Abstract
PURPOSE
Strategies to inhibit the epidermal growth factor receptor (EGFR) using the tyrosine kinase inhibitor (TKI) erlotinib have been associated with limited clinical efficacy in head and neck squamous cell carcinoma (HNSCC). Co-activation of alternative kinases may contribute to erlotinib resistance.
EXPERIMENTAL DESIGN
We generated HNSCC cells expressing dominant-active c-Src (DA-Src) to determine the contribution of c-Src activation to erlotinib response.
RESULTS
Expression of DA-Src conferred resistance to erlotinib in vitro and in vivo compared with vector-transfected control cells (VC). Phospho-Met was strongly upregulated by DA-Src, and DA-Src cells did not produce hepatocyte growth factor (HGF). Knockdown of c-Met enhanced sensitivity to erlotinib in DA-Src cells in vitro, as did combining a c-Met or c-Src inhibitor with erlotinib. Inhibiting EGFR resulted in minimal reduction of phospho-Met in DA-Src cells, whereas complete phospho-Met inhibition was achieved by inhibiting c-Src. A c-Met inhibitor significantly sensitized DA-Src tumors to erlotinib in vivo, resulting in reduced Ki67 labeling and increased apoptosis. In parental cells, knockdown of endogenous c-Src enhanced sensitivity to erlotinib, while treatment with HGF to directly induce phospho-Met resulted in erlotinib resistance. The level of endogenous phospho-c-Src in HNSCC cell lines was also significantly correlated with erlotinib resistance.
CONCLUSIONS
Ligand-independent activation of c-Met contributes specifically to erlotinib resistance, not cetuximab resistance, in HNSCC with activated c-Src, where c-Met activation is more dependent on c-Src than on EGFR, providing an alternate survival pathway. Addition of a c-Met or c-Src inhibitor to erlotinib may increase efficacy of EGFR inhibition in patients with activated c-Src.
INTRODUCTION
EGFR is a receptor tyrosine kinase that is overexpressed in about 90% of HNSCC. Previous studies have shown that overexpression of EGFR in HNSCC correlates with regional lymph node metastasis and poor clinical outcome (1). The contribution of EGFR to proliferation, cell cycle regulation, invasion and angiogenesis, and its accessible location on the cell surface makes it an attractive target for therapeutic intervention (2). EGFR small molecule TKIs, such as gefitinib and erlotinib, are known to selectively inhibit the tyrosine kinase domain of EGFR by competitive binding to the ATP-binding region. Despite frequent overexpression of EGFR in various human cancers, including HNSCC, EGFR TKIs have demonstrated limited clinical efficacy to date, implicating intrinsic resistance mechanisms (3). There are over 50 clinical trials currently underway in different disease settings of HNSCC using erlotinib, so understanding intrinsic resistance to erlotinib could help identify a subset of patients most likely to respond. Unlike non-small cell lung cancer (NSCLC), where EGFR activating mutations confer sensitivity to EGFR TKIs (4), HNSCC very rarely contain EGFR activating mutations. Co-activation of several receptor and non-receptor tyrosine kinases has been implicated in the limited response to targeting a single pathway in cancer models (5). However, the precise mechanisms determining EGFR TKI sensitivity in HNSCC remain incompletely understood.
Elevated expression of the non-receptor tyrosine kinase c-Src and/or increased Src kinase activity has been reported in a wide variety of human cancers, including breast, colon, lung, and head and neck cancers (6–8). Activated c-Src (phospho-c-Src) expression is associated with poor differentiation and lymph node metastases in HNSCC patients (8). We previously reported that c-Src activation enhances cellular invasion and proliferation in HNSCC cells in vitro (9). The contribution of c-Src activation to EGFR inhibitor response in head and neck cancer has not been previously explored.
In this study, we tested the hypothesis that activation of c-Src contributes to EGFR TKI resistance, and that targeting alternative pathways activated by c-Src may improve treatment responses. The c-Met tyrosine kinase pathway can activate many of the same signaling pathways as EGFR and dysregulated c-Met signaling has been linked to numerous epithelial carcinomas, including HNSCC (10). Increased c-Met activity has been shown in other cancers to contribute to EGFR TKI resistance (11). We previously showed ligand-independent activation of c-Met by EGFR in a series of HNSCC cell lines, and observed enhanced anti-tumor effects with the combination of a c-Met TKI and the EGFR TKI gefitinib (12). Using a cell line that over-expresses activated c-Src, we report here a novel mechanism of erlotinib resistance in HNSCC that involves ligand-independent activation of c-Met by c-Src. These cells were not resistant to cetuximab. Response to erlotinib in these cells can be significantly improved by the addition of a selective c-Met inhibitor to erlotinib treatment. Combining erlotinib with the Src inhibitor dasatinib also was more effective than erlotinib alone in HNSCC cells over-expressing activated c-Src. These results provide preclinical evidence that the addition of either a c-Met or c-Src inhibitor to erlotinib may be a promising therapeutic strategy in HNSCC patients whose tumors show high levels of activated c-Src in conjunction with EGFR expression.
MATERIALS AND METHODS
Reagents and antibodies
Erlotinib and the highly selective c-Met inhibitor PF04217903 (which lacks inhibitory activity against RON, ALK and other kinases, ref. 13) were purchased from ChemieTek (Indianapolis, IN, USA), dissolved in DMSO, and stored as 10mM and 100mM stock solutions at −20°C for in vitro use. Cetuximab was from Bristol Myers Squibb (New York, NY, USA) as a 2mg/ml solution in sterile saline. PF04217903 was dissolved in water at 25mg/ml and erlotinib was dissolved in 20% trappsol at 4mg/ml for in vivo use. Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA, USA). Antibodies for phospho-Src Family (Tyr416), total Src (32G6 monoclonal antibody), phospho-EGFR (Tyr845), phospho-EGFR (Tyr1068), and phospho-Met (Tyr1234/1235) were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Purified mouse anti-EGFR antibody was purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Total c-Met (C-12) antibody was from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA), while β-tubulin antibody was from Abcam (Cambridge, UK). Secondary antibodies were from Bio-Rad Laboratories (Hercules, CA, USA).
Cell lines
All HNSCC cell lines were of human origin and obtained from the following sources: 686LN (14), G. Chen (Emory University); FaDu, American Type Culture Collection (ATCC, Rockville, MD, USA); UM-SCC-22A, UM-SCC-22B and UM-SCC1, T. Carey (University of Michigan); PCI-15B, T. Whiteside (University of Pittsburgh); CAL-33, G. Milano (Centre Antoine-Lacassagne, Nice, France); and HN-5, J. Myers (MD Anderson). All cell lines were validated by genotyping within 2 months of performing the experiments (15).
Generation of stable HNSCC cell lines expressing dominant-active c-Src
686LN cells were transfected with a plasmid expressing dominant-active c-Src (DA-Src) carrying a mutation at tyrosine 529 to phenylalanine, which eliminates the inhibitory phosphotyrosine at position 529. Corresponding control vector, pUSEamp, and the DA-Src plasmids were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY, USA). Three days after transfection, cells were subjected to selection medium containing 200μg/ml neomycin for an additional 10 days followed by validation.
Western blotting
Cell lysates were prepared as described (16). Forty micrograms of total protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting as previously reported (17).
HGF ELISA
Cell culture media was harvested and analyzed in triplicate by Quantikine Human HGF ELISA (R&D Systems, Minneapolis, MN USA) according to the manufacturer’s instructions.
Matrigel invasion assay
Invasion assays were performed using Matrigel-coated modified Boyden chamber inserts (BD Biosciences, Bedford, MA, USA). Cells (1.25×104) were seeded onto the upper chamber. Both the upper and lower chambers contained drug treatment. The lower chamber also contained 10% FBS which served as a chemoattractant. Cells were incubated for 24 hrs at 37°C in a 5% CO2 incubator. Non-invading cells retained in the upper chamber were removed and the invaded cells were fixed and stained with Hema 3 (Fisher Scientific, Hampton, NH, USA). Invaded cell number was normalized to cell proliferation (normalized to O.D. by MTT assay for each respective treatment group). Six randomly selected fields were counted under the microscope at 200X magnification. The mean ± SE was calculated from three independent experiments.
MTT assay
Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyl tetrazolium bromide (MTT) assay. Cells were plated in triplicate (3×104/well in 24-well plates) overnight followed by drug treatment at various concentrations as indicted in the legends. MTT solution (5 mg/ml of MTT in PBS; Sigma-Aldrich, St. Louis, MO, USA) was added for the appearance of colored formazan product, which was then dissolved in DMSO, and subjected to colorimetric measurement at 570 nm with a plate reader (BioTek Instruments, Inc. Winooski, VT, USA). Percentage growth inhibition was calculated as (ODvehicle−ODdrug)/ODvehicle × 100%. For Figure 5D, cells were plated in 24 well plates in the presence of recombinant human HGF (50ng/mL) or vehicle control. Cells were subjected to drug treatment in the continued presence of vehicle or HGF for 48 hrs before analysis with MTT.
Figure 5.
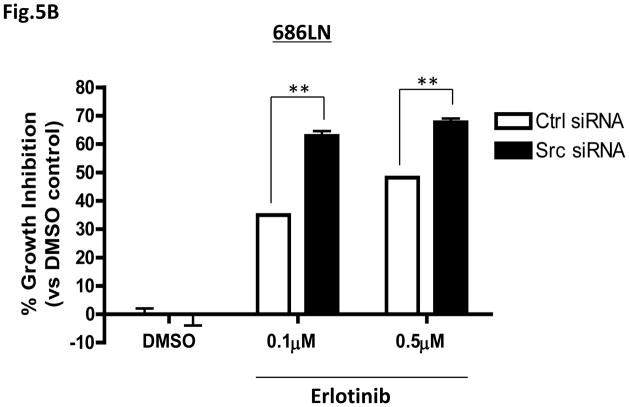
Specific knockdown of c-Src by siRNA enhances HNSCC cell sensitivity to erlotinib. (A) 686LN and FaDu HNSCC cells (1×105/well) were transfected with c-Src siRNA or control siRNA (300pmoles) for 48 hrs. Knockdown of c-Src was observed in both cells lines by Western blotting. Representative Western blotting results are shown. β-tubulin was used as loading control. Densitometry results are shown beneath each blot compared to β-tubulin. Similar results were observed in 3 independent experiments. (B,C) c-Src siRNA or control siRNA transfectants (686LN and FaDu) were treated with DMSO or erlotinib at the indicated doses (EC50 and sub-EC50 doses for respective cell lines). Cell viability was assessed by MTT assay after 48 hrs. The results from three independent experiments are shown. P<0.0001***, P<0.01** (D) 686LN parental cells were plated in 24 well plates in the presence of recombinant human HGF (50ng/ml) or vehicle control. Cells were subjected to erlotinib treatment in the continued presence of vehicle or HGF for 48 hrs before analysis with MTT. Confirmation of phospho-c-Met activation was confirmed by Western blotting (inset). P<0.0001.
siRNA transfection
Small interfering RNAs (siRNAs) were purchased from Dharmacon, (Lafayette, CO, USA). The target sequence for human specific c-Src siRNA (D-003175-05) was 5′ GAGAACCUGGUGUGCAAAG-3′. c-Met was targeted using a mixture of four siRNAs provided as a single reagent (L-003156-00). Control siRNA from Dharmacon (D-001210-01 for c-Src, D-001810-10-05 for c-Met) was used as a control for non-sequence-specific effects. siRNA (300pmoles) was transfected in HNSCC cells using Lipofectamine 2000 according to the manufacturer’s instructions.
In vivo studies
Six week old female athymic nude mice (nu/nu) were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Stable cell lines VC-2 or DA-Src-5 were inoculated into the flanks of athymic nude mice (1 × 106cells/tumor) with matrigel (BD Biosciences). Tumor nodules were palpable on day 5 after inoculation and mice were randomized into four groups. Mice were treated 5 days per week with 40mg/kg erlotinib, 15mg/kg PF04217903 or vehicle (20% trappsol in H2O, Sigma-Aldrich) by oral gavage. In a separate experiment, mice were treated 5 days per week with 0.03mg cetuximab i.p. or vehicle control. Tumor size was measured two times a week with digital calipers. Tumor volume was calculated as: volume=length × width2/2. At the end of the treatment period, the animals were killed and tumors were removed and fixed in 10% buffered formalin for immunostaining. Animal care was in strict compliance with the institutional guidelines established by the University of Pittsburgh.
Immunohistochemistry
Slides were deparaffinized with xylenes and rehydrated before heat-induced antigen retrieval. Non-specific binding was blocked for 45 min at room temperature. Sections were incubated with primary antibodies for Ki67 (1:50, GeneