Abstract
Topoisomerase II (TOP2)-targeting poisons such as anthracyclines and etoposide are commonly used for cancer chemotherapy and kill tumor cells by causing accumulation of DNA double-strand breaks (DSBs). Several lines of evidence indicate that overexpression of TOP2A, the gene encoding topoisomerase IIα, increases sensitivity of tumor cells to TOP2 poisons but it is not clear why some TOP2A-overexpressing (TOP2A-High) tumors respond poorly to these drugs. In this study, we identified that TOP2A expression is induced by DLX4, a homeoprotein that is overexpressed in breast and ovarian cancers. Analysis of breast cancer datasets revealed that TOP2A-High cases that also highly expressed DLX4 responded more poorly to anthracycline-based chemotherapy than TOP2A-High cases that expressed DLX4 at low levels. Overexpression of TOP2A alone in tumor cells increased the level of DSBs induced by TOP2 poisons. In contrast, DLX4 reduced the level of TOP2 poison-induced DSBs irrespective of its induction of TOP2A. DLX4 did not stimulate homologous recombination-mediated repair of DSBs. However, DLX4 interacted with Ku proteins, stimulated DNA-dependent protein kinase activity, and increased erroneous end-joining repair of DSBs. Whereas DLX4 did not reduce levels of TOP2 poison-induced DSBs in Ku-deficient cells, DLX4 stimulated DSB repair and reduced the level of TOP2 poison-induced DSBs when Ku was reconstituted in these cells. Our findings indicate that DLX4 induces TOP2A expression, but reduces sensitivity of tumor cells to TOP2 poisons by stimulating Ku-dependent repair of DSBs. These opposing activities of DLX4 could explain why some TOP2A-overexpressing tumors are not highly sensitive to TOP2 poisons.
Keywords: chemosensitivity, topoisomerase II, DNA repair, homeobox genes
INTRODUCTION
DNA double-strand breaks (DSBs) are the most lethal form of DNA damage and are primarily repaired by two pathways. The homologous recombination (HR) pathway is a high fidelity repair mechanism that utilizes the sister chromatid as a template. The canonical non-homologous end-joining (NHEJ) pathway is initiated by the binding of Ku heterodimers to DNA ends which recruit the catalytic subunit of DNA-dependent protein kinase (DNA-PK) to form a complex that enables ligation of DNA ends with little or no homology (1,2). Cells that are defective in the HR or NHEJ pathways are highly sensitive to poisons that target topoisomerase II (TOP2) (3–6). TOP2 is an enzyme that induces transient DSBs to relieve torsional stress imposed on DNA during replication (7). Of the two mammalian TOP2 isoforms, TOP2α is essential for cell growth (7). TOP2 poisons such as etoposide and anthracyclines (e.g. doxorubicin, epirubicin) are commonly used as anticancer drugs. TOP2 poisons trap the enzyme in a complex with DNA at a step after DSBs have occurred, thereby causing DSBs to accumulate to lethal levels (8). Experimental studies have demonstrated that overexpressing TOP2α in cells increases TOP2 poison-induced cell death, whereas down-regulating TOP2α decreases sensitivity (9–11). On the other hand, there have been conflicting studies regarding the clinical value of TOP2α in predicting responsiveness to TOP2 poisons. The TOP2A gene that encodes TOP2α maps to 17q21-22, a region that is amplified in ~12% of breast and ovarian cancers (12,13). Whereas several studies have reported that TOP2A amplification identifies cancer patients who respond favorably to anthracycline-based chemotherapy (13,14), others have found the TOP2A status has no predictive value (15–17). However, a molecular explanation for the discordance between these experimental and clinical studies has not been identified.
Homeobox genes constitute a gene super-family encoding transcription factors (often termed homeoproteins) that control developmental patterning (18–20). Increasing evidence indicates that aberrant expression of many homeobox genes in tumors deregulates cell proliferation and cell survival (19,20). For example, the homeobox gene CDX2 inhibits cell proliferation and is down-regulated in many colon cancers (21,22). Overexpression of SIX1 in ovarian cancers confers resistance to TRAIL-mediated apoptosis (23), whereas loss of BARX2 in ovarian cancers is associated with cisplatin-resistance (24). However, only few bona fide transcriptional targets of homeoproteins have been identified, and the mechanisms of these regulatory factors in tumorigenesis remain poorly understood.
The DLX4 gene (also reported as DLX7, BP1) is a member of the DLX family of homeobox genes that controls diverse developmental processes including skeletal patterning and neurogenesis (25). DLX4 is absent from most normal adult tissues, but is frequently expressed in breast and ovarian cancers (26,27) and also in lung cancers and leukemias (28,29). We previously identified that DLX4 inhibits gene responses that are central to the TGF-β cytostatic program (30), and also stimulates tumor angiogenesis by inducing expression of fibroblast growth factor-2 and vascular endothelial growth factor (27). Others have found that DLX4 is more highly expressed in a doxorubicin-resistant subclone of MCF-7 breast cancer cells than in the parental cell line (26), raising the possibility that DLX4 also modulates sensitivity to specific chemotherapeutic agents. In this study, we identified that TOP2A is a transcriptional target of DLX4, but that induction of TOP2A by DLX4 is not associated with increased sensitivity to TOP2 poisons. DLX4 was found to interact with Ku proteins, stimulate Ku-dependent repair of DSBs and reduce levels of DSBs induced by TOP2 poisons. These apparently opposing activities of DLX4 could explain why some tumors have elevated TOP2A expression but are not sensitive to TOP2 poisons.
MATERIALS AND METHODS
Reagents
Sources of antibodies (Abs) were as follows: DLX4 (Abcam, Abnova); TOP2α (TopoGEN); Ku70, Ku80 (Thermo Fisher Scientific); DNA-PK catalytic subunit (DNA-PKcs) (Cell Signaling Technology); DNA ligase IV, XRCC4, FLAG, actin (Sigma-Aldrich); histone H2A (Millipore). Doxorubicin, etoposide, paclitaxel, 5-fluorouracil, cyclophosphamide, 5-bromo-4-chloro-3-indolyl-β-D-galacto-pyranoside (X-gal) and isopropyl-L-thio-β-D-galacto-pyranoside (IPTG) were purchased from Sigma-Aldrich.
Plasmids
All shRNAs and cDNAs encoding Ku80 and TOP2α-GFP fusion protein were purchased from OriGene Technology. pUC19 plasmid was purchased from Invitrogen. BRCA1 and I-Sce I plasmids (31) and full-length and truncated DLX4 expression plasmids (30) have been previously described. pGL2 plasmids containing wild-type TOP2A promoter sequences were provided by William Beck (University of Illinois). The motif ATATAAAAG in the TOP2A promoter (nucleotides −127 to −119) was mutated to ACAGAGACG by using the QuikChange II Site-directed Mutagenesis Kit (Agilent Technologies).
Cell lines
Parental MDA-MB-468 and XR-V15B cells, authenticated by STR analysis, were purchased from American Type Culture Collection and passaged within 6 months of receipt. Parental TOV112D cells (provided by Ju-Seog Lee, MD Anderson Cancer Center) and U2OS cells were also tested by STR analysis. The stable +DLX4 MDA-MB-468 and U2OS DR-GFP reporter cell lines are described in our previous studies (30,31). Cell lines were cultured in the following media supplemented with 10% FBS and penicillin/streptomycin: MDA-MB-468 (RPMI 1640); U2OS (McCoys’ 5A); XR-V15B (DMEM); TOV112D (1:1 mixture of MCDB 105 medium and Medium 199). Lipofectamine 2000 reagent (Invitrogen) was used for transfecting cell lines.
Cell viability and cell death assays
Cells were plated in 96-well plates (2,000 per well) and incubated for 2 d with drugs at concentrations ranging from 10−4 to 104 µM. Cell viability was measured by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche). IC50 values were calculated from the average of dose-response curves obtained in 3 independent experiments. Cell death was measured by assaying mono- and oligo- nucleosomes in cell lysates by using Cell Death Detection ELISA (Roche).
Chromatin immunoprecipitation (IP) and luciferase reporter assays
Chromatin IP assays were performed by using the ChIP Assay kit (Upstate Biotechnology). Sheared chromatin was incubated with DLX4 Ab and DNA purified from precipitated protein-DNA complexes. A 375 bp fragment of the TOP2A promoter was amplified by using the following primers: forward, 5’-GAAGCTAAGGCTCCCATTCC-3’; reverse, 5’-CGTCCAGAAGAACCAATCGT-3’. As a negative control, a 166 bp GAPDH fragment was amplified using the following primers: forward, 5’-TACTAGCGGTTTTACGGGCG-3’; reverse, 5’-TCGAACAGGAGGAGCAGAGAGCGA-3’. Cells were co-transfected with pGL2 firefly luciferase reporter plasmids and with pRL-CMV Renilla luciferase reporter plasmid (Promega) for normalizing transfection efficiency. Luciferase activities were assayed using the Dual-luciferase reporter assay kit (Promega).
Comet assay
DSBs were measured by neutral comet assay using the Comet Assay kit (Trevigen). Comet images were captured by fluorescence microscopy. Tail moments (percentage of DNA in tail×tail length) were quantified using CometScore software (www.autocomet.com). For each cell line, 3 independent assays were performed where comet tails were analyzed in a minimum of 50 randomly selected cells in each assay.
HR assay
Cells of the U2OS DR-GFP reporter cell line were transfected with I-Sce I plasmid to generate a DSB within the SceGFP reporter gene. At 1 d thereafter, cells were transfected with empty vector, DLX4 or BRCA1 plasmids and cultured for 2 d. Repair of the DSB was assayed by PCR and flow cytometric analysis of GFP fluorescence intensity as previously described (31).
End-joining assay
Cells were suspended in lysis buffer (10 mM HEPES, pH 7.9, 0.5 M sucrose, 1 mM dithiothreitol (DTT), 5 mM MgCl2, 0.2% Nonidet P-40) and centrifuged at 800×g for 5 min. Pellets were suspended in nuclear buffer (10 mM HEPES, pH 7.9, 25% glycerol, 420 mM NaCl, 1 mM DTT, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2% Nonidet P-40) and centrifuged at 12,000×g for 10 min. Supernatants were dialyzed at 4°C in dialysis buffer (20 mM HEPES, pH 7.9, 10% glycerol, 100 mM KCl, 1 mM DTT, 0.2 mM EDTA). Eco RI-digested pUC19 DNA (100 ng) was incubated at 20°C for 16 h with dialyzed nuclear extract (15 µg) in a reaction containing 50 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 5 mM ATP, 50 µM dNTPs, 1 mM DTT. E. coli strain DH5α (Invitrogen) was transformed with 10 ng of pUC19 DNA and plated on LB agar plates containing ampicillin, X-gal and IPTG. Numbers of blue and white colonies were counted on each plate. Plasmid DNA was isolated from individual colonies and sequenced.
IP and immunoblotting
Whole cell extracts were prepared by lysing cells in M-PER buffer (Pierce Biotechnology). Nuclear extracts were prepared as previously described (30). Where indicated, genomic DNA was removed by treatment with benzonase. For protein IP, extracts were pre-cleared with protein G agarose and 1 mg of extract was incubated with Abs indicated in the text. For DNA IP, nuclear extracts were pre-cleared with streptavidin agarose and incubated with biotinylated oligonucleotide duplexes as previously described (30). Immunoprecipitates were subjected to SDS-PAGE and immunoblotting using PVDF membrane (GE Healthcare).
DNA-PK assay
Nuclear extracts were prepared as for IP assays and incubated with DEAE Sepharose® Fast Flow (GE Healthcare) to remove genomic DNA. DEAE sepharose was removed by centrifugation and extracts dialyzed in buffer containing 20 mM Tris-HCl, pH 8.0, 10% glycerol, 100 mM NaCl, 1% Nonidet P-40, 2 mM EDTA. DNA-PK assays were performed by using the SignaTECT® DNA-PK Assay System (Promega).
Bioinformatic analysis
DLX4 and TOP2A transcript levels in tumors were analyzed in publicly available gene expression datasets as described in Supplementary Methods.
Statistical analysis
Statistical analysis was performed using STATISTICA6 software (StatSoft Inc.). Statistical significance of data was assessed by two-tailed Student’s t-test unless noted otherwise. Data represent mean + s.d. Significance of differences in gene expression between groups of patients was assessed by Mann-Whitney U-test. Fisher exact test was used for evaluating differences in response rates between groups. P values of >0.05 were considered not significant.
RESULTS
DLX4 overexpression in tumor cells selectively decreases TOP2 poison-sensitivity
Increasing evidence indicates that various homeobox genes modulate responsiveness of cells to diverse anti-proliferative and apoptotic signals (23,24,30). We initially investigated the possibility that DLX4 modulates the sensitivity of tumor cells to commonly used chemotherapeutic agents by using a MDA-MB-468 breast cancer cell line that we transduced with DLX4 in earlier studies (30). Expression of DLX4 significantly reduced sensitivity of MDA-MB-468 cells to doxorubicin (P=0.005), but did not alter sensitivity to paclitaxel (microtubule disruptor), 5-fluorouracil (pyrimidine analog) or cyclophosphamide (alkylating agent) (Supplementary Table I). Similar results were obtained when DLX4 was overexpressed in U2OS osteosarcoma cells (Supplementary Table I). These findings suggest that DLX4 overexpression selectively decreases TOP2 poison-sensitivity.
DLX4 induces TOP2A promoter activity and TOP2α levels
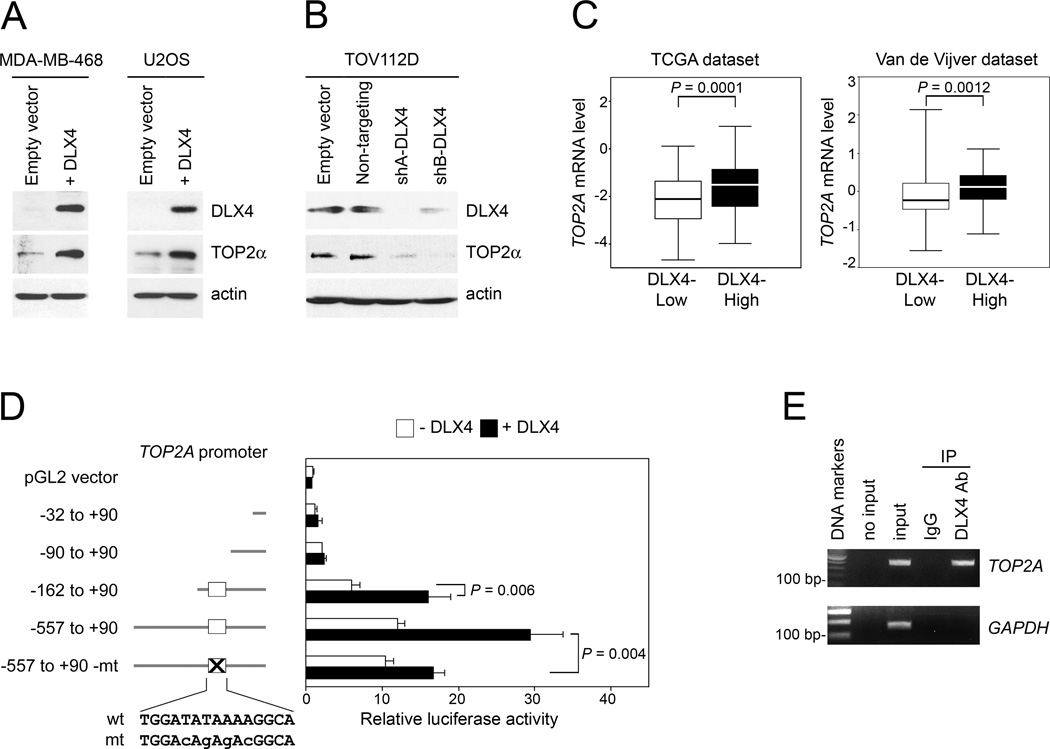
Because down-regulation of TOP2α has been reported to reduce sensitivity to TOP2 poisons (10,11), we evaluated whether DLX4 inhibits TOP2α expression. Contrary to our expectation, enforced expression of DLX4 induced TOP2α levels (Fig. 1A). Conversely, TOP2α levels decreased when endogenous DLX4 was knocked-down by shRNAs that targeted two different sites in DLX4 but were not affected by non-targeting shRNA (Fig. 1B). When breast cancer cases in two independent cohorts were stratified according to expression of DLX4 in tumors, levels of TOP2A transcripts were found to be significantly higher in DLX4-High than in DLX4-Low tumors in each of these cohorts (the Cancer Genome Atlas (TCGA) dataset, P=0.0001; Van de Vijver dataset (32), P=0.0012; Fig. 1C). TOP2A transcript levels were also higher in DLX4-High than in DLX4-Low tumors in TCGA datasets of ovarian and lung cancers (ovarian cancers, P=0.022; lung cancers, P=0.028; Supplementary Fig. S1). Because homeoproteins are thought to function as transcription factors (18), we evaluated the effect of DLX4 on TOP2A promoter activity in luciferase reporter assays. DLX4 stimulated TOP2A promoter activity through a region located between positions −162 to −90 relative to the transcription start site (P=0.006; Fig. 1D). Binding of endogenous DLX4 to this region was detected by chromatin IP (Fig. 1E). An AT-rich motif located within the responsive region is homologous to the DLX4-binding site in the β-globin promoter that is the only well-characterized target of DLX4 (33). Mutation of the AT-rich motif significantly reduced the ability of DLX4 to stimulate TOP2A promoter activity (P=0.004; Fig. 1D). Together, our findings indicate that TOP2A is a transcriptional target of DLX4, but that DLX4 reduces TOP2 poison-sensitivity irrespective of its induction of TOP2A.
Fig. 1. DLX4 induces TOP2A promoter activity and TOP2α levels.
[A,B] Western blot of DLX4 and TOP2α levels in [A] vector-control and DLX4-overexpressing MDA-MB-468 and U2OS cell lines, and [B] TOV112D cells transfected with empty vector, non-targeting shRNA and shRNAs targeting two different sites of DLX4 (shA-DLX4, shB-DLX4). [C] Breast cancer cases from the TCGA Project (n=537) and study of Van de Vijver et al (32) (n=295) were stratified according to DLX4 expression in tumors, where DLX4 transcript levels in each dataset were defined as High (> upper quartile) and Low (< lower quartile). Significance of differences in TOP2A transcript levels (log2 scale) between upper and lower quartile sub-groups was evaluated by Mann-Whitney U-test. [D] U2OS cells that lacked DLX4 (white bar) or expressed DLX4 (black bar) were transfected with pGL2 luciferase reporter plasmids containing the indicated regions of the TOP2A promoter. Wild-type (capitals) and mutated (small case) sequences of the DLX4-binding site (white box) are indicated. Shown are average relative luciferase activities of 3 independent experiments. [E] Detection of binding of endogenous DLX4 to the TOP2A promoter in TOV112D cells by chromatin IP. Input corresponds to 1% of the chromatin solution before IP.
DLX4 is associated with reduced responsiveness to anthracycline-based chemotherapy
Several studies have published datasets of transcriptional profiles of breast cancers linked to outcomes following anthracycline chemotherapy in combination with 5-fluorouracil, cyclophosphamide and/or a taxane (34–37). Because DLX4 did not affect sensitivity of tumor cells to 5-fluorouracil, cyclophosphamide or paclitaxel (Supplementary Table I), we analyzed four independent breast cancer datasets to gain insight into the relationship between DLX4 and TOP2A expression and anthracycline-sensitivity. Cases in each cohort were classified into groups according to levels of DLX4 and TOP2A transcripts in tumors. Response rates of a given group were found to be consistent across all four cohorts (Table I). Because the sizes of several groups were too small to compare in an individual cohort, response rates were compared between groups of cases from the four cohorts combined. The response rate was higher in the TOP2A-High group than in the TOP2A-Low group (P=0.00001; Table I). However, the response rate was lower in the sub-group of TOP2A-High cases that were also DLX4-High than in the sub-group of TOP2A-High cases that were DLX4-Low (P=0.043; Table I). These findings raise the possibility that TOP2A-High tumors that also highly express DLX4 respond to anthracyclines more poorly than TOP2A-High tumors that have low levels of DLX4 expression.
Table I.
Response rates of breast cancer patients grouped by TOP2A and DLX4 expression levels1
| GSE25055 | GSE22093 | GSE20194 | GSE4779 | Combined | P-value2 | |
|---|---|---|---|---|---|---|
| TOP2A-Low | 4/76 (5.3%) | 2/24 (8.3%) | 5/69 (7.2%) | 7/25 (28.0%) | 18/194 (9.3%) | 0.00001 |
| TOP2A-High | 26/76 (34.2%) | 11/24 (45.8%) | 19/69 (27.5%) | 10/25 (40.0%) | 66/194 (34.0%) | |
| DLX4-Low | 15/76 (19.7%) | 7/24 (29.2%) | 17/69 (24.6%) | 9/25 (36.0%) | 48/194 (24.7%) | 0.18 |
| DLX4-High | 13/76 (17.1%) | 2/24 (8.3%) | 12/69 (17.4%) | 9/25 (36.0%) | 36/194 (18.6%) | |
| TOP2A-High + DLX4-Low | 6/13 (46.2%) | 2/5 (40.0%) | 6/13 (46.2%) | 4/9 (44.4%) | 18/40 (45.0%) | 0.043 |
| TOP2A-High + DLX4-High | 5/22 (22.7%) | 2/9 (22.2%) | 3/13 (23.1%) | 2/7 (28.6%) | 12/51 (23.5%) | |
Breast cancer cases from four independent datasets (refs. 34–37) were classified by levels of TOP2A and DLX4 transcripts in tumors. Transcript levels of each gene were defined as High (> upper quartile) and Low (< lower quartile) in each cohort. Shown are numbers and proportions of patients, within a given group defined by transcript levels of TOP2A and/or DLX4, who had a pathologic complete response following anthracycline-based chemotherapy. Details of datasets and analysis are described in Supplementary Methods.
Differences in response rates between the indicated groups of cases from the four cohorts combined were evaluated by two-tailed Fisher exact test.
DLX4 reduces DSBs and cell death induced by TOP2 poisons
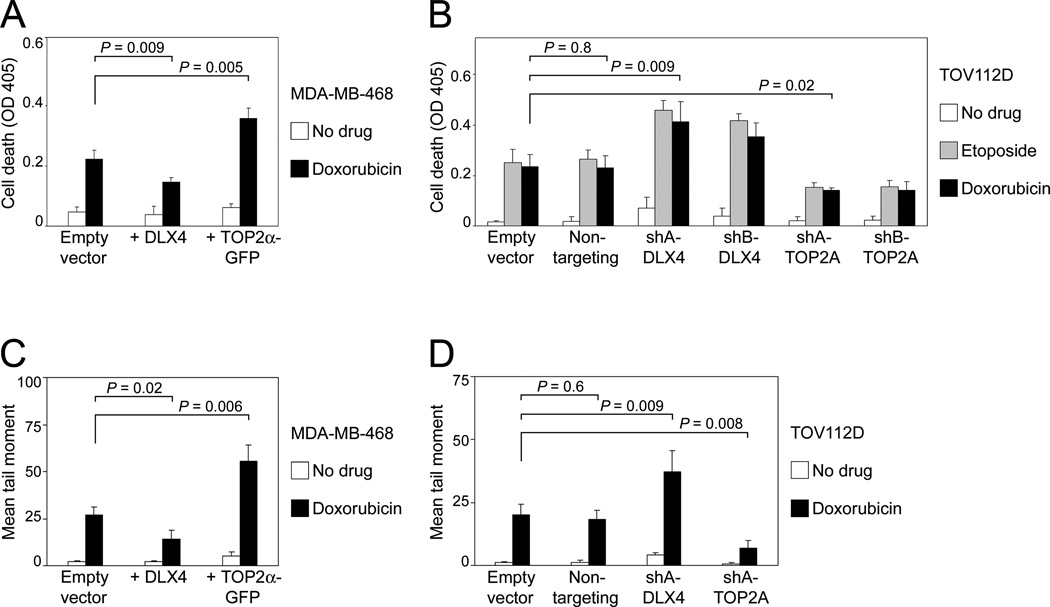
To determine whether tumor cells that highly express both TOP2A and DLX4 are less sensitive to TOP2 poisons than tumor cells that highly express TOP2A but not DLX4, we initially evaluated levels of doxorubicin-induced cell death in MDA-MB-468 cells in which TOP2α alone was overexpressed (Supplementary Fig. S2A) and in which TOP2α was induced by DLX4 (Fig. 1A). As compared to vector-control cells, cell death was increased in cells that overexpressed TOP2α alone (P=0.005) but was reduced in cells that overexpressed DLX4 (P=0.009) (Fig. 2A). To confirm our findings, we evaluated cell death in TOV112D ovarian cancer cells in which TOP2α was knocked-down by TOP2A shRNAs (Supplementary Fig. S2B) and in which TOP2α was down-regulated by DLX4 knockdown (Fig. 1B). Doxorubicin-induced cell death was reduced when TOP2α was knocked-down by TOP2A shRNAs (P=0.02) but was increased when DLX4 was knocked-down (P=0.009) (Fig. 2B). Similar results were obtained when TOV112D cells were treated with etoposide (Fig. 2B). As compared to vector-control MDA-MB-468 cells, higher levels of doxorubicin-induced DSBs were detected by neutral comet assays in MDA-MB-468 cells that overexpressed TOP2α (P=0.006), whereas lower levels were detected in cells that overexpressed DLX4 (P=0.02) (Fig. 2C). Conversely, levels of DSBs were reduced in TOV112D cells when TOP2α was knocked-down (P=0.008) but were increased when DLX4 was knocked-down (P=0.009) (Fig. 2D). These findings indicate that DLX4, notwithstanding its induction of TOP2α, inhibits TOP2 poison-induced cell death by reducing accumulation of DSBs.
Fig. 2. DLX4 reduces TOP2 poison-induced DSBs and cell death.
[A,B] Cell death was assayed by ELISA in tumor cells at 2 d after treatment with TOP2 poisons. Shown are average results of 3 independent experiments. [A] Vector-control, +DLX4 and +TOP2α MDA-MD-468 cells were incubated without and with doxorubicin (100 nM). [B] TOV112D cells were transfected with empty vector, non-targeting shRNA, shRNAs targeting DLX4 (shA-DLX4, shB-DLX4) and TOP2A (shA-TOP2A, shB-TOP2A). At 1 d thereafter, doxorubicin (200 nM) or etoposide (500 nM) was added to cells. [C,D] DSBs were assayed by neutral comet assay in [C] MDA-MB-468 and [D] TOV112D cells at 1 d after doxorubicin treatment. Shown are mean tail moments of 3 independent assays where 50 randomly selected cells were scored in each assay.
DLX4 does not stimulate HR repair but stimulates error-prone end-joining repair of DSBs
In mammalian cells, DSBs are primarily repaired by HR and by end-joining (1,2). To determine whether DLX4 stimulates HR-mediated repair of DSBs, we used a U2OS cell line that stably expresses a DR-GFP reporter cassette. Transient expression of the restriction enzyme I-Sce I in this cell line induces a DSB within the SceGFP gene. Repair of the DSB by HR is facilitated by using the adjacent iGFP gene (encoding a truncated GFP) as a template and results in restoration of GFP expression by the SceGFP gene (Supplementary Fig. S3A). Restoration of GFP expression (measured as % of GFP+ cells) was compared when DLX4 and BRCA1, a well-characterized regulator of HR, were expressed in the reporter cell line (Supplementary Fig. S3B). Restoration of GFP expression was markedly increased by BRCA1 (from 5.3% to 17.1% GFP+ cells) but not by DLX4 (from 5.3% to 5.9% GFP+ cells) (Supplementary Fig. S3C). PCR amplification was performed to confirm increased repair of the SceGFP gene in cells when BRCA1 but not DLX4 was overexpressed (Supplementary Fig. S3D). These findings indicate that DLX4 does not stimulate HR-mediated repair of DSBs.
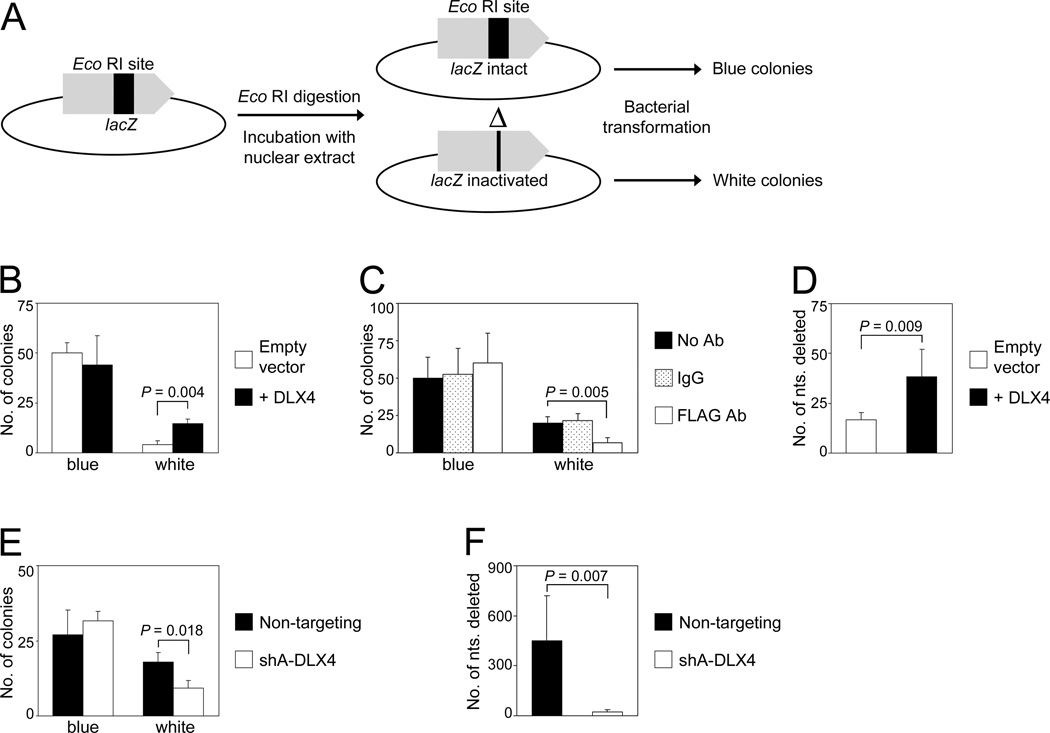
Whereas HR is a high fidelity repair mechanism, repair of DSBs by end-joining is error-prone (1,2). To investigate the effect of DLX4 on end-joining, nuclear extracts of vector-control U2OS cells and U2OS cells that expressed FLAG-tagged DLX4 (+DLX4) were assayed for the ability to rejoin an Eco RI-cut DSB within the lacZ gene of pUC19 plasmid DNA. Frequency and efficacy of plasmid repair were measured by the formation of blue colonies (correct repair) and white colonies (misrepair) following transformation of E. coli (Fig. 3A). As compared to vector-control U2OS extracts, extracts of +DLX4 U2OS cells exhibited an overall increase in repair activity, but this increase was due to a higher frequency of misrepair (P=0.004; Fig. 3B). This increase in misrepair was inhibited when extracts were depleted of FLAG-tagged DLX4 by using Ab to FLAG (P=0.005; Fig. 3C). To confirm that DLX4 stimulates erroneous repair, we sequenced pUC19 DNA isolated from individual white colonies. Representative examples of sequences surrounding the Eco RI-cut DSB and tracts of nucleotide microhomology identified around breakpoint junctions are shown in Supplementary Fig. S4A. As compared to assays using vector-control U2OS extracts, the size of deletions surrounding the DSB were significantly larger in assays using +DLX4 U2OS extracts (P=0.009; Fig. 3D). To confirm our findings, we performed end-joining assays using extracts of TOV112D cells that were transfected with non-targeting and DLX4-specific shRNAs. Knockdown of endogenous DLX4 reduced the frequency of misrepair (P=0.018, Fig. 3E), and also reduced the size of deletions surrounding the DSB (P=0.007; Fig. 3F; Supplementary Fig. S4B). These findings indicate that DLX4 stimulates erroneous end-joining repair of DSBs.
Fig. 3. DLX4 stimulates erroneous end-joining repair of DSBs.
[A] Schematic diagram of the end-joining assay. Repair of the Eco RI-cut lacZ gene of pUC19 DNA incubated in nuclear extracts was assayed by formation of blue colonies (correct repair) and white colonies (misrepair) following transformation of E. coli and plating on plates containing X-gal and IPTG. [B] Colony formation in end-joining assays using nuclear extracts of vector-control U2OS cells and U2OS cells that expressed FLAG-tagged DLX4 (+DLX4). [C] Colony formation in end-joining assays using nuclear extracts of U2OS cells that expressed FLAG-tagged DLX4, where extracts were depleted using FLAG Ab, IgG or left untreated. [D] Numbers of nucleotides surrounding the Eco RI-cut DSB that were deleted in pUC19 DNA isolated from individual white colonies of end-joining assays using U2OS extracts. [E] Colony formation in end-joining assays using nuclear extracts of TOV112D cells transfected with non-targeting and DLX4 shRNAs. [F] Numbers of deleted nucleotides in pUC19 DNA isolated from white colonies of end-joining assays using TOV112D extracts. Shown are average results of 3 independent experiments.
DLX4 interacts with the NHEJ machinery and stimulates DNA-PK activity
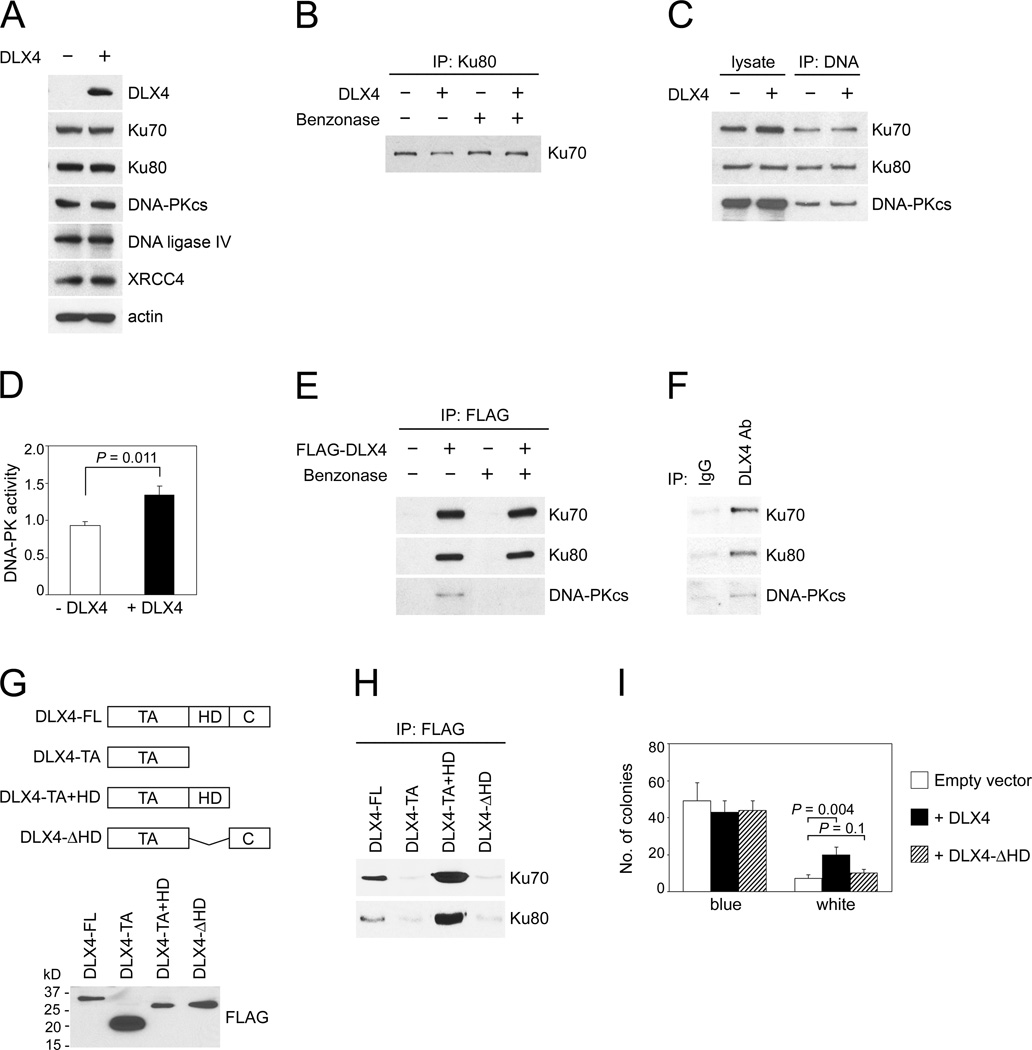
To determine the mechanism by which DLX4 stimulates end-joining repair, we initially evaluated the effect of DLX4 on expression of components of the canonical NHEJ machinery. DLX4 did not alter expression levels of Ku70 (also known as XRCC6), Ku80 (also known as XRCC5, Ku86), DNA-PKcs, XRCC4 or DNA ligase IV (Fig. 4A). DLX4 also did not affect interactions between Ku70 and Ku80 (Fig. 4B). Furthermore, DLX4 neither affected binding of Ku proteins to DNA ends nor the interaction of DNA-PKcs with Ku-DNA complexes (Fig. 4C). On the other hand, DLX4 increased DNA-PK activity (P=0.011; Fig. 4D). Interaction of FLAG-tagged DLX4 with DNA-PKcs was not detected in the absence of DNA and only weakly detected in the presence of DNA (Fig. 4E). However, interactions of FLAG-tagged DLX4 with Ku70 and with Ku80 were strongly detected both in the absence and presence of DNA (Fig. 4E). Interactions of endogenous DLX4 with these NHEJ proteins were confirmed in IP assays using extracts of TOV112D cells (Fig. 4F). We compared the abilities of full-length and truncated FLAG-tagged DLX4 proteins to interact with Ku70 and Ku80 (Fig. 4G). The N-terminal transcriptional activation domain (TA) of DLX4 did not interact with Ku70 or Ku80 (Fig. 4H). However, deletion of the DNA-binding homeodomain of DLX4 (DLX4-ΔHD) abolished Ku-binding ability (Fig. 4H), indicating that DLX4 interacts with Ku proteins through its homeodomain. As compared to vector-control U2OS cells, error-prone end-joining was significantly increased in U2OS cells that expressed full-length DLX4 (P=0.004) but not in cells that expressed the DLX4-ΔHD form (Fig. 4I). These findings indicate that DLX4 interacts with Ku proteins and stimulates DNA-PK activity, and that the ability of DLX4 to stimulate end-joining repair of DSBs depends on its interaction with Ku proteins.
Fig. 4. DLX4 interacts with the NHEJ machinery and stimulates DNA-PK activity.
[A] Western blot of levels of canonical NHEJ components in vector-control (-DLX4) and +DLX4 U2OS cells. [B] Interaction of Ku80 with Ku70 was assayed by IP in extracts treated with benzonase to remove DNA or left untreated. [C] Interactions of Ku proteins and DNA-PKcs with DNA ends were assayed by incubating biotinylated oligomer duplexes with U2OS nuclear extracts. Following DNA pull-down, DNA-associated proteins were analyzed by immuno-blotting. [D] DNA-PK activity in U2OS nuclear extracts (expressed as pmol ATP/minute/µg of protein). [E] Interactions of FLAG-tagged DLX4 with Ku70, Ku80 and DNA-PKcs were assayed in U2OS whole cell extracts (treated with benzonase or left untreated) by IP using FLAG Ab and immunoblotting using Abs to NHEJ components. [F] Interactions of endogenous DLX4 with Ku proteins and DNA-PKcs were assayed by IP in TOV112D extracts (not treated with benzonase). [G] Western blot of FLAG-tagged proteins containing full-length DLX4 (FL), transactivation domain (TA), homeodomain (HD) and C-terminal tail (C) expressed in U2OS cells. [H] Interactions of FLAG-tagged DLX4 proteins with Ku proteins were detected by IP as in [E]. [I] Colony formation in end-joining assays using extracts of U2OS cells that were transfected with empty vector, full-length DLX4 and a truncated form lacking the Ku-interacting domain (DLX4-ΔHD). Shown are average results of 3 independent experiments.
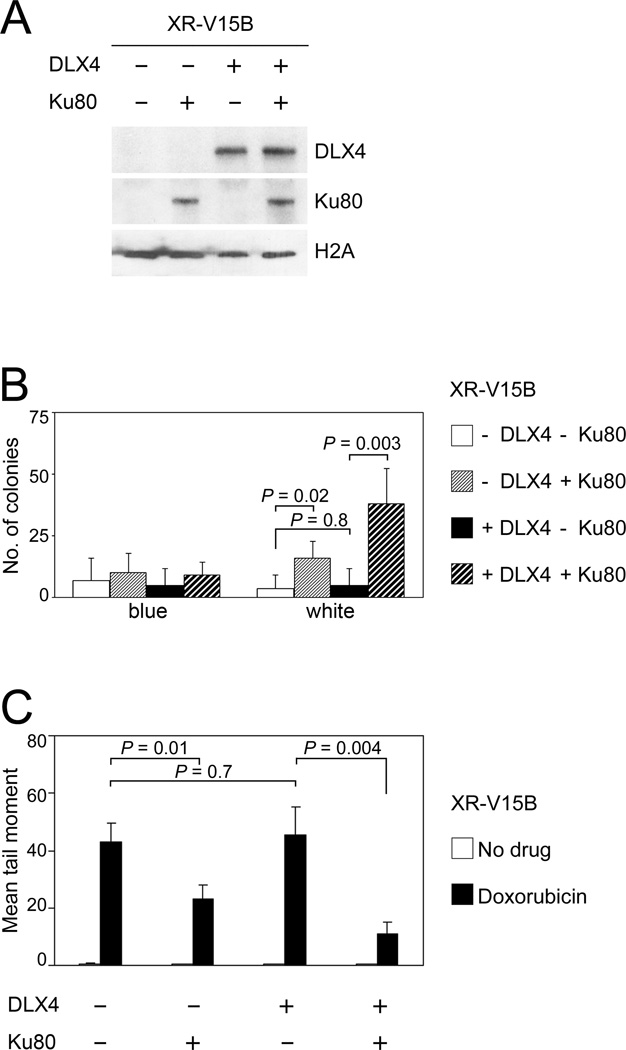
The ability of DLX4 to reduce levels of TOP2 poison-induced DSBs is Ku-dependent
In subsequent experiments, we investigated whether the ability of DLX4 to reduce levels of TOP2 poison-induced DSBs is due to its stimulation of Ku-dependent NHEJ activity. We firstly evaluated the effect of DLX4 on end-joining repair of DSBs in Ku-deficient cells. Cells of the Ku80-deficient cell line XR-V15B (38) were transfected with Ku80 and/or DLX4 (Fig. 5A). Very little end-joining activity was detected in vector-control XR-V15B cells (Fig. 5B). Reconstitution of Ku80 in XR-V15B cells increased end-joining activity (P=0.02), but expression of DLX4 alone had no stimulatory effect (Fig. 5B). In contrast, DLX4 increased end-joining activity in XR-V15B cells that were reconstituted with Ku80 (P=0.003; Fig. 5B). Expression of DLX4 alone in XR-V15B cells did not reduce the levels of DSBs induced by doxorubicin (Fig. 5C). However, significantly lower levels of doxorubicin-induced DSBs were detected when DLX4 was expressed in XR-V15B cells that were reconstituted with Ku80 (P=0.004; Fig. 5C). Together, our findings support a model in which DLX4 induces TOP2A expression, but reduces levels of TOP2 poison-induced DSBs by interacting with Ku proteins and stimulating Ku-dependent end-joining repair of DSBs.
Fig. 5. DLX4 reduces levels of TOP2 poison-induced DSBs by stimulating Ku-dependent NHEJ activity.
Cells of the Ku80-deficient cell line XR-V15B were transfected with Ku80 and/or DLX4. [A] Western blot of DLX4 and Ku80 levels in XR-V15B nuclear extracts. [B] Colony formation in end-joining assays using XR-V15B nuclear extracts. Shown are average results of 3 independent experiments. [C] DSBs were assayed in XR-V15B cells by neutral comet assays at 1 d after doxorubicin treatment (500 nM). Shown are mean tail moments of 3 independent assays where 50 randomly selected cells were scored in each assay.
DISCUSSION
TOP2A has been the most extensively studied gene for determining responsiveness to TOP2 poisons, but there have been conflicting studies regarding its clinical value as a predictive marker (13–17). Reasons that have been proposed to explain this discordance include differences in techniques that evaluated the TOP2A status in tumors and the possible importance of co-amplification of HER2 (17). In this study, we identified that TOP2A is a transcriptional target of DLX4, a homeoprotein that is overexpressed in various types of tumors including breast and ovarian cancers. Our study supports prior experimental evidence that overexpression of TOP2A alone enhances sensitivity of cells to TOP2 poisons, whereas down-regulating TOP2A increases resistance (9–11). However, notwithstanding its induction of TOP2A, DLX4 inhibited levels of TOP2 poison-induced DSBs and cell death. These apparently opposing activities of DLX4 might provide a molecular explanation as to why some tumors have elevated levels of TOP2A expression but respond poorly to TOP2 poisons.
The importance of NHEJ in repairing TOP2 poison-induced DNA damage has been supported by several studies. NHEJ-deficient cells are highly sensitive to TOP2 poisons (3,6) and inhibition of DNA-PK activity increases sensitivity to TOP2 poisons (5). Our studies demonstrated that DLX4 does not stimulate HR-mediated DSB repair, but interacts with Ku proteins and stimulates end-joining repair of DSBs in a Ku-dependent manner. DLX4 did not stimulate end-joining in the absence of its Ku-interacting domain. Whereas levels of end-joining activity and TOP2 poison-induced DSBs were not affected by DLX4 in Ku-deficient cells, DLX4 stimulated DSB repair and reduced the level of TOP2 poison-induced DSBs when Ku was reconstituted in these cells. These results support a model in which DLX4 inhibits accumulation of TOP2 poison-induced DSBs by stimulating the canonical NHEJ pathway but not alternate Ku-independent end-joining pathways (2). DNA-PKcs is activated when recruited to DNA-bound Ku complexes (1). Because DLX4 neither stimulated expression of NHEJ components, Ku70-Ku80 dimerization, the binding of Ku proteins to DNA nor the interaction of DNA-PKcs with Ku-DNA complexes, it is as yet unclear how DLX4 stimulates DNA-PK activity. Because DLX4 can bind Ku proteins in the absence of DNA, it is possible that this binding induces conformational changes in Ku-DNA complexes that enhance DNA-PK activity. This suggests that DLX4 stimulates DNA repair in a non-transcriptional capacity. There is evidence that some homeoproteins have non-transcriptional functions (39,40). However, the possibility that DLX4 might also regulate transcription of genes that control DNA-PK activity cannot at this present time be excluded.
