genotyping by PCR was done as described previously (Jiang et al., 2008).
2.6 Isolation of BMDMs
Bone marrow derived primary macrophages were isolated from mouse femurs and cultured as described (Jiang et al., 2008; Takeshita et al., 2000).
2.7 Assay of BRET in live cells
BRET (bioluminescence resonance energy transfer) sensors for cAMP and PKA, CAMYEL and pm-AKARB respectively, were introduced to BMDMs through retroviral infection. Procedures for retroviral infection of BMDMs and BRET assays for measuring cAMP responses in live cells were described before (Jiang et al., 2008; Jiang et al., 2007).
2.8 Cytokine measurement
BMDMs were plated at 1–1.5×105 cells per well on a 48-well plate the day before the assay. Cells were serum starved for 2 hours, then stimulated with various ligands for 4 hours. Medium was collected at the end of the 4-hour incubation and secreted cytokines were quantified using an ELISA kit following the manufacturer’s protocols. Cells remaining in the wells were disrupted with lysis buffer containing 1% Triton X-100 and total protein was measured using Precision Red (Cytoskeleton Inc.).
2.9 Phagocytosis assay
BMDMs were plated at 5×104 cells per well on a 96-well plate the day before the assay. Cells were serum starved for 4 hours and either not treated or pretreated with 1% paraformaldehyde or 10 μM cytochalasin D for 5 minutes. FITC-zymosan particles were then added to cells at a 10:1 ratio. After 20 minutes of incubation at 37 °C, cells were washed extensively with 1x PBS and extracellular FITC was quenched with trypan blue solution. Residual trypan blue was removed by extensive wash with 1x PBS. BMDMs were lysed (20 mM HEPES, 0.2% NP-40, 50 mM NaCl, 2.5 mM MgCl2) and the fluorescent signal was read on a plate reader. Each condition was done in duplicate per experiment.
2.10 Analysis of protein phosphorylation
BMDMs were plated at 5×105 cells per well on a 24-well plate the day before the assay. Cells were serum starved for 2 hours and then stimulated with various ligands for 5 minutes. Stimulation was stopped by placing the cells on ice and washing the cells with ice-cold PBS. The cells were lysed in 1% Triton-X lysis buffer (20 mM HEPES, 20 mM NaCl, 5 mM EDTA) containing protease inhibitors and phosphatase inhibitor cocktails I & II. After clearance of particulates, lysates were mixed with SDS sample buffer and heated at 95°C for 5 minutes. The samples were analyzed following standard SDS-PAGE and western blot protocols with antibodies for detecting phosphorylation of PKA substrates with the RRXS*/T* motif.
2.11 Zymosan induced peritonitis and flow cytometry analysis of peritoneal exudates
Mice at 6 to 10 weeks of age were injected with 1 mg of zymosan into the peritoneal cavity. Age matched mutant mice and their wild type littermates were grouped together for each experiment. At 4-, 24-, or 72-hours post injection, mice were euthanized and the peritoneal exudates were harvested by lavage (Cash et al., 2009). The cells were then counted and stained with a mixture of antibodies against cell surface markers for neutrophils and macrophages, including APC-Gr1 (BD), FITC-F4/80 (BioLegend), and CD11b-PE (BD). After staining, the cells were fixed in 1% paraformaldehyde and analyzed with a FACSCalibur flow cytometer and FlowJo software.
3. Results
3.1 Zymosan induces increases in cAMP and activation of PKA in wild type BMDMs
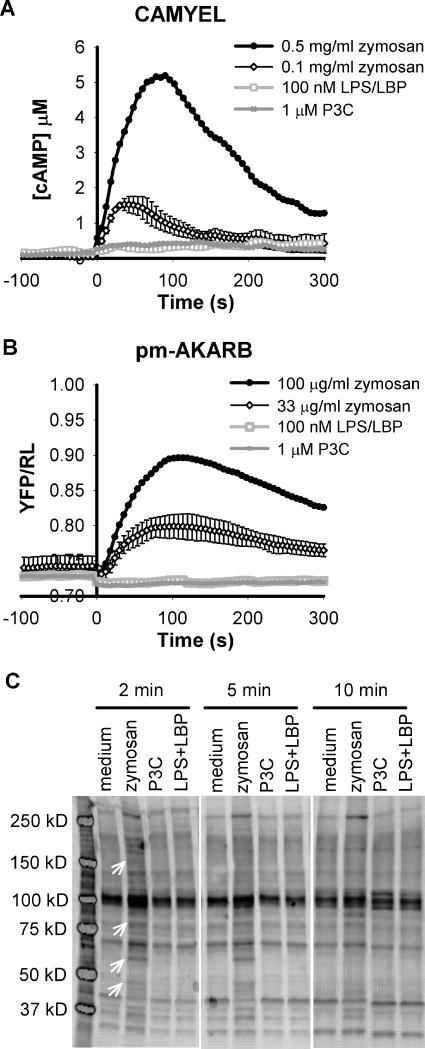
It has been reported that activation of the Toll-like receptor (TLR) signaling pathways has various effect on cAMP metabolism in macrophage cells (Jin and Conti, 2002; Moon et al., 2011; Osawa et al., 2006; Wang et al., 2010). To examine this effect directly we measured intracellular cAMP changes in response to several TLR ligands in live cells using bone marrow derived macrophages (BMDMs) that express a BRET sensor for cAMP, CAMYEL (cAMP BRET sensor using YFP-Epac-Luciferase) (Jiang et al., 2007). Activation of the TLR3 or TLR4 pathway with their respective ligands, poly I:C or LPS, did not acutely affect the intracellular concentration of cAMP (up to 15 minutes post ligand addition). Interestingly, when the cells were treated with zymosan particles, a TLR2 ligand, there was a transient rise of cAMP that peaked at about 1 to 2 minutes post ligand addition (Figure 1A). However, activation of the TLR2 pathway using soluble ligands such as PAM3Cys-SKKKKx3HCl (P3C) failed to induce any transient changes in intracellular cAMP concentration in BMDMs (Figure 1A).
Figure 1.
Zymosan induces cAMP responses and activation of PKA in wild type BMDMs. BMDMs were isolated from 6–10 week old wild type mice and infected with retrovirus carrying the BRET sensor for cAMP, CAMYEL (A), or the BRET sensor for PKA activity, pm-AKARB (B). Intracellular cAMP and activated PKA were measured using BRET assays. At time 0, cells were treated with zymosan at the concentrations indicated, 100 ng/ml LPS in the presence of 0.25 nM LBP, or 1 μM P3C. Each trace is the average of two to three independent experiments and error bars represent the standard deviation. Errors are similar for all conditions and shown on only one trace for clarity. (C). BMDMs were stimulated with 0.5 mg/ml zymosan, 1 μM P3C, or 10 ng/ml LPS + 25 pM LBP as indicated for 2, 5, or 10 min. Cell lysates were harvested and analyzed by western blot. Phosphorylation of PKA substrates was detected using an antibody selective for phospho-PKA substrates. Arrows indicate unique bands present only in zymosan treated cell lysates.
To evaluate the physiological consequence of zymosan induced rises in cAMP, we measured the ability of zymosan to activate PKA, a downstream target of cAMP, with a plasma membrane anchored BRET sensor for PKA, pm-AKARB (A kinase activity reported by BRET). This BRET sensor is engineered based on an established FRET sensor for PKA, AKAR2 (Zhang et al., 2005), and localization of the sensor to the plasma membrane greatly enhanced the signal-to-noise ratio of this sensor (Supplementary Figure S1). Since activation of PKA occurs at much lower concentrations of cAMP than detected by the CAMYEL sensor, which utilizes the lower affinity Epac protein for binding cAMP (Jiang et al., 2007), pm-AKARB has the potential to detect more subtle changes in intracellular cAMP concentration. As shown in Figure 1B, zymosan particles at 33 μg/ml induced significant changes in the BRET signal of pm-AKARB in BMDMs and 100 μg/ml zymosan produced a robust response. On the other hand, other TLR ligands including the TLR2 ligand, P3C, still failed to trigger any response in BMDMs when measured with pm-AKARB (Figure 1B). Activation of PKA by zymosan was validated by using western blots to detect the phosphorylation pattern of PKA substrates. In Figure 1C, an antibody specific for substrates phosphorylated by PKA detected a distinct pattern of bands in lysates from zymosan treated BMDMs; these bands appear within 2 min and some are gone or declining by 10 min, thus reflecting the time course of increases in cAMP and PKA activity. The phosphorylated proteins were absent in BMDMs treated with P3C or LPS. Taken together, our data consistently demonstrate that zymosan uniquely regulates intracellular cAMP concentration and activation of PKA in BMDMs.
Because zymosan particles are a crude preparation from yeast cell wall and are insoluble in water, we tested for possible effects of contaminating material as well as non-specific effects on the BRET assay. First, zymosan particles were still capable of triggering the response in BMDMs after soaking and extensive washing with buffer (data not shown). Zymosan particles from a different lot were also capable of inducing the cAMP response and activation of PKA in BMDMs, although the efficacy varied (Supplementary Figure S3). Based on these observations, we think the effect of zymosan is not due to contamination. Second, zymosan particles do not appear to interfere with the BRET assay by selectively obscuring emission signals from the sensors. This is based on the fact that both the CAMYEL and pm-AKARB sensors detected consistent responses to zymosan, even though their changes in BRET are opposite in response to stimuli (Jiang et al., 2007) (Supplementary Figure S1).
