Abstract
Sustained stimulation of the intrarenal/intratubular renin–angiotensin system in a setting of elevated arterial pressure elicits renal vasoconstriction, increased sodium reabsorption, proliferation, fibrosis, and eventual renal injury. Activation of luminal AT1 receptors in proximal and distal nephron segments by local Ang II formation stimulates various transport systems. Augmented angiotensinogen (AGT) production by proximal tubule cells increases AGT secretion contributing to increased proximal Ang II levels and leading to spillover of AGT into the distal nephron segments, as reflected by increased urinary AGT excretion. The increased distal delivery of AGT provides substrate for renin, which is expressed in principal cells of the collecting tubule and collecting ducts, and is also stimulated by AT1 receptor activation. Renin and prorenin are secreted into the tubular lumen and act on the AGT delivered from the proximal tubule to form more Ang I. The catalytic actions of renin and or prorenin may be enhanced by binding to prorenin receptors on the intercalated cells or soluble prorenin receptor secreted into the tubular fluid. There is also increased luminal angiotensin converting enzyme in collecting ducts facilitating Ang II formation leading to stimulation of sodium reabsorption via sodium channel and sodium/chloride co-transporter. Thus, increased collecting duct renin contributes to Ang II-dependent hypertension by augmenting distal nephron intra-tubular Ang II formation leading to sustained stimulation of sodium reabsorption and progression of hypertension.
Keywords: Collecting duct renin, Prorenin receptor, Angiotensin II, Angiotensinogen, Angiotensin-converting enzyme, Hypertension
Introduction
Although every organ system in the body has elements of the renin–angiotensin system (RAS), local synthesis is of major significance in the regulation of the angiotensin I (Ang I) and II (Ang II) levels in the kidneys. The kidneys have the capacity to accumulate circulating Ang II via Ang II type 1a (AT1a) receptor-mediated internalization as well as by de novo formation of Ang peptides with eventual compartmentalization in the tubular, cellular, and interstitial compartments [46, 70, 71, 73]. The localization and cellular processing of RAS components have provided new aspects of the functional concept of a “self-contained” renal RAS in the tubular compartments not only in the proximal tubules [85] but also in the distal nephron segments [20, 73, 75, 89]. The demonstration that increased expression and secretion of renin and prorenin by distal nephron segments contributes to augmentation of intrarenal and intratubular Ang II content in Ang II-dependent hypertension has provided a novel mechanism for renin from collecting duct origin to exert a critical role in the development and progression of hypertension [42, 73, 91, 94]. While the regulation of juxtaglomerular (JG) renin has been extensively studied, our understanding of the mechanisms regulating collecting duct renin remains incomplete. This review focuses on recent findings regarding the mechanisms that regulate renin in distal nephron segments and their relevance to the development and progression of hypertension.
Renin distribution
Although, in the strictest sense, renin is not a hormone, it can be considered as such because of its role in regulating Ang I generation and because it is subject to tight control. Hence, the plasma renin concentration or activity is often used as a measure of the overall activity of the RAS. In most species, renin synthesized by the JG cells is the primary source of both circulating and intrarenal renin levels [7, 29]. However, some strains of mice also produce substantial amounts of renin in the submandibular and submaxillary glands as expression of the duplicated renin gene, Ren-2 [9, 21]. Ren-2 is also present in the kidneys although in smaller quantities than Ren-1. In addition, adrenal glands express substantial renin protein and mRNA levels [18, 69], but it is not clear if the renin in adrenal glands and other tissues contributes to the circulating renin levels. What has been generally demonstrated is that in species other than mice, bilateral nephrectomy lowers the circulating renin levels to the undetectable range within 48 h [80]. Likewise, the plasma concentrations of Ang I and Ang II fall to non-detectable levels by 48 h after bilateral nephrectomy [80].
The secreted active form of renin contains 339–343 amino acid residues after proteolytic removal of the 43-aminoacid residue at the N-terminus (prorenin). Although circulating active renin is derived mainly from the kidneys, the kidneys and other tissues also secrete prorenin into the circulation and its concentration may exceed that of renin [98]. Besides serving as the precursor for active renin, circulating prorenin is taken up by some tissues where it may contribute to the local synthesis of angiotensin peptides [86]. For example, the heart does not produce renin under normal conditions and its transcript is undetectable or extremely low [19, 39]. Nevertheless, transgenic rats expressing the Ren-2d renin gene characterized by high circulating prorenin levels in the absence of the cardiac transgene have prorenin internalized into cardiomyocytes [82]. This effect is followed by the generation of angiotensins and development of cardiac injury suggesting that uptake of circulating prorenin but not active renin may play an important role in cardiac hypertrophy.
While it is often assumed that most of the renin is secreted directly into the lumen of the afferent arterioles by the JG cells, this concept neglects the fact that this route requires that renin, a glycoprotein with a molecular weight of 37,000–40,000 Da, would have to penetrate the endothelial layer of the afferent arterioles in order to be secreted directly into the blood stream. Thus, it seems more likely that renin synthesized by JG cells is secreted primarily into the interstitial spaces and then enters the circulation via diffusion across the peritubular capillaries [63, 64]. Indeed, renin activity in blood from efferent arterioles is lower than renal venous blood and only slightly greater than in aortic blood [63]. Furthermore, renal interstitial fluid renin concentrations as reflected from measurements in renal lymph are much higher than either renal venous or arterial blood concentrations [108, 109]. Nevertheless, there is measureable renin in proximal tubular fluid indicating that significant filtration of renin does occur [53] or that renin is synthesized and secreted by proximal tubule cells [30]. Another possible explanation is that renin released by the JG cells diffuses along the terminal sections of the afferent arterioles towards the glomerular tuft where the arterioles have endothelial fenestrations which would presumably allow passage of renin directly into the blood stream and enter the glomerular circulation [53, 95]. Endothelial fenestrations are present on the afferent arteriole facing the JG granular cells thus providing a structural basis by which renin can be secreted directly into the afferent arteriolar lumen [95]. To the extent that this occurs, the blood perfusing the glomeruli would have enhanced concentrations of renin which could then filter across the glomerular capillaries to provide renin to the proximal tubular fluid [53].
The findings of very high renin concentrations in the renal lymph support the conclusion that most of the renin is secreted into the interstitial compartments, but it is also apparent that it quickly gains access to the intravascular compartment, presumably by diffusing from the interstitial fluid across the peritubular capillaries, and exits the kidney via the renal vein [108, 109]. Because of the close association between JG renin secretion and the plasma renin levels, plasma renin activity (PRA) is often used as an index of the level of renin secretory activity by the JG cells.
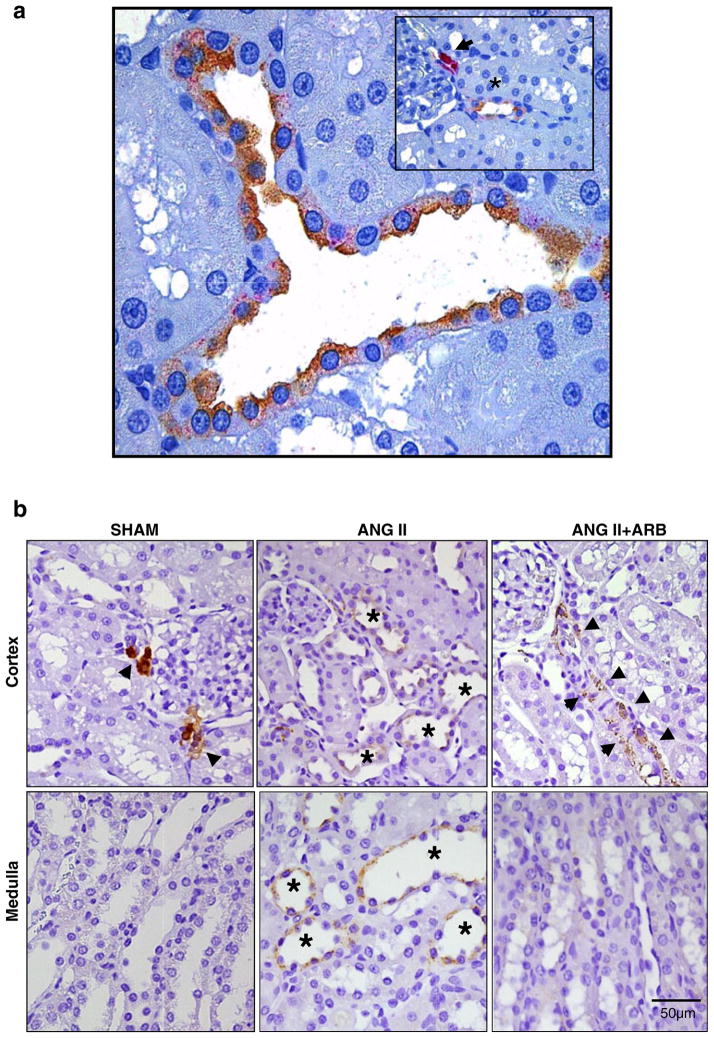
The presence of renin in tubular fluid may be a consequence of renin production by cells other than those of the JG cells. Using antibodies against renin from a JG cell tumor, Menard et al. [58] reported staining in interlobular arteries and afferent arterioles of a patient with a partially infarcted kidney. Kidneys from rats treated chronically with angiotensin-converting enzyme (ACE) inhibitors also exhibit renin immunoreactivity of the afferent arteriole extending well beyond the JG loci up towards the interlobular artery, suggesting that ACE inhibition induces a recruitment of cells that in the basal state were not expressing the renin gene [24]. Positive renin immunoreactivity has also been observed in cells of glomeruli and of proximal and distal nephron segments [10, 30, 62, 100–102]. Originally, these findings were interpreted as primarily reflecting uptake of the filtered renin [101]. However, renin mRNA has been demonstrated in mesangial cells, proximal tubular cells, connecting tubule cells, and cortical and medullary collecting duct cells [12, 23, 30, 50–52, 62, 92, 94]. Although not abundant, renin mRNA and protein levels have been demonstrated in proximal tubule cells [12, 30, 62, 100]. Renin may also be filtered, thus providing an additional source of proximal tubule renin [53]. Regardless of source, proximal tubule renin contributes importantly to the local generation of Ang I from AGT produced and secreted by proximal tubule cells [85]. Ang I may be formed either intracellularly or in the tubular lumen. Once formed, the Ang I can be readily converted to Ang II by the abundant ACE on the brush border of the proximal tubule [71–73]. Recent studies also reported renin protein expression using immunohistochemistry and Western blot techniques in M-1 cells, a cortical collecting duct cell line from mouse origin [42, 51]. Lalouel et al. [51] emphasized the potential significance of renin in collecting duct cells in generating Ang I in distal nephron segments. As shown in Fig. 1a, renin in distal nephron segments is specifically localized to principal cells of connecting tubules and collecting duct [42, 51, 91]. These findings, along with the demonstrations that intrarenal AGT expression and secretion and collecting duct renin expression are markedly augmented in Ang II-dependent hypertension (Fig. 1b) [27, 45, 46, 60], support the hypothesis that abundant substrate and renin are available for intratubular Ang II production in distal nephron segments of the kidney [73].
Fig. 1.
Renin immunostaining in juxtaglomerular cells collecting duct cells. a Co-localization of aquaporin-2 (brown) and renin (red) in a rat paraffin kidney section. Aquaporin-2, a marker of principal cells, is co-expressed with renin. Insert shows specific immunostaining of renin in juxtaglomerular cells (arrowhead) and a collecting duct in the proximity (asterisk), displaying double staining. b Compared to sham-operated rats (left panels), renin immunostaining is increased in the cortical (upper panels) and medullary (low panels) collecting ducts of chronic Ang II-infused rats (middle panels). AT1R blockade (ARB) blunted this effect (right panels). Notice the differential effect of Ang II on juxtaglomerular renin (arrowheads) versus collecting duct renin (asterisks). Adapted from Prieto-Carrasquero et al. (2004, 2005)
Enhancement of collecting duct renin in Ang II-dependent hypertension: interaction with prorenin receptor
As previously indicated, a substantial fraction of the Ang II present in renal tissues is generated locally from AGT delivered to the kidney as well as from AGT locally produced by proximal tubule cells [44, 57]. Thus, it has been proposed that renin in distal nephron segments provides a pathway for Ang I generation from proximally delivered AGT [75, 89, 94]. Renin expressed in principal cells of connecting tubules and cortical and medullary collecting ducts is intriguing because it appears to be regulated differently than renin in JG cells [91, 94, 101] (Fig. 1b). Renin protein and message in the principal cells are upregulated by chronic infusions of Ang II [91, 92]. In contrast to the inhibitory effect that Ang II exerts on JG renin, chronic Ang II infusions stimulate renin in renal medullary tissue [91, 92]. The finding that increases in collecting duct renin transcript and protein levels in chronic Ang II-infused rats are prevented by AT1 receptor blockers (ARB) suggests an AT1 receptor-mediated mechanism [92]; however, these data did not distinguish between the stimulatory effects of Ang II versus those due to the associated increases in arterial blood pressure.
The two-kidney, one-clip (2K1C) Goldblatt hypertensive rat model was used to dissect the effects of high Ang II levels, which are present in both kidneys of 2K1C rats, from the effects of exposure to elevated arterial pressure, which is restricted to the non-clipped kidney (NCK). During 2K1C Goldblatt hypertension, a cascade of events initiated by the increased renin secretion from the clipped kidney (CK) is followed by early increases in circulating Ang II, which ultimately inhibit JG renin synthesis and release in the NCK. High circulating Ang II levels return back toward normal after 2–3 weeks of renal unilateral clipping [28]. In this model, there were increases in renin message and protein in principal cells from both clipped and NCK, thus indicating that Ang II stimulates collecting duct renin independently of blood pressure [90]. In particular, the augmented gene expression of renin in the collecting ducts of both kidneys in 2K1C Goldblatt hypertensive rats supports the conclusion that the enhancement of local synthesis and stimulation of renin in the distal nephron segments occurs independently of blood pressure. Associated with the increased renin, there were also increases in Ang II and ACE but suppression of Ang 1–7 levels and ACE2 activity in medullary tissue from both clipped and NCK [87].