Several other homeoproteins, such as CDX2 and HOXB7, have been reported to interact with Ku proteins (41,42). Interestingly, CDX2 inhibits DNA repair in intestinal cells (41). HOXB7 has been reported to stimulate DNA repair (42), but it is not known whether HOXB7 promotes correct or erroneous repair. An intriguing finding of our study is that DLX4 increases both the frequency and magnitude of erroneous end-joining. NHEJ depends on DNA ends being held in alignment and in proximity in order for end-processing and ligation to occur (1). Ku proteins not only serve as ‘scaffolding’ proteins to bring DNA ends together but also have lyase activity (43). DLX4 did not block ligation but caused larger deletions at breakpoint junctions. The interaction of DLX4 with Ku proteins might alter alignment of DNA ends and also end-processing. On the other hand, it is possible that binding of CDX2 to Ku proteins sterically blocks alignment or processing of DNA ends so that ligation does not occur. The opposite effects of DLX4 and CDX2 on end-joining might be due to different conformational changes in Ku-DNA complexes.
An implication of our finding that DLX4 increases end-joining infidelity is that DLX4 over-expression in tumor cells might promote genomic instability. The role of NHEJ in maintaining genomic stability is unclear. Deficiency in NHEJ components has been reported to increase chromosomal aberrations (44,45), but NHEJ has also been found to promote genomic rearrangements (46). We observed large deletions in end-joining assays using control TOV112D cells (Fig. 3F). This suggests that TOV112D cells have constitutively overactive NHEJ and is consistent with findings that this cell line has numerous chromosomal aberrations (47). Several studies indicate that error-prone repair is increased in tumors. Leukemic cells exhibit higher NHEJ activity and misrepair frequency than normal hematopoietic cells (48,49). CDX2 inhibits end-joining in intestinal cells and is down-regulated in colon cancers (22,41). The susceptibility of Cdx2+/− mice to intestinal cancer has been attributed in part to increased genomic instability (21). Conversely, DLX4 stimulates erroneous end-joining and is overexpressed in tumors. Because the precise functions of DLX4 in normal development are not known, it is not clear how its DNA repair activity is related to its developmental functions. One possibility is that the ability of DLX4 to stimulate repair of DSBs might have evolved as a protective mechanism against the potentially lethal effects of its induction of TOP2α.
The DLX4 and TOP2A genes are both located on 17q21-22 region, a region that is amplified in ~12% of breast and ovarian cancers (12,13). However, DLX4 levels are elevated in >50% of breast and ovarian cancers (26,27), indicating that DLX4 overexpression is not solely due to gene amplification. TOP2α levels in breast cancers also do not correlate with TOP2A gene copy number (50). The frequency of co-amplification of DLX4 and TOP2A is not known, but co-amplification might account for elevated expression of both genes in a subset of tumors. Our findings that DLX4 induces TOP2α levels could explain at least in part why TOP2α levels are elevated in tumors in which the TOP2A gene is not amplified. Deletion of TOP2A also occurs in 6–14% of breast cancers (13,14,16), and could explain why a subset of tumors that highly express DLX4 have low TOP2A mRNA levels. TOP2α levels are regulated by other mechanisms in addition to transcription (17). Further investigation of TOP2α protein levels in tumors is therefore needed. Based on our findings, evaluation of DLX4 levels in tumors that have elevated TOP2α protein levels but respond poorly to TOP2 poisons warrants further investigation.
In summary, our study identifies two opposing activities of DLX4 in inducing TOP2A expression and stimulating repair of DSBs that could explain why some TOP2A-overexpressing tumors respond poorly to TOP2 poisons. Our study also supports increasing evidence that homeoproteins confer survival advantages to tumor cells by diverse mechanisms. Further studies of DLX4 and other homeoproteins that control DNA repair and cell survival could provide important insights into predicting responsiveness of tumor cells to chemotherapeutic agents.
Supplementary Material
Acknowledgements
Bon Q. Trinh is a Vietnam Education Foundation Fellow. STR analysis was performed by the MD Anderson Cancer Center Characterized Cell Line Facility that is supported by Cancer Center Core grant NCI #CA016672. We thank Ju-Seog Lee, Yun Yong Park and Sang Bae Kim (MD Anderson Cancer Center) for assistance with downloading gene expression datasets and helpful suggestions, and Guang Peng and Hui Dai (MD Anderson Cancer Center) for advice regarding repair assays.
Grant support: This work was supported by U.S. National Institutes of Health grant CA141078 (to H. Naora).
Abbreviations
- Ab
antibody
- DNA-PK
DNA-dependent protein kinase
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- DSB
DNA double-strand break
- HR
homologous recombination
- IP
immunoprecipitation
- NHEJ
non-homologous end-joining
- TOP2
topoisomerase II
- TOP2α
topoisomerase IIα
Footnotes
Conflict of interest: The authors declare that no conflict of interest exists.
REFERENCES
- 1.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 2.Kasparek TR, Humphrey TC. DNA double-strand break repair pathways, chromosomal rearrangements and cancer. Semin Cell Dev Biol. 2011;22:886–897. doi: 10.1016/j.semcdb.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Adachi N, Suzuki H, Iiizumi S, Koyama H. Hypersensitivity of nonhomologous DNA end-joining mutants to VP-16 and ICRF-193: implications for the repair of topoisomerase II-mediated DNA damage. J Biol Chem. 2003;278:35897–35902. doi: 10.1074/jbc.M306500200. [DOI] [PubMed] [Google Scholar]
- 4.Malik M, Nitiss JL. DNA repair functions that control sensitivity to topoisomerase II-targeting drugs. Eukaryot Cell. 2004;3:82–90. doi: 10.1128/EC.3.1.82-90.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willmore E, de Caux S, Sunter NJ, Tilby MJ, Jackson GH, Austin CA, et al. A novel DNA-dependent protein kinase inhibitor, NU7026, potentiates the cytotoxicity of topoisomerase II poisons used in the treatment of leukemia. Blood. 2004;103:4659–4665. doi: 10.1182/blood-2003-07-2527. [DOI] [PubMed] [Google Scholar]
- 6.Malik M, Nitiss KC, Enriquez-Rios V, Nitiss JL. Roles of nonhomologous end-joining pathways in surviving topoisomerase II-mediated DNA damage. Mol Cancer Ther. 2006;5:1405–1414. doi: 10.1158/1535-7163.MCT-05-0263. [DOI] [PubMed] [Google Scholar]
- 7.Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitiss JL, Liu YX, Harbury P, Jannatipour M, Wasserman R, Wang JC. Amsacrine and etoposide hypersensitivity of yeast cells overexpressing DNA topoisomerase II. Cancer Res. 1992;52:4467–4472. [PubMed] [Google Scholar]
- 10.Gudkov AV, Zelnick CR, Kazarov AR, Thimmapaya R, Suttle DP, Beck WT, et al. Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci USA. 1993;90:3231–3335. doi: 10.1073/pnas.90.8.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess DJ, Doles J, Zender L, Xue W, Ma B, McCombie WR, et al. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc Natl Acad Sci USA. 2008;105:9053–9058. doi: 10.1073/pnas.0803513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T, Imoto I, Kosugi Y, Ishiwata I, Inoue S, Takayama M, et al. A novel amplification at 17q21-23 in ovarian cancer cell lines detected by comparative genomic hybridization. Gynecol Oncol. 2001;81:172–177. doi: 10.1006/gyno.2001.6132. [DOI] [PubMed] [Google Scholar]