3.2 The increase in cAMP and activation of PKA by zymosan is dependent on AC7
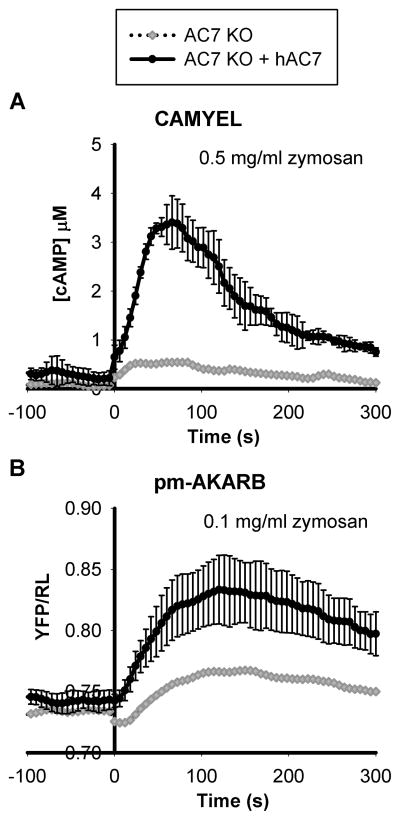
One of the major adenylyl cyclases expressed in BMDMs is AC7. We have previously shown that AC7 integrates inputs from multiple signaling pathways to regulate cAMP responses in BMDMs (Jiang et al., 2008). Does AC7 also play a role in mediating zymosan induced cAMP responses in BMDMs? To test this, AC7 deficient BMDMs were isolated from a conditional knockout mouse strain generated in our laboratory in which AC7 is specifically deleted in the myeloid lineage (Materials and Methods, Supplementary Figure S2). The amount of AC7 transcript in those BMDMs was about 10% of that in wild type cells as assessed by qPCR (Supplementary Figure S2C). As shown in Figure 2, when AC7 deficient BMDMs expressing the CAMYEL or pm-AKARB sensors were treated with zymosan, only small responses were detected. The residual responses were presumably due to incomplete deletion of the AC7 allele in a small percentage of cells. To confirm the role of AC7 for normal cAMP responses, we introduced human AC7 cDNA into AC7 deficient BMDMs. Re-expression of AC7 partially rescued the zymosan induced cAMP response and PKA activation in AC7 deficient BMDMs (Figure 2, compare with WT in Figure 1). Therefore, the effect of zymosan on the rise of intracellular cAMP and activation of PKA is largely mediated through AC7.
Figure 2.
Zymosan induced cAMP and activation of PKA is dependent on the expression of AC7. BMDMs were isolated from 6–10 week old mice with AC7 specifically knocked out in the myeloid lineage. AC7 deficient BMDMs were infected with retrovirus carrying the BRET sensor for cAMP, CAMYEL (A), or the BRET sensor for PKA, pm-AKARB (B), with or without the addition of retrovirus containing the expression of human AC7 cDNA. After 4 days of culture post infection, cells were treated with 0.5 mg/ml or 0.1mg/ml zymosan at time 0 and intracellular cAMP and PKA activity measured using BRET assays. Each trace is the average of two independent experiments and error bars represent the standard deviation.
3.3 AC7 deficient BMDMs show increased production of TNFα in response to zymosan
Zymosan has been widely used as a TLR2 ligand to trigger inflammatory responses in innate immune cells. However, it is well documented that the ability of zymosan to stimulate inflammatory cytokine responses appears to be weaker than other soluble stimuli of TLR2 (Underhill, 2003). Because cAMP has been shown to dampen the TLR signaling pathway and the production of proinflammatory cytokines, we tested if the concomitant stimulation of cAMP and activation of PKA by zymosan could modulate its effect on signaling through TLR2. AC7 deficient BMDMs provided an opportunity to examine this hypothesis. Zymosan would activate the TLR2 signaling pathway in AC7 deficient BMDMs without causing rises in cAMP and activation of PKA.
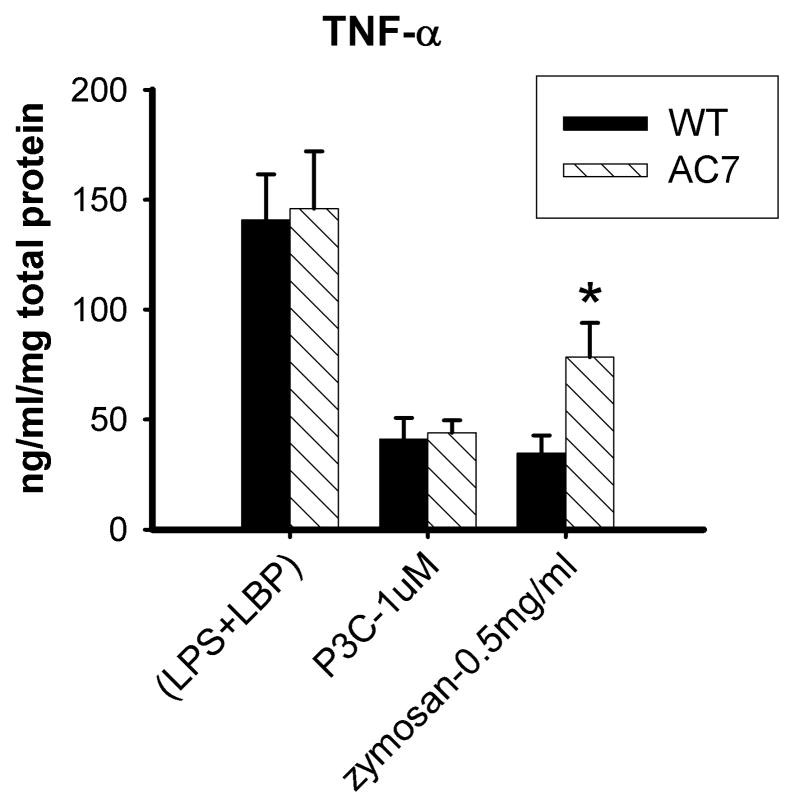
We compared the production of the proinflammatory cytokine, TNFα, in response to zymosan in wild type vs. AC7 deficient BMDMs (Figure 3). Zymosan induced much stronger production of TNFα in AC7 deficient BMDMs than in wild type cells. Stimulation with P3C, a soluble TLR2 ligand that has no effect on cAMP, produced similar amounts of TNFα in wild type and mutant BMDMs. Consistent with our previous result, TNFα production caused by LPS, a TLR4 ligand, was also similar in both cell types (Duan et al., 2010). Thus the TLR signaling pathways appear to function normally in AC7 deficient BMDMs. The difference in response to zymosan stimulation is likely caused by the differential cAMP response to zymosan in wild type and AC7 deficient cells. In wild type cells, the effect of zymosan on TLR2 signaling is likely dampened by its concomitant activation of cAMP and PKA.
Figure 3.
Zymosan induced production of TNFα in wild type and AC7 deficient BMDMs. BMDMs were isolated from 6–10 week old mice deficient of AC7 or their wild type littermates. After 7 to 10 days in culture, the cells were serum starved for 2 hours before being stimulated with the indicated ligands, 10 ng/ml LPS + 25 pM LBP, 1 μM P3C, or 0.5 mg/ml zymosan. After 4 hours of stimulation, accumulation of TNFα in the medium was measured using ELISA. There was no detectable TNFα in medium prior to ligand treatment (less than 8 pg/ml). The measured cytokine was normalized against the amount of total protein per well (indicator of total numbers of cells). Error bars indicate the standard deviation of 3 independent experiments (each from a different isolation of BMDMs). A single asterisk indicates a p value equal to 0.01.
3.4 AC7 deficient mice display prolonged inflammation in zymosan induced peritonitis
The mouse model of zymosan induced peritonitis is a well characterized system for studying the resolution of inflammation in a physiological context. Injection of zymosan particles into the mouse peritoneal cavity typically triggers an acute but relatively mild inflammatory response in the peritoneal cavity that results in recruitment of neutrophils and macrophages within 4 to 24 hours; the inflammation is typically resolved around 72 to 96 hours post injection. We reasoned that in the absence of a cAMP response, zymosan could trigger a prolonged inflammatory response.
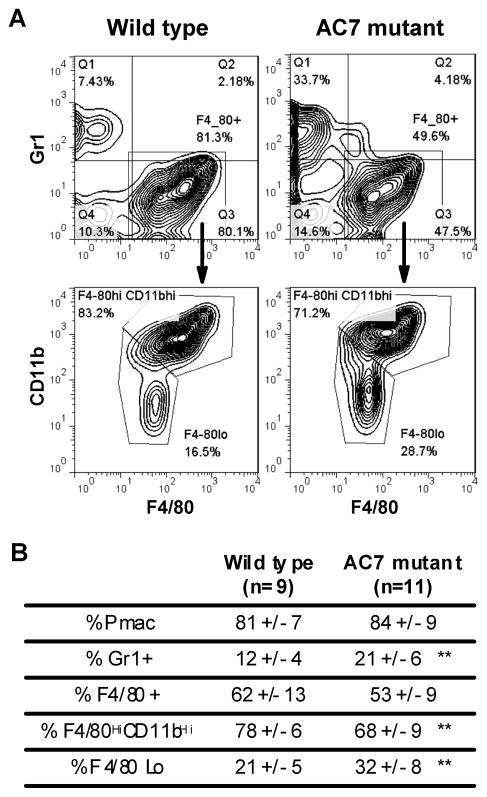
To test this idea, we used the conditional knockout mouse strain with AC7 specifically deleted in the myeloid lineage (Supplementary Figure S2). Injection of zymosan into the peritoneal cavity of mutant mice or their wild type littermates elicited recruitment of neutrophils and macrophage cells to the site of inflammation. At 4 hours or 24 hours post injection, no significant difference in the percentages of neutrophils and macrophages recruited to the peritoneal cavity was observed between the two populations (data not shown). However, at 72 hours post injection, there were significantly higher percentages of neutrophils present in the peritoneal cavities of AC7 deficient mice than in their wild type littermates (Figure 4). The composition of F4/80 positive macrophage cells was also different. Compared to their wild type litter mates, there was a higher percentage of F4/80Lo cells and a lower percentage F4/80HiCD11bHi cells in AC7 mutant mice. This is indicative of the presence of more inflammatory monocytes and less resident macrophages in the mutant mice (Ghosn et al., 2010; Rosas et al., 2010; Taylor et al., 2003). Taken together, it appears that mice deficient of AC7 in the myeloid lineage displayed prolonged inflammation and delayed resolution in the zymosan induced peritonitis model.
Figure 4.
AC7 deficient mice display prolonged inflammation in the zymosan induced peritonitis model. Mice deficient of AC7 in the myeloid lineage and their wild type littermates were injected with 1 mg of zymosan into the peritoneal cavity at age 6 to 10 weeks. At 72-hour post injection mice were euthanized and the peritoneal exudates were harvested by lavage (Cash et al., 2009). The cells were then counted, stained with neutrophil and macrophage markers (APC-Gr1, CD11b-PE and FITC-F4/80), and analyzed with a FACSCalibur flow cytometer and FlowJo software. Representative graphs of FACS analysis are shown in (A). Analysis to determine percentages of neutrophils and macrophages present at different stages during zymosan induced peritonitis is presented in (B). Double asterisks indicate a p value less than 0.01 based on t-test.
3.5 Characterization of the zymosan associated factor that triggers cAMP responses and activation of PKA
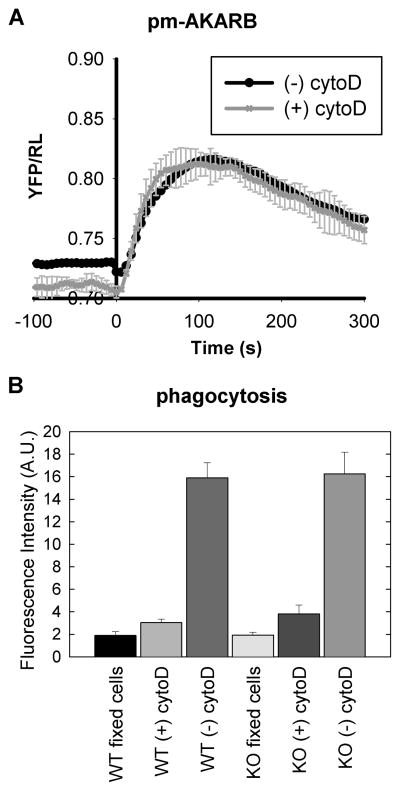
Zymosan particles are known to bind to several cell surface receptors on macrophage cells and trigger two major responses, activation of the TLR2 pathway and phagocytosis. Activation of the TLR2 pathway alone using soluble stimuli, such as P3C, does not stimulate intracellular cAMP responses or activation of PKA in BMDMs (Figure 1). To determine if phagocytosis is required for the effect of zymosan on cAMP and PKA, we treated BMDMs with an inhibitor of actin polymerization, cytochalasin D, to block phagocytosis and then stimulated the cells with zymosan. As shown in Figure 5, in the presence of cytochalasin D, the cells still displayed robust activation of PKA in response to zymosan. Futhermore, phagocytosis in AC7 deficient BMDMs is the same as WT cells; this indicates that altered binding of zymosan to receptors is not a reason for decreased cAMP responses in KO cells. This suggests that the effect of zymosan on PKA activation is independent of the particles being phagocytosed and is likely due to a receptor-mediated response.
Figure 5.
Phagocytosis is not required for zymosan induced PKA activation. (A). BMDMs expressing the pm-AKARB sensor were stimulated with 33 μg/ml zymosan with or without the pretreatment of 10 μM cytochalasin D for 5 minutes. Activation of PKA was recorded as the change in BRET signal of the sensor. (B). Phagocytosis of zymosan particles into WT or AC7 deficient (KO) BMDMs was determined with FITC-labeled zymosan. BMDMs were either first fixed using 1% paraformaldehyde, treated with 10 μM cytochalasin D for 5 minutes, or incubated without treatment. Then FITC-zymosan particles were added to cells at ~10:1 ratio. After 20 minutes of incubation at 37°C, cells were washed extensively and extracellular FITC was quenched with trypan blue. BMDMs were lysed and fluorescence was read on a plate reader. Each condition was done in duplicate per experiment and error bars represent the standard deviation of 3 independent experiments.