Rohrwasser et al. [94] reported in vitro renin secretion by isolated connecting tubules. Recently, we described the augmentation of renin secretion by the collecting duct cells in chronic Ang II-infused hypertensive rats [54] since they exhibited increased urinary renin content even though PRA was suppressed. Urinary renin levels are also elevated in diabetic patients [104], which occur in parallel with the known intrarenal RAS activation in diabetes. Although the presence of renin mRNA in the renal medullary tissues of Ang II-dependent hypertensive rats indicates local synthesis, it is possible that renin protein uptake by the collecting duct cells also contributes to the increased local renin. This is a possibility to be considered in light of the recently discovered prorenin/renin receptor (PRR) detected in mesangial cells, cortical renal arteries, and distal tubules of the kidney [14, 79]. However, evidence indicating that the PRR is not a receptor dedicated for renin clearance [8, 40] and that PRR is expressed by the intercalated cells and not the principal cells [1, 25] rule out the possibility that the presence of renin in principal cells is due to PRR uptake.
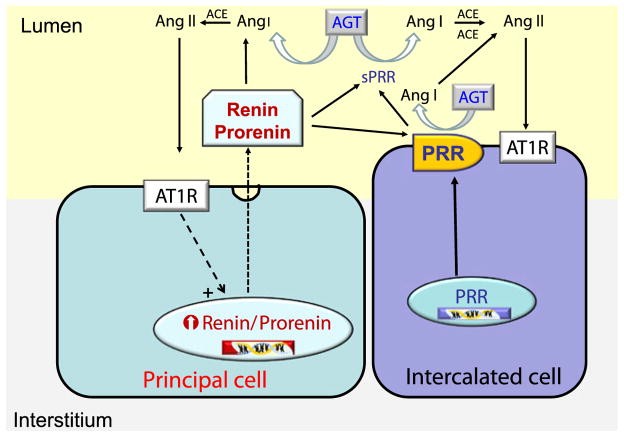
The discovery of the PRR brings a new perspective regarding the possible role of PRR in a setting of activated intrarenal RAS. The PRR was described as an associated protein with the V-ATPase (vacuolar H+-ATPase), giving the name to the gene, Atp6ap2 (ATPase 6 accessory protein 2/prorenin receptor). The PRR binds renin and prorenin with an affinity in the nanomolar range and their binding triggers a range of cellular events via mitogen-activated kinases (MAPKs) and extracellular-signal-regulated kinases (ERK 1/2). Importantly, although the functional roles of the PRR remains to be clarified, its actions have been linked to diabetic nephropathy [97]. Despite the low levels of renin in patients with diabetic nephropathy, high levels of prorenin are detected in these patients associated with occurrence of microvascular complications, microalbuminuria, and retinopathy [17, 41]. It has been suggested that blockade of prorenin binding to the PRR receptor using a PRR handle-region peptide is able to prevent and even reverse diabetic nephropathy [34]; however, these findings remain controversial because other studies have failed to show an effect of the handle-region peptide [67]. Nonetheless, evidence showing the presence of PRR in podocytes, mesangial cells, and particularly in the intercalated type-A cells of distal nephron segments [25, 35, 76, 78] is of great relevance since the synthesis of prorenin is also augmented in the collecting ducts during diabetic nephropathy, which may lead to activation of signaling pathways that can promote tubular damage [42]. This evidence suggests that interactions between the PRR and renin or prorenin in plasma, interstitial space, or in tubular fluids contribute to the generation of additional Ang II levels. In fact, prorenin levels in the plasma are not correlated with plasma renin activity in some diseases [16], which suggest complex interactions depending on the status of prorenin or PRR. We have demonstrated that the PRR is augmented in the collecting ducts of rats infused with Ang II during 14 days [25]; however, the pattern between mRNA and post-transcriptional events seems to be different, making the PRR/prorenin interaction complex especially in diabetic nephropathy and hypertension. Interestingly, binding of renin and prorenin to PRR induces a fourfold increase in the catalytic efficiency of angiotensinogen conversion to Ang I [76, 79]. Thus, renin/prorenin interaction with its receptor may provide a novel mechanism for tissue Ang I generation on the cell surface [25, 76–78]. As shown in Fig. 2, apical PRR is co-expressed in cells expressing the anion exchanger type 1 (AE-1; basolateral membrane).
Fig. 2.
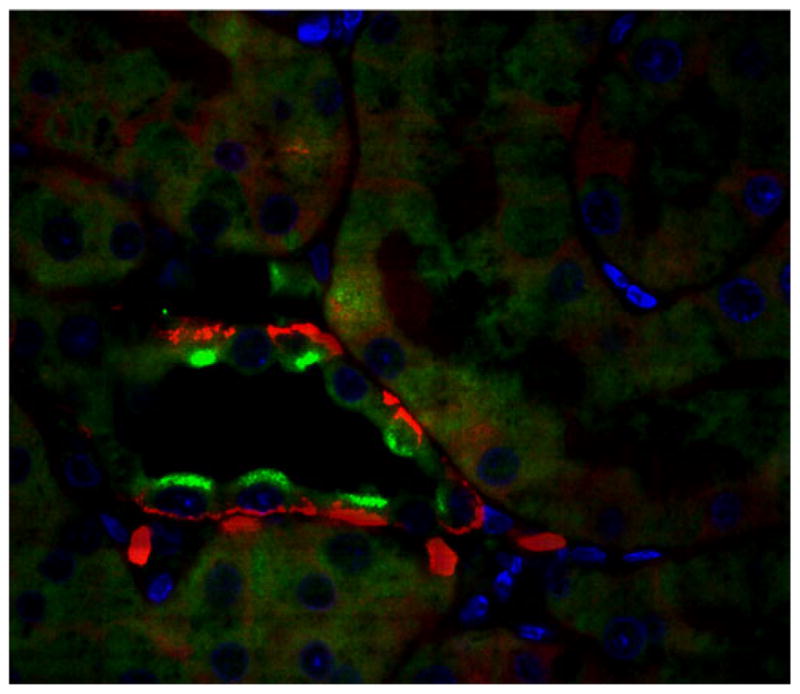
The prorenin receptor immunoexpression in the type-A intercalated cells of the collecting duct. Microphotograph of a rat paraformaldehyde perfused kidney section (3 μm) immunofluorescence staining of prorenin receptor located on the apical membrane (green, Alexa Fluor 488; Invitrogen) of intercalated type-A cells co-localizing with basolateral anion exchanges type-1 (AE1, red Alexa Fluor 594; Invitrogen) Bue (4′,6-diamidino-2-phenylindole-DAPI), a nuclei marker. In this preparation, a goat anti-PRR (1:400 dilution; Abcam) and a rabbit anti-AE1 (1:500 dilution; Alpha Diagnostic International) were used. Image was visualized using an oil immersion × 100 objective of a Nikon Eclipse 50i fluorescence microscope attached to a DS digital camera
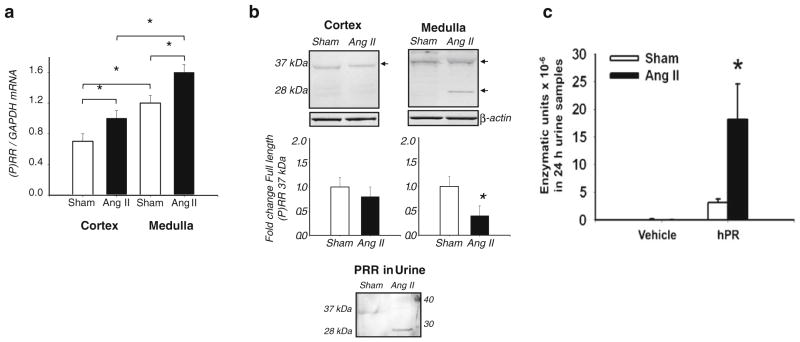
The PRR increases Ang II tissue generation by binding renin or prorenin and increasing its catalytic efficiency, thus leading to enhanced Ang II formation and contributing to increased blood pressure [25, 49, 79]. We have found increased PRR protein and transcript levels in hypertensive chronic Ang II-infused rats [25] and Cyp1a1Ren2 transgenic rats [88], and significant enhancement of specific PRR immunoreactivity in the collecting duct of both kidneys of 2K1C Goldblatt rats [75]. Nevertheless, Muller et al. [67] reported that 2K1C Goldblatt rats do not exhibit upregulation of the PRR transcript in either kidney. Discrepancies found in the upregulation of the PRR expression in both kidneys of Goldblatt hypertensive rats may be due to the differences in sensitivity of the methods used in those reports. One possibility is that enhanced prorenin synthesized and secreted by principal cells is anchored by the PRR present in the intercalated cells of the collecting duct, thus increasing the Ang I formation in distal nephron segments. Recent studies demonstrated that chronic Ang II-infused hypertensive rats exhibit augmentation of the PRR mRNA levels (Fig. 3a) [25]. Interestingly, the renal inner medullary tissues and the urine of Ang II hypertensive rats exhibit the sPRR form while those samples from sham-operated do not (Fig. 3b, c). Although the role of the PRR remains controversial and further studies are needed to provide direct evidence in support of its function, our findings are consistent with the notion that prorenin secreted by the principal cells of the collecting ducts binds to the PRR located on the apical membrane of the intercalated cells or to the sPRR present in the tubular fluid, thus enhancing local renin enzymatic activity leading to increased Ang I and Ang II formation in the lumen of distal nephron segments [25, 88]. These findings, along with the demonstration that renin is found on the luminal side of connecting tubule and collecting duct cells [91, 94], indicate that renin is secreted into the distal tubular lumen. Its local interaction with PRR thus contributes to the increased renin activity in the tubular fluid of collecting duct segments leading to formation of Ang I and elevated Ang II (Fig. 4).
Fig. 3.
Kidney PRR expression levels in the renal cortex and medulla of angiotensin II-infused rats. a PRR mRNA levels are significantly increased in the renal cortex and medulla of Ang II-infused rats compared to sham-operated rats. b Upper panels, PRR protein immunoblots from Ang II-infused and sham-operated rats show the specific PRR band of 37 kDa in both cortex and medulla. Middle panels show that the levels of full length of PRR (37-kDa band) in the renal cortex of sham and Ang II rats are similar, while they are significantly decreased in the medulla of Ang II-infused rats. The presence of the sPRR form (28-kDa band) became apparent only in the renal medullary tissues of Ang II hypertensive rats. In contrast, sPRR was not detected in the medulla of sham rats nor in the cortex of either group. Lower panel displays the PRR immunoblot using urines of Ang II-infused and sham rats, which indicate that the sPRR form (28-kDa band) is only detected in the luminal fluid of Ang II-infused rats. c In Sprague–Dawley rat urines collected with protease inhibitor cocktail, the amounts of Ang I forming enzymatic units excreted per day × 10–6 in Ang II-infused hypertensive rats compared with sham-operated rats were increased in the absence of human recombinant prorenin (hPR) and increased further in the presence of 22 pmol hPR. In samples spiked with hPR, the background renin and non-specific enzyme activity were subtracted. These results indicate that the presence of sPRR in the urine of Ang II-infused rats contributes to enhance renin enzymatic activity in the luminal fluid of distal nephron segments. MW molecular weight standard (adapted from Gonzalez et al. 2011), MW molecular weight
Fig. 4.
Proposed mechanism for the contribution of renin and prorenin in the collecting duct to intratubular Ang II formation. In Ang II-dependent hypertension, Ang II type 1 receptor (AT1R)-dependent stimulation of renin and prorenin synthesis and secretion by principal cells in the collecting ducts interact with the prorenin receptor (PRR) on the plasma membrane of intercalated cells or with the soluble form of the PRR (sPRR) to further increase the Ang I formation upon the catalytic action on angiotensinogen (AGT) delivered from proximal segments
Regulation of renin in the collecting duct during Ang II-dependent hypertension
The mechanisms responsible for the upregulation of renin in the collecting ducts during Ang II-dependent hypertension remain unclear. Increased renin synthesis is mediated by AT1 receptors independent of changes in blood pressure [90]. In vitro studies also suggest that collecting duct renin synthesis and secretion can be directly increased by Ang II [42]; however, the mechanisms involved are not well delineated. AT1 receptor activation suppresses renin synthesis in JG cells via protein kinase C (PKC) and Ca++ [43, 68]; however, in vivo and in vitro evidence suggests that the mechanisms of regulation of collecting duct renin are different from those described for JG cells. AT1 receptors are expressed in principal cells and activate the epithelial sodium channel (ENaC), stimulating sodium reabsorption [55, 83, 99]. In Ang II-dependent hypertension, elevated plasma Ang II levels stimulate aldosterone secretion leading to activation of mineralocorticoid receptors (MR) with corresponding increases in Na+ reabsorption through ENaC in principal cells [3, 56, 111]. The effects of aldosterone are additive to those of luminal Ang II which increases the open probability and induces translocation of the α-ENaC subunit to the apical membrane [55]. Thus, the effects of Ang II complement those of aldosterone to regulate overall ENaC activity.
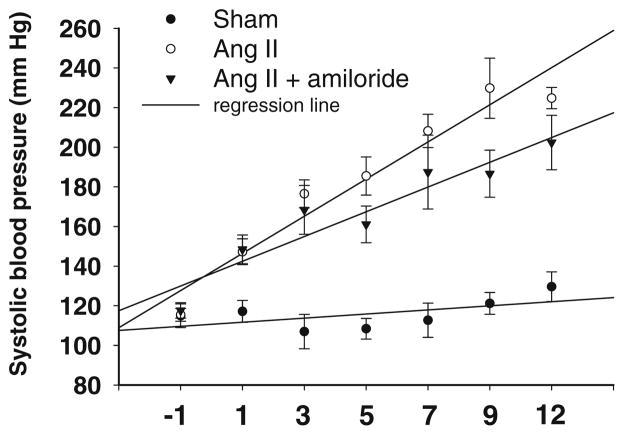
Further studies were performed to determine if increased collecting duct renin in Ang II-infused rats is due to enhanced MR activity and/or ENaC stimulation. Studies showing that chronic Ang II infusion enhances distal nephron sodium reabsorption [3, 96, 111] suggest that enhanced collecting duct renin expression may be responsible for generating the increased Ang II levels that lead to enhanced sodium reabsorption. In vivo results demonstrate that amiloride, a specific ENaC inhibitor, reduced the rate of progression of hypertension (Fig. 5) and enhanced the natriuresis in Ang II-infused rats, suggesting a significant contribution of ENaC to the increased sodium reabsorption in the collecting duct during chronic Ang II infusions [26]. Thiazide treatment during early stages of hypertension also reduced the hypertension in Cyp1a1-Ren2 rats [2]. Despite these effects, renin mRNA levels and urinary renin content were similarly increased in rats subjected to chronic treatment with Ang II and Ang II plus amiloride even though PRA was completely suppressed. Moreover, in vitro activation of MR by aldosterone at concentrations that increase α-ENaC mRNA expression did not alter renin mRNA or protein levels. Rohrwasser et al. [94] demonstrated in mice that a high-sodium diet plus amiloride treatment increased renin immunoreactivity in connecting tubule cells although there were decreases in JG renin; however, connecting tubule cell renin immunoreactivity in mice did not exhibit significant variation in response to changes in dietary sodium [52, 94]. These results indicate that the magnitude of sodium reabsorption by the collecting duct is not a modulator of renin synthesis or excretion.