- 13.O’Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, et al. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst. 2009;101:644–650. doi: 10.1093/jnci/djp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Press MF, Sauter G, Buyse M, Bernstein L, Guzman R, Santiago A, et al. Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol. 2011;29:859–867. doi: 10.1200/JCO.2009.27.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arriola E, Moreno A, Varela M, Serra JM, Falo C, Benito E, et al. Predictive value of HER-2 topoisomerase IIalpha in response to primary doxorubicin in breast cancer. Eur J Cancer. 2006;42:2954–2960. doi: 10.1016/j.ejca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Harris LN, Broadwater G, Abu-Khalaf M, Cowan D, Thor AD, Budman D, et al. Topoisomerase II alpha amplification does not predict benefit from dose-intense cyclophosphamide, doxorubicin, and fluorouracil therapy in HER2-amplified early breast cancer: results of CALGB 8541/150013. J Clin Oncol. 2009;27:3430–3436. doi: 10.1200/JCO.2008.18.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oakman C, Moretti E, Galardi F, Santarpia L, Di Leo A. The role of topoisomerase II alpha and HER-2 in predicting sensitivity to anthracyclines in breast cancer patients. Cancer Treat Rev. 2009;35:662–667. doi: 10.1016/j.ctrv.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, et al. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 19.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 20.Samuel S, Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;41:2428–2437. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Δ716 Cdx2+/− compound mutant mice. Nat Genet. 2003;35:323–330. doi: 10.1038/ng1265. [DOI] [PubMed] [Google Scholar]
- 22.Ee HC, Erler T, Bhathal PS, Young GP, James RJ. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am J Pathol. 1995;147:586–592. [PMC free article] [PubMed] [Google Scholar]
- 23.Behbakht K, Qamar L, Aldridge CS, Coletta RD, Davidson SA, Thorburn A, et al. Six1 overexpression in ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and is associated with poor survival. Cancer Res. 2007;67:3036–3042. doi: 10.1158/0008-5472.CAN-06-3755. [DOI] [PubMed] [Google Scholar]
- 24.Sellar GC, Watt KP, Li L, Nelkin BD, Rabiasz GJ, Porteous DJ, et al. The homeobox gene BARX2 can modulate cisplatin sensitivity in human epithelial ovarian cancer. Int J Oncol. 2002;21:929–933. [PubMed] [Google Scholar]
- 25.Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–4386. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- 26.Fu SW, Schwartz A, Stevenson H, Pinzone JJ, Davenport GJ, Orenstein JM, et al. Correlation of expression of BP1 a homeobox gene, with estrogen receptor status in breast cancer. Breast Cancer Res. 2003;5:R82–R87. doi: 10.1186/bcr602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara F, Samuel S, Liu J, Rosen D, Langley RR, Naora H. A homeobox gene related to Drosophila Distal-less promotes ovarian tumorigenicity by inducing expression of vascular endothelial growth factor and fibroblast growth factor-2. Am J Pathol. 2007;170:1594–1606. doi: 10.2353/ajpath.2007.061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomida S, Yanagisawa K, Koshikawa K, Yatabe Y, Mitsudomi T, Osada H, et al. Identification of a metastasis signature and the DLX4 homeobox protein as a regulator of metastasis by combined transcriptome approach. Oncogene. 2007;26:4600–4608. doi: 10.1038/sj.onc.1210242. [DOI] [PubMed] [Google Scholar]
- 29.Haga SB, Fu S, Karp JE, Ross DD, Williams DM, Hankins WD, et al. BP1 a new homeobox gene, is frequently expressed in acute leukemias. Leukemia. 2000;14:1867–1875. doi: 10.1038/sj.leu.2401912. [DOI] [PubMed] [Google Scholar]
- 30.Trinh BQ, Barengo N, Naora H. Homeodomain protein DLX4 counteracts key transcriptional control mechanisms of the TGF-β cytostatic program and blocks the antiproliferative effect of TGF-β. Oncogene. 2011;30:2718–2729. doi: 10.1038/onc.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng G, Yim EK, Dai H, Jackson AP, Burgt I, Pan MR, et al. BRIT1/MCPH1 links chromatin remodelling to DNA damage response. Nat Cell Biol. 2009;11:865–872. doi: 10.1038/ncb1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 33.Chase MB, Fu S, Haga SB, Davenport G, Stevenson H, Do K, et al. BP1, a homeodomain-containing isoform of DLX4, represses the beta-globin gene. Mol Cell Biol. 2002;22:2505–2514. doi: 10.1128/MCB.22.8.2505-2514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305:1873–1881. doi: 10.1001/jama.2011.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwamoto T, Bianchini G, Booser D, Qi Y, Coutant C, Shiang CY, et al. Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer. J Natl Cancer Inst. 2011;103:264–272. doi: 10.1093/jnci/djq524. [DOI] [PubMed] [Google Scholar]
- 36.Popovici V, Chen W, Gallas BG, Hatzis C, Shi W, Samuelson FW, et al. Effect of training-sample size and classification difficulty on the accuracy of genomic predictors. Breast Cancer Res. 2010;12:R5. doi: 10.1186/bcr2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 38.Errami A, Smider V, Rathmell WK, He DM, Hendrickson EA, Zdzienicka MZ, et al. Ku86 defines the genetic defect and restores X-ray resistance and V(D)J recombination to complementation group 5 hamster cell mutants. Mol Cell Biol. 1996;16:1519–1526. doi: 10.1128/mcb.16.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topisirovic I, Kentsis A, Perez JM, Guzman ML, Jordan CT, Borden KL. Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels. Mol Cell Biol. 2005;25:1100–1112. doi: 10.1128/MCB.25.3.1100-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubnau J, Struhl G. RNA recognition and translational regulation by a homeodomain protein. Nature. 1996;379:694–699. doi: 10.1038/379694a0. [DOI] [PubMed] [Google Scholar]
- 41.Renouf B, Soret C, Saandi T, Delalande F, Martin E, Vanier M, et al. Cdx2 homeoprotein inhibits non-homologous end joining in colon cancer but not in leukemia cells. Nucleic Acids Res. 2012;40:3456–3469. doi: 10.1093/nar/gkr1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin E, Wu X, Zhu T, Cheung JC, Chen H, Lorincz A, et al. A role for the HOXB7 homeodomain protein in DNA repair. Cancer Res. 2007;67:1527–1535. doi: 10.1158/0008-5472.CAN-06-4283. [DOI] [PubMed] [Google Scholar]
- 43.Roberts SA, Strande N, Burkhalter MD, Strom C, Havener JM, Hasty P, et al. Ku is a 5’-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Difilippantonio MJ, Zhu J, Chen HT, Meffre E, Nussenzweig MC, Max EE, et al. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature. 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson DO, Sekiguchi JM, Chang S, Frank KM, Gao Y, DePinho RA, et al. The nonhomologous end-joining pathway of DNA repair is required for genomic stability and the suppression of translocations. Proc Natl Acad Sci USA. 2000;97:6630–6633. doi: 10.1073/pnas.110152897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothkamm K, Kühne M, Jeggo PA, Löbrich M. Radiation-induced genomic rearrangements formed by nonhomologous end-joining of DNA double-strand breaks. Cancer Res. 2001;61:3886–3893. [PubMed] [Google Scholar]
- 47.Dafou D, Ramus SJ, Choi K, Grun B, Trott DA, Newbold RF, et al. Chromosomes 6 and 18 induce neoplastic suppression in epithelial ovarian cancer cells. Int J Cancer. 2009;124:1037–1044. doi: 10.1002/ijc.24058. [DOI] [PubMed] [Google Scholar]
- 48.Gaymes TJ, Mufti GJ, Rassool FV. Myeloid leukemias have increased activity of the nonhomologous end-joining pathway and concomitant DNA misrepair that is dependent on the Ku70/86 heterodimer. Cancer Res. 2002;62:2791–2797. [PubMed] [Google Scholar]
- 49.Brady N, Gaymes TJ, Cheung M, Mufti GJ, Rassool FV. Increased error-prone NHEJ activity in myeloid leukemias is associated with DNA damage at sites that recruit key nonhomologous end-joining proteins. Cancer Res. 2003;63:1798–1805. [PubMed] [Google Scholar]
- 50.Mueller RE, Parkes RK, Andrulis I, O’Malley FP. Amplification of the TOP2A gene does not predict high levels of topoisomerase II alpha protein in human breast tumor samples. Genes Chromosomes Cancer. 2004;39:288–297. doi: 10.1002/gcc.20008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.