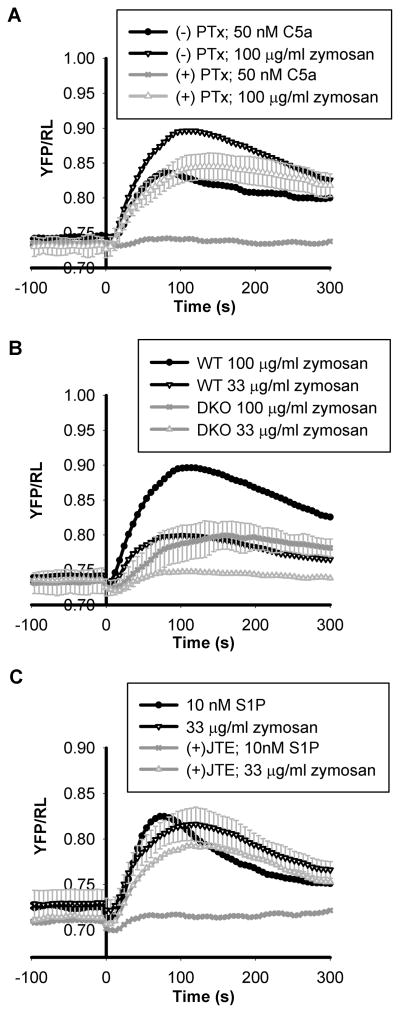
Since zymosan induced activation of PKA is dependent on the activity of AC7 and AC7 mediates cAMP responses from multiple GPCR signaling pathways in BMDMs, we tested whether zymosan functions through a G-protein signaling pathway. Disabling of Gαi function using pertussis toxin completely abolished activation of PKA by C5a in wild type BMDMs, but only reduced responses to zymosan stimulation by ~20% (Figure 6A). Interestingly, BMDMs deficient of both Gα12 and Gα13 proteins (DKO) showed a larger reduction in activation of PKA by zymosan (Figure 6B). The deficient cells were minimally responsive to lower doses of zymosan (33 μg/ml zymosan), but partially responsive (~30%) to higher doses (100 μg/ml zymosan). Therefore, zymosan appears to elicit activation of cAMP and PKA, in part, through the G12/13 pathway, and possibly the Gi pathway.
Figure 6.
Characterization of zymosan associated factor capable of inducing PKA activation. (A). Wild type BMDMs carrying the pm-AKARB sensor were stimulated with 50 nM C5a or 100 μg/ml zymosan with or without pre-incubation with 100 ng/ml pertussis toxin for 16 hours; activation of PKA was measured by BRET assay. (B). Wild type BMDMs and BMDMs deficient of both Gα12 and Gα13 were stimulated with 33 μg/ml or 100 μg/ml zymosan as indicated and activation of PKA was recorded by BRET assay. DKO denotes double knockout of Gα12 and Gα13. (C). BMDMs expressing the pm-AKARB sensor were stimulated with 33 μg/ml zymosan or 10 nM S1P with or without the presence of 2 μM JTE-013, an antagonist to S1P2 receptor. Each trace is the average of 2–3 independent experiments and error bars represent the standard deviation.
We have previously shown that the bioactive phospholipid, sphingosine-1-phosphate (S1P), acts via the S1P2 receptor, Gα13, and AC7 to increase intracellular cAMP concentration in BMDMs (Jiang et al., 2008; Jiang et al., 2007). To ensure that the effect of zymosan is different than that of S1P, we used a specific antagonist against the S1P2 receptor, JTE-013, to block the effect of S1P on PKA activation (Figure 6C). However, JTE-013 did not block the PKA response to zymosan (Figure 6C), indicating that the effect of zymosan is distinct from that of S1P and is not mediated through the S1P2 receptor.
4. Discussion
The pleiotropic function of cAMP signaling in many cell types makes it a prime target during microbial invasion of host cells (Finlay and McFadden, 2006; Hajishengallis and Lambris, 2011; McDonough and Rodriguez, 2012). In fact, enzymes involved in every step of cAMP metabolism, including Gαs, adenylyl cyclases, and phosphodiesterases, are targeted by various pathogens (Ahuja et al., 2004; Cassel and Pfeuffer, 1978; Hosono and Suzuki, 1985). Novel mechanisms on how microbes hijack the cAMP metabolic pathway are still being discovered (Pulliainen et al., 2012). In this study, we reveal a novel regulation of cAMP synthesis and PKA activation by the yeast cell wall component, zymosan. We show that zymosan can increase intracellular cAMP and activate PKA in mouse macrophage cells (Figure 1). Both responses depend on the expression of AC7, a key isoform of adenylyl cyclase expressed in BMDMs (Figure 2).
Elevation of cAMP in macrophages dampens their responses to TLR stimuli and leads to reduced production of proinflammatory cytokines (Duan et al., 2010; Natarajan et al., 2006; Wall et al., 2009). Not surprisingly, BMDMs devoid of AC7 produced a much higher level of TNFα in response to zymosan when compared to wild type cells although both types of cells respond similarly to stimulation with P3C (Figure 3). This result indicates that the ability of zymosan particles to stimulate cAMP and activate PKA has a real physiological consequence of dampening the inflammatory response in macrophage cells. It is conceivable that fungi such as yeast use this strategy to change host immune responses and reduce detection for early proliferation. This result could also help explain why the ability of zymosan to stimulate the TLR2 pathway appears less potent than that of other soluble stimuli of the TLR2 receptor (Underhill, 2003).