Fig. 5.
Systolic blood pressure in control rats (sham-operated), Ang II-infused and Ang II-infused treated with amiloride. The rate of progression of systolic blood pressures measured by tail-cuff methods was reduced in Ang II hypertension rats treated with amiloride (5 mg/kg/ day; Sigma) compared to Ang II rats. Ang II was subcutaneously administered via minipump at a dose of 80 ngmin−1kg−1 for 14 days. Regression line curve (R2) is shown for each group (n= 10)
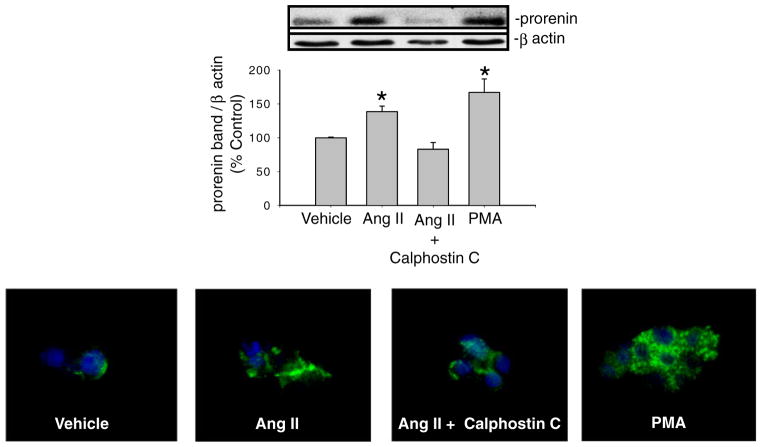
To evaluate the possible mechanism involved in the AT1 receptor-dependent renin upregulation in collecting duct, we examined the presence of functional AT1 receptors in inner medullary collecting duct (IMCD) cells [26]. Intracellular Ca++ measurements in response to Ang II incubation demonstrated increases in intracellular Ca++ levels, showing that AT1 receptors are functionally expressed in the plasma membrane of IMCD cells. Immunofluorescence studies in vitro also demonstrated the presence of renin/prorenin in principal cells of the collecting duct as previously shown [91]. Furthermore, AT1 receptor blockade in primary cultures of IMCD cells demonstrated a direct AT1 receptor-dependent mechanism. Since PKC and Ca++ inhibit JG renin expression [43, 68], we also investigated this possibility. In rat IMCD cells expressing functional AT1 receptors (Fig. 6), we found that PKC activation with phorbol 12-myristate 13-acetate (PMA) actually increased renin mRNA and prorenin protein levels while calphostin C prevented the Ang II-mediated stimulation, thus suggesting that the downstream pathway in the stimulation of renin in the collecting duct cells is mediated by PKC and opposite to that occurring in JG cells [26]. Calphostin C inhibits the classical (α) and the novel (δ, ε, η) but not the atypical (ζ) PKC isoforms [93] described in the rat IMCD cells [13]. Treatment with PMA specifically activates classical and novel PKC isoforms [13]; thus, it is unlikely that the atypical ζ PKC isoform is involved [93]. Taken together, the evidence indicates that AT1 receptors are the main regulator of renin/ prorenin synthesis and secretion in the collecting duct and that this occurs independently of MR activation or ENaC activity via a PKC-dependent pathway [26].
Fig. 6.
PKC-dependent stimulation of renin in rat inner medullary collecting duct primary cultured cells. Relative abundance of prorenin protein in response to Ang II, Ang II + calphostin C, and PMA (phorbol 12-myristate 13-acetate) treatments in freshly isolated inner medullary collecting duct (IMCD) cells assessed by Western blot and immunofluorescence.*P<0.05 versus vehicle treated cells (adapted from Gonzalez et al. 2011)
Role of collecting duct renin in determining regional differences in intrarenal angiotensin peptide content during Ang II-dependent hypertension
Intrarenal Ang II is not distributed in a homogenous fashion but is compartmentalized in both a regional and segmental manner [70]. However, there is still no consensus regarding the differential levels of intrarenal Ang II contents between the renal cortex and the renal medulla [37, 72]. The renal cortex has abundant expression of AGT and ACE in PT cells and renin in JG cells. Nonetheless, ACE activity is fivefold higher in the renal medulla than in the renal cortex [37]. Angiotensin 1–7, a putative vasodilator and anti-proliferative peptide formed by ACE2 acting on Ang II, and ACE2, the homolog of ACE that cleaves Ang I and Ang II into Ang 1–9 and Ang 1–7, are abundant throughout the renal tubules of the cortex and outer medulla [5, 103, 110]. In 2K1C Goldblatt hypertensive rats, we found reduced ACE2 transcript levels associated with decreased Ang 1–7 immunoreactivity in the renal cortexes compared to kidneys of sham rats [87]. Moreover, in the chronic Ang II-infused rat model, we found that the kidney Ang II contents are significantly higher in the renal medulla than in the cortex [75]. During Ang II-dependent hypertension, enhancement of renin mRNA and protein in the collecting duct cells, as reflected in increased renin activity in the urine [54], may lead to increased intratubular Ang II content, thus contributing to an imbalance in the content of Ang II and Ang 1–7 via differential regulation of ACE and ACE2 leading to increased intrarenal formation of Ang II [87]. Further evidence demonstrating that Ang II upregulates ACE and downregulates ACE2 in various tissues [22, 38, 47] highlights the importance of the recent discovery of the Ang II-degrading enzyme, ACE2, in the metabolic pathway of Ang II in hypertension. The abundant ACE in renal medullary collecting duct cells further contributes to the increased Ang II levels in the renal medulla of Ang II-dependent hypertensive rats and ultimately to the stimulated ENaC activity in cortical collecting duct cells [6, 48, 83]. Under this scenario, stimulation of sodium reabsorption in the collecting duct may explain the attenuation of the pressure-natriuretic response to elevations in arterial blood pressure and the development and maintenance of hypertension in these hypertensive models [28, 32, 61, 74, 111].
Contribution of collecting duct renin in Ang II-dependent hypertension
Abnormalities in the renal regulation of sodium balance seem to be a common pathway in the development of hypertension, particularly during inappropriate activation of the intrarenal RAS, since it controls renal sodium and water balance. In the kidney, the function of the collecting duct is crucial for fine tuning of sodium reabsorption in which Ang II plays a critical role. Ang II increases NaCl reabsorption in the collecting duct via direct stimulation of several transporters including ENaC [48, 55, 83], thus contributing to the development of hypertension [73]. In addition, chloride must also be absorbed to maintain the pressor effect of sodium. In the collecting duct, pendrin may provide a major route for chloride absorption. Pendrin expression and activity in turn is stimulated by Ang II and aldosterone [81, 105, 106]. In Ang II-dependent hypertension, the evidence indicates that collecting duct renin contributes to the maintenance and increase in intrarenal Ang II generation [73, 87, 89]. In chronic Ang II-infused rats and mice, despite marked suppression of PRA and renin in JG cells, the intra-renal levels of Ang II are much higher than expected due to AT1a receptor-mediated internalization [112, 114] and local Ang II de novo generation [73, 111]. In contrast to the suppression of JG renin and PRA, in Ang II-dependent hypertension there is stimulation of renin and prorenin synthesis and secretion in the principal cells of the collecting ducts but not in other nephron segments [89–92]. In the 2K1C rat model, during the initial phase of hypertension, the Ang II-dependent alterations in renal hemodynamics and tubular reabsorption of the NCK are due to the elevations in circulating Ang II [15, 84]. The NCK is highly responsive to pharmacological blockade of the RAS [31, 33, 36, 84, 113]. Administration of ACE inhibitors or AT1 receptor antagonists increases renal blood flow and glomerular filtration rate, reduces fractional sodium reabsorption, and decreases the sensitivity of the tubuloglomerular feedback mechanism [4, 31]. This responsiveness of the NCK to blockade of the RAS persists even during the maintenance phase of hypertension when plasma renin and Ang II concentrations return towards normal [28, 66]. The observation that the NCK is highly Ang II dependent even when circulating Ang II levels return towards normal suggests that there is dissociation between the circulating Ang II levels and the renal Ang II dependency. Importantly, the fact that the Ang II content of the NCK is elevated even at a time when JG renin content and its transcript levels are markedly decreased [28, 59, 65] suggests a unique mechanism responsible for the enhanced intrarenal Ang II in NCK. Thus, it is unlikely that circulating Ang II sustains the upregulation of distal nephron renin since plasma Ang II concentrations in 2K1C hypertensive rats return toward control levels [28]. However, because increases in plasma Ang II concentration occur during the early phases following renal unilateral clipping [107], this could play a role in initiating the increases of intrarenal Ang II by augmentation of intrarenal AGT [44] and AT1 receptor-mediated uptake [114]. Accordingly, the initial event caused by unilateral renal artery clipping may initiate the cascade responsible for intrarenal Ang II augmentation in the NCK [11, 28]. It is also unlikely that circulating renin or prorenin is the stimulus for the upregulation of renin in the collecting duct cells since chronic Ang II-infused rats exhibit stimulation of renin in distal nephron segments in a setting of marked suppression of PRA [91].
In both of these models of Ang II-dependent hypertension, the local amplification mechanism of intrarenal Ang II on distal nephron renin may allow a moderate increase in Ang II to further augment the medullary intratubular and interstitial Ang II levels in order to achieve rapid homeostatic regulation of sodium balance as needed in a setting of volume depletion. Although this effect appears to be a positive feedback under pathologic conditions, the physiologic consequences of this mechanism would be to prevent or minimize volume and sodium depletion by enhancing sodium reabsorption to reestablish sodium balance and, ultimately, to allow renin to return to normal levels. Thus, in physiological conditions, this may represent a feed-forward mechanism that anticipates and prevents volume depletion [91]. However, during inappropriate activation of the RAS such as following unilateral renal artery clipping, chronic exogenous infusions of Ang II, or kidney diseases, this stimulus would be sustained leading to further increases in local Ang II levels through the coordinated actions of AGT in the proximal tubule cells and renin in the distal nephron segments.
Acknowledgments
The authors received support from the National Heart, Lung, and Blood Institute (HL-26371), the Institutional Award Program of the National Center for Research Resources, Centers of Biomedical Research Excellence (P20 RR-017659), and the American Heart Association (09BGIA2280440). We thank Debbie Olavarrieta for preparation of the manuscript.
Footnotes
This article is published as part of the special issue on the Renin–Angiotensin System.
Contributor Information
Minolfa C. Prieto, Department of Physiology and Hypertension and Renal Center of Excellence, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70112, USA
Alexis A. Gonzalez, Department of Physiology and Hypertension and Renal Center of Excellence, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70112, USA. Instituto de Química, Facultad de Ciencias, Pontificia Universidad Católica de Valparaíso, Valparaiso, Chile
L. Gabriel Navar, Email: navar@tulane.edu, Department of Physiology and Hypertension and Renal Center of Excellence, Tulane University School of Medicine, 1430 Tulane Avenue, New Orleans, LA 70112, USA. Department of Physiology, Tulane University Health Sciences Center, 1430 Tulane Avenue, New Orleans, LA 70112, USA.
References
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 2.Ashek A, Menzies RI, Mullins LJ, Bellamy CO, Harmar AJ, Kenyon CJ, Flatman PW, Mullins JJ, Bailey MA. Activation of thiazide-sensitive co-transport by angiotensin II in the cyp1a1-Ren2 hypertensive rat. PLoS One. 2012;7:e36311. doi: 10.1371/journal.pone.0036311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 4.Braam B, Navar LG, Mitchell KD. Modulation of tubuloglomerular feedback by angiotensin II type 1 receptors during the development of Goldblatt hypertension. Hypertension. 1995;25:1232–1237. doi: 10.1161/01.hyp.25.6.1232. [DOI] [PubMed] [Google Scholar]
- 5.Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, Penninger J, Ferrario CM. Enhanced renal immunocytochemical expression of ANG-(1–7) and ACE2 during pregnancy. Hypertension. 2003;42:749–753. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- 6.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 7.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 8.Catanzaro DF. Physiological relevance of renin/prorenin binding and uptake. Hypertens Res. 2005;28:97–105. doi: 10.1291/hypres.28.97. [DOI] [PubMed] [Google Scholar]
- 9.Catanzaro DF, Mullins JJ, Morris BJ. The biosynthetic pathway of renin in mouse submandibular gland. J Biol Chem. 1983;258:7364–7368. [PubMed] [Google Scholar]
- 10.Celio MR, Inagami T. Angiotensin II immunoreactivity co-exists with renin in the juxtaglomerular granular cells of the kidney. Proc Natl Acad Sci USA. 1981;78:3897–3900. doi: 10.1073/pnas.78.6.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervenka L, Wang C-T, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Harris MP, Rose D, Smart A, He XR, Kretzler M, Briggs JP, Schnermann J. Renin and renin mRNA in proximal tubules of the rat kidney. J Clin Invest. 1994;94:237–243. doi: 10.1172/JCI117312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol. 1998;274:F564–F572. doi: 10.1152/ajprenal.1998.274.3.F564. [DOI] [PubMed] [Google Scholar]
- 14.Danser AHJ, van Kats JP, Admiraal PJJ, Derkx FHM, Lamers JMJ, Verdouw PD, Saxena PR, Schalekamp MADH. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- 15.DeForrest J, Knappenberger R, Antonaccio M, Ferrone R, Creekmore J. Angiotensin II is a necessary component for the development of hypertension in the two kidney, one clip rat. Am J Cardiol. 1982;49:1515–1517. doi: 10.1016/0002-9149(82)90373-3. [DOI] [PubMed] [Google Scholar]
- 16.Deinum J, Ronn B, Mathiesen E, Derkx FH, Hop WC, Schalekamp MA. Increase in serum prorenin precedes onset of micro-albuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia. 1999;42:1006–1010. doi: 10.1007/s001250051260. [DOI] [PubMed] [Google Scholar]
- 17.Deinum J, Tarnow L, van Gool JM, de Bruin RA, Derkx FH, Schalekamp MA, Parving HH. Plasma renin and prorenin and renin gene variation in patients with insulin-dependent diabetes mellitus and nephropathy. Nephrol Dial Transplant. 1999;14:1904–1911. doi: 10.1093/ndt/14.8.1904. [DOI] [PubMed] [Google Scholar]
- 18.Dzau VJ, Ellison KE, Brody T, Ingelfinger J, Pratt RE. A comparative study of the distributions of renin and angiotensinogen messenger ribonucleic acids in rat and mouse tissues. Endocrinology. 1987;120:2334–2338. doi: 10.1210/endo-120-6-2334. [DOI] [PubMed] [Google Scholar]
- 19.Ekker M, Tronik D, Rougeon F. Extrarenal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci USA. 1989;86:5155–5158. doi: 10.1073/pnas.86.13.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol. 2012;74:325–349. doi: 10.1146/annurev-physiol-020911-153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field LJ, McGowan RA, Dickinson DP, Gross KW. Tissue and gene specificity of mouse renin expression. Hypertension. 1984;6:597–603. doi: 10.1161/01.hyp.6.4.597. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert RE, Wu LL, Kelly DJ, Cox A, Wilkinson-Berka JL, Johnston CI, Cooper ME. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol. 1999;155:429–440. doi: 10.1016/S0002-9440(10)65139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859–864. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594–599. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin–angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010;298:F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–767. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 29.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 30.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 31.Huang W-C, Bell PD, Harvey D, Mitchell KD, Navar LG. Angiotensin influences on tubuloglomerular feedback mechanism in hypertensive rats. Kidney Int. 1988;34:631–637. doi: 10.1038/ki.1988.227. [DOI] [PubMed] [Google Scholar]
- 32.Huang W-C, Navar LG. Effects of unclipping and converting enzyme inhibition on bilateral renal function in 2 kidney 1 clip Goldblatt hypertensive rats. Kidney Int. 1983;23:816–822. doi: 10.1038/ki.1983.100. [DOI] [PubMed] [Google Scholar]
- 33.Huang W-C, Ploth DW, Bell PD, Work J, Navar LG. Bilateral renal function responses to converting enzyme inhibitor (SQ 20881) in two kidney one clip Goldblatt hypertensive rats. Hypertension. 1981;3:285–293. doi: 10.1161/01.hyp.3.3.285. [DOI] [PubMed] [Google Scholar]
- 34.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H. Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med. 2008;86:629–635. doi: 10.1007/s00109-008-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imamura A, Mackenzie HS, Lacy ER, Hutchison FN, Fitzgibbon WR, Ploth DW. Effects of chronic treatment with angiotensin converting enzyme inhibitor or an angiotensin receptor antagonist in two-kidney, one-clip hypertensive rats. Kidney Int. 1995;47:1394–1402. doi: 10.1038/ki.1995.196. [DOI] [PubMed] [Google Scholar]
- 37.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol. 2002;283:F1003–F1010. doi: 10.1152/ajprenal.00322.2001. [DOI] [PubMed] [Google Scholar]
- 38.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 39.Iwai N, Inagami T. Quantitative analysis of renin gene expression in extrarenal tissues by polymerase chain reaction method. J Hypertens. 1992;10:717–724. [PubMed] [Google Scholar]
- 40.Jan Danser AH, Batenburg WW, van Esch JH. Prorenin and the (pro)renin receptor—an update. Nephrol Dial Transplant. 2007;22:1288–1292. doi: 10.1093/ndt/gfl846. [DOI] [PubMed] [Google Scholar]
- 41.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641–646. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 42.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klar J, Sigl M, Obermayer B, Schweda F, Kramer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension. 2005;46:1340–1346. doi: 10.1161/01.HYP.0000192025.86189.46. [DOI] [PubMed] [Google Scholar]
- 44.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin–angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 47.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 49.Krebs C, Hamming I, Sadaghiani S, Steinmetz OM, Meyer-Schwesinger C, Fehr S, Stahl RA, Garrelds IM, Danser AH, van Goor H, Contrepas A, Nguyen G, Wenzel U. Antihy-pertensive therapy upregulates renin and (pro)renin receptor in the clipped kidney of Goldblatt hypertensive rats. Kidney Int. 2007;72:725–730. doi: 10.1038/sj.ki.5002408. [DOI] [PubMed] [Google Scholar]
- 50.Lai KN, Leung JCK, Lai KB, To WY, Yeung VTF, Lai FMM. Gene expression of the renin–angiotensin system in human kidney. J Hypertens. 1998;16:91–102. doi: 10.1097/00004872-199816010-00014. [DOI] [PubMed] [Google Scholar]
- 51.Lalouel J-M, Rohrwasser A, Terreros D, Morgan T, Ward K. Angiotensinogen in essential hypertension: from genetics to nephrology. J Am Soc Nephrol. 2001;12:606–615. doi: 10.1681/ASN.V123606. [DOI] [PubMed] [Google Scholar]
- 52.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 53.Leyssac PP. Changes in single nephron renin release are mediated by tubular fluid flow rate. Kidney Int. 1986;30:332–339. doi: 10.1038/ki.1986.189. [DOI] [PubMed] [Google Scholar]
- 54.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion associated with augmented urinary angiotensin (Ang) II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol. 2011;301:F1195–F1201. doi: 10.1152/ajprenal.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J Biol Chem. 2012;287:660–671. doi: 10.1074/jbc.M111.298919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney [see comments] J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menard J, N’Goc PW, Bariety J, Guyenne PT, Corvol P. Direct radioimmunoassay and immunocytochemical localization of renin in human kidneys. Clin Sci (Lond) 1979;57(Suppl 5):105s–108s. doi: 10.1042/cs057105s. [DOI] [PubMed] [Google Scholar]
- 59.Mendelsohn FAO. Failure of suppression of intrarenal angiotensin II in the contralateral kidney of one clip, two kidney hypertensive rats. Clin Exp Pharmacol Physiol. 1980;7:219–223. doi: 10.1111/j.1440-1681.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 60.Milani CJ, Kobori H, Mullins JJ, Mitchell KD. Enhanced urinary angiotensinogen excretion in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent malignant hypertension. Am J Med Sci. 2010;340:389–394. doi: 10.1097/MAJ.0b013e3181eabd28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, diagnosis, and management. Raven; New York: 1995. pp. 1437–1450. [Google Scholar]
- 62.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan T, Davis JM. Renin secretion at the individual nephron level. Pflügers Arch. 1975;359:23–31. doi: 10.1007/BF00581275. [DOI] [PubMed] [Google Scholar]
- 64.Morgan T, Gillies A. Factors controlling the release of renin: a micropuncture study in the cat. Pflügers Arch. 1977;368:13–18. doi: 10.1007/BF01063449. [DOI] [PubMed] [Google Scholar]
- 65.Morishita R, Higaki J, Okunishi H, Tanaka T, Ishii K, Nagano M, Mikami H, Ogihara T, Murakami K, Miyazaki M. Changes in gene expression of the renin–angiotensin system in two-kidney, one clip hypertensive rats. J Hypertens. 1991;9:187–192. doi: 10.1097/00004872-199102000-00015. [DOI] [PubMed] [Google Scholar]
- 66.Morton JJ, Wallace ECH. The importance of the renin–angiotensin system in the development and maintenance of hypertension in the two-kidney, one-clip hypertensive rat. Clin Sci (Lond) 1983;64:359–370. doi: 10.1042/cs0640359. [DOI] [PubMed] [Google Scholar]
- 67.Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, Luft FC, Hilgers KF. (Pro)renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension. 2008;51:676–681. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- 68.Muller MW, Todorov V, Kramer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflugers Arch. 2002;444:499–505. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 69.Naruse M, Takii Y, Inagami T. Renin exists in high concentration in the adrenal gland of the rat. Biomed Res. 1981;2:583–586. [Google Scholar]
- 70.Navar LG, Harrison-Bernard LM, Imig JD. Compartmentalization of intrarenal angiotensin II. In: Ulfendahl HR, Aurell M, editors. Renin–angiotensin. Portland, London: 1998. pp. 193–208. [Google Scholar]
- 71.Navar LG, Harrison-Bernard LM, Wang C-T, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 72.Navar LG, Imig JD, Zou L, Wang C-T. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 73.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin–angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Navar LG, Ploth DW. Pathophysiology of renovascular hypertension. In: Izzo JL, Black HR, Sica DA, editors. Hypertension primer: the essentials of high blood pressure. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 162–165. [Google Scholar]
- 75.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180–186. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen G. Renin/prorenin receptors. Kidney Int. 2006;69:1503–1506. doi: 10.1038/sj.ki.5000265. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen G, Burckle C, Sraer JD. The renin receptor: the facts, the promise and the hope. Curr Opin Nephrol Hypertens. 2003;12:51–55. doi: 10.1097/00041552-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen G, Burckle CA, Sraer JD. Renin/prorenin-receptor biochemistry and functional significance. Curr Hypertens Rep. 2004;6:129–132. doi: 10.1007/s11906-004-0088-3. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nussberger J, Fluckiger JP, Hui KY, Evequoz D, Waeber B, Brunner HR. Angiotensin I and II disappear completely from circulating blood within 48 hours after binephrectomy: improved measurement of angiotensins in rat plasma. J Hypertens Suppl. 1991;9:S230–S231. [PubMed] [Google Scholar]
- 81.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–F920. doi: 10.1152/ajprenal.00361.2006. [DOI] [PubMed] [Google Scholar]
- 82.Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MG, Mullins JJ. Functional significance of prorenin internalization in the rat heart. Circ Res. 2002;90:1135–1141. doi: 10.1161/01.res.0000019242.51541.99. [DOI] [PubMed] [Google Scholar]
- 83.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 84.Ploth DW, Roy RN. Renin-angiotensin influences on tubuloglomerular feedback activity in the rat. Kidney Int. 1982;22 (Suppl 12):S114–S121. [PubMed] [Google Scholar]
- 85.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, Bachmann S, Theilig F. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prescott G, Silversides DW, Reudelhuber TL. Tissue activity of circulating prorenin. Am J Hypertens. 2002;15:280–285. doi: 10.1016/s0895-7061(01)02284-1. [DOI] [PubMed] [Google Scholar]
- 87.Prieto MC, Gonzalez-Villalobos RA, Botros FT, Martin VL, Pagan J, Sato R, Lara LS, Feng Y, Fernandez F, Kobori H, Casarini DE, Navar LG. Reciprocal changes in renal ACE/Ang II and ACE2/Ang 1–7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol. 2011 Jan 5;300:F749–F755. doi: 10.1152/ajprenal.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of renin and prorenin receptor in the collecting ducts of Cyp1a1-Ren2 rats contribute to development and progression of malignant hypertension. Am J Physiol Renal Physiol. 2010;300:F581–F588. doi: 10.1152/ajprenal.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3:96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roffey J, Rosse C, Linch M, Hibbert A, McDonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr Opin Cell Biol. 2009;21:268–279. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 94.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin–angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 95.Rosivall L, Taugner R. The morphological basis of fluid balance in the interstitium of the juxtaglomerular apparatus. Cell Tissue Res. 1986;243:525–533. doi: 10.1007/BF00218059. [DOI] [PubMed] [Google Scholar]
- 96.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, Mcdonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl(−) cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 97.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. (Pro)renin receptor-mediated signal transduction and tissue renin–angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009;58:1625–1633. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sealey JE, Laragh JH. Prorenin” in human plasma? Circ Res. 1975;36:10–16. doi: 10.1161/01.res.36.6.10. [DOI] [PubMed] [Google Scholar]
- 99.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol. 2012;302:F679–F687. doi: 10.1152/ajprenal.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tank JE, Henrich WL, Moe OW. Regulation of glomerular and proximal tubule renin mRNA by chronic changes in dietary NaCl. Am J Physiol Renal Physiol. 1997;273:F892–F898. doi: 10.1152/ajprenal.1997.273.6.F892. [DOI] [PubMed] [Google Scholar]
- 101.Taugner R, Hackenthal E, Inagami T, Nobiling R, Poulsen K. Vascular and tubular renin in the kidneys of mice. Histochemistry. 1982;75:473–484. doi: 10.1007/BF00640599. [DOI] [PubMed] [Google Scholar]
- 102.Taugner R, Mannek E, Nobiling R, Buhrle CP, Hackenthal E, Ganten D, Inagami T, Schroder H. Coexistence of renin and angiotensin II in epitheloid cell secretory granules of rat kidney. Histochemistry. 1984;81:39–45. doi: 10.1007/BF00495399. [DOI] [PubMed] [Google Scholar]
- 103.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 104.van den Heuvel M, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, van den Meiracker AH, Danser AH. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin–angiotensin–aldosterone system activity and the efficacy of renin–angiotensin–aldosterone system blockade in the kidney. J Hypertens. 2011;29:2147–2155. doi: 10.1097/HJH.0b013e32834bbcbf. [DOI] [PubMed] [Google Scholar]
- 105.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 106.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol. 2011;301:F1314–F1325. doi: 10.1152/ajprenal.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol Renal Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 108.Wilcox CS, Dzau VJ. Effect of captopril on the release of the components of the renin–angiotensin system into plasma and lymph. J Am Soc Nephrol. 1992;2:1241–1250. doi: 10.1681/ASN.V271241. [DOI] [PubMed] [Google Scholar]
- 109.Wilcox CS, Peart WS. Release of renin and angiotensin II into plasma and lymph during hyperchloremia. Am J Physiol Renal Physiol. 1987;253:F734–F741. doi: 10.1152/ajprenal.1987.253.4.F734. [DOI] [PubMed] [Google Scholar]
- 110.Yagil Y, Yagil C. Hypothesis: ACE2 modulates blood pressure in the mammalian organism. Hypertension. 2003;41:871–873. doi: 10.1161/01.HYP.0000063886.71596.C8. [DOI] [PubMed] [Google Scholar]
- 111.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009;54:120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 113.Zimmerman BG, Arendshorst WJ, DiBona GF, Hostetter TH, Ploth DW, Raij L. Renal functional derangements in hypertension. Federation Proc. 1986;45(12):2661–2664. [PubMed] [Google Scholar]
- 114.Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal ANG II augmentation in ANG II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]