The model of zymosan induced peritonitis in mice was used to explore the physiological effect of zymosan induced cAMP responses and PKA activation in vivo. Mice deficient of AC7 in the myeloid lineage displayed prolonged inflammation and failed to resolve inflammation in a timely manner (Figure 4). We have previously shown that mice deficient of AC7 in the immune system were hypersensitive to LPS induced endotoxic shock (Duan et al., 2010). Together with the current result, it highlights the importance of AC7 in regulating proper innate immune responses in vivo. However, the prolonged inflammation caused by zymosan in AC7 deficient mice may not be solely due to the initial lack of a cAMP response to zymosan in the macrophage cells. Inflammatory responses and resolution of inflammation in vivo are regulated by complex signaling pathways, many of which exert their functions through modulating cAMP concentration and PKA activity (Bystrom et al., 2008; Castro et al., 2005; Nathan and Ding, 2010). Lack of AC7 in macrophage cells could result in reduced cAMP responses at both the inflammation and resolution phases of the process and lead to a more profound phenotype. A better way to address the specific role of zymosan induced cAMP and PKA activation would be to use zymosan particles devoid of cAMP stimulating activity while retaining their effectiveness for activating the TLR2 pathway.
What could be the factor that allows zymosan particles to increase cAMP in BMDMs? We believe that this is mediated by a cell surface receptor for several reasons. First, phagocytosis is not required for the effect, indicating the zymosan particles are capable of signaling from outside the cells. Second, G proteins (G12/13 and possibly Gi) and AC7 are required for the cAMP and PKA responses induced by zymosan, strongly suggesting the involvement of G-protein coupled receptors. Zymosan particles are mainly composed of polycarbohydrates including β-glucan, mannan, and chitin (Di Carlo and Fiore, 1958; Underhill, 2003). They are recognized by various cell surface receptors, such as Dectin-1 and integrin Mac-1. However, it is unclear if any of those receptors are coupled to G proteins like Gi or G12/13 or are capable of triggering cAMP responses. Zymosan is also capable of activating the TLR2 receptor. Although we showed that activation of TLR2 alone by other soluble factors did not stimulate cAMP and PKA, we cannot rule out the possibility that the TLR2 receptor could still be involved in zymosan induced cAMP and PKA responses. For example, the bacterium Porphyromonas gingivalis is a major oral and systemic pathogen capable of raising intracellular cAMP concentration in macrophage cells, an action that requires the expression of TLR2 receptor (Wang et al., 2010). Conceivably, activation of TLR2 receptor by specific ligands could lead to transactivation of G12/13 and/or Gi coupled receptors, which in turn regulate cAMP synthesis. This remains to be further elucidated.
5. Conclusions
The yeast cell wall component, zymosan, induces a variety of cellular responses in macrophage cells. Here we reported a novel discovery that zymosan induces cAMP increase and PKA activation in primary macrophage cells. We demonstrated that this response is mediated by AC7 and heterotrimeric G proteins, primarily G13, and it does not require phagocytosis of zymosan particles. Lack of cAMP and PKA response leads to increased TNFa production in response to zymosan in cultured BMDMs. Mice lack of AC7 in the myeloid lineage displayed prolonged inflammation in zymosan induced peritonitis. We propose that zymosan induced increases in cAMP and activation of PKA serve as a mechanism to dampen inflammatory responses in host cells, which consequently favors the survival of microbes.
Supplementary Material
Highlights.
Zymosan particles increase cAMP and activation of PKA in macrophage cells
These responses depend on AC7 and heterotrimeric G proteins, primarily G12/13
Macrophage cells deficient of AC7 produce more TNFα in response to zymosan
Mice without myeloid AC7 show prolonged inflammation in zymosan induced peritonitis
Acknowledgments
We thank Dr. Michelle Tallquist for pPGKneo.F2L2.DTA vector, Dr. Rhonda Bassel-Duby for FLPe mouse strain, Dr. Edward Wakeland for the LysMcre mouse strain, and Dr. Nina Wettschureck for LysMcre; G12−/−; Gα13fl/fl knockout mice. We are grateful for the help of Dr. Robert Hammer and the transgenic technology core at UT Southwestern Medical Center during the generation of AC7 conditional knockout strain. We thank Dr. Pila Estess for help with flow cytometry and Dr. Dorothy Yuan for stimulating discussions. This work was supported by NIH grants GM084098-01A1 (L. I. J. and P. C. S), GM084098-01A1S1 (L. I. J. and P. C. S.), and the Alfred and Mabel Gilman Chair in Molecular Pharmacology (P.C.S.).
Abbreviations
- AC
adenylyl cyclase
- BMDM
bone marrow derived macrophages
- PKA
protein kinase A
- BRET
bioluminescence resonance energy transfer
- CAMYEL
cAMP BRET sensor using YFP-Epac-Luciferase
- pm-AKARB
plasma membrane anchored-A kinase activity reported by BRET
- S1P
sphingosine-1-phosphate
- PGE2
prostaglandin E2
- RL
Renilla luciferase
- YFP
yellow fluorescent protein
- LBP
LPS binding proteins
- P3C
PAM3Cys-SKKKKx3HCl
Appendix A. Supplementary data
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lily I. Jiang, Email: Lily.Jiang@UTSouthwestern.edu.
Paul C. Sternweis, Email: Paul.Sternweis@UTSouthwestern.edu.
Jennifer E. Wang, Email: Jennifer.Wang@UTSouthwestern.edu.
References
- Ahuja N, Kumar P, Bhatnagar R. The adenylate cyclase toxins. Crit Rev Microbiol. 2004;30:187–96. doi: 10.1080/10408410490468795. [DOI] [PubMed] [Google Scholar]
- Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–22. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrom J, Evans I, Newson J, Stables M, Toor I, vanRooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–27. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash JL, White GE, Greaves DR. Chapter 17. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol. 2009;461:379–96. doi: 10.1016/S0076-6879(09)05417-2. [DOI] [PubMed] [Google Scholar]
- Cassel D, Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978;75:2669–73. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A, Jerez MJ, Gil C, Martinez A. Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: Advances in the development of specific phosphodiesterase inhibitors. Medicinal Research Reviews. 2005;25:229–244. doi: 10.1002/med.20020. [DOI] [PubMed] [Google Scholar]
- Di Carlo FJ, Fiore JV. On the composition of zymosan. Science. 1958;127:756–7. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- Duan B, Davis R, Sadat EL, Collins J, Sternweis PC, Yuan D, Jiang LI. Distinct roles of adenylyl cyclase VII in regulating the immune responses in mice. J Immunol. 2010;185:335–44. doi: 10.4049/jimmunol.0903474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Vacher J. Targeted expression of Cre recombinase in macrophages and osteoclasts in transgenic mice. Genesis. 2005;41:138–45. doi: 10.1002/gene.20108. [DOI] [PubMed] [Google Scholar]
- Finlay BB, McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–82. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Ghosn EE, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–73. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono K, Suzuki H. Morphological transformation of Chinese hamster cells by acylpeptides, inhibitors of cAMP phosphodiesterase, produced by Bacillus subtilis. J Biol Chem. 1985;260:11252–5. [PubMed] [Google Scholar]
- Jiang LI, Collins J, Davis R, Fraser ID, Sternweis PC. Regulation of cAMP responses by the G12/13 pathway converges on adenylyl cyclase VII. J Biol Chem. 2008;283:23429–23439. doi: 10.1074/jbc.M803281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, Hsueh R, Rebres RA, Ross EM, Taussig R, Fraser I, Sternweis PC. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem. 2007;282:10576–84. doi: 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–33. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–42. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 2012;10:27–38. doi: 10.1038/nrmicro2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–22. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- Moon EY, Lee YS, Choi WS, Lee MH. Toll-like receptor 4-mediated cAMP production up-regulates B-cell activating factor expression in Raw264.7 macrophages. Exp Cell Res. 2011;317:2447–55. doi: 10.1016/j.yexcr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation--overview of mechanisms of action in T cells. Cell Signal. 2011;23:1009–16. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Natarajan M, Sternweis PC, Lin K-M, Hsueh RC, Ranganathan R Laboratories TAfCS. A global analysis of cross-talk in a mammalian cellular signalling network. Nature Cell Bio. 2006;8:571–580. doi: 10.1038/ncb1418. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Osawa Y, Lee HT, Hirshman CA, Xu D, Emala CW. Lipopolysaccharide-induced sensitization of adenylyl cyclase activity in murine macrophages. Am J Physiol Cell Physiol. 2006;290:C143–51. doi: 10.1152/ajpcell.00171.2005. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M. Putting on the brakes: cyclic AMP as a multipronged controller of macrophage function. Sci Signal. 2009;2:pe37. doi: 10.1126/scisignal.275pe37. [DOI] [PubMed] [Google Scholar]
- Pulliainen AT, Pieles K, Brand CS, Hauert B, Bohm A, Quebatte M, Wepf A, Gstaiger M, Aebersold R, Dessauer CW, Dehio C. Bacterial effector binds host cell adenylyl cyclase to potentiate Galphas-dependent cAMP production. Proc Natl Acad Sci U S A. 2012;109:9581–6. doi: 10.1073/pnas.1117651109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas M, Thomas B, Stacey M, Gordon S, Taylor PR. The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B. J Leukoc Biol. 2010;88:169–80. doi: 10.1189/jlb.0809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–32. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15:1477–88. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–7. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res. 2003;9:176–80. doi: 10.1179/096805103125001586. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–5. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–73. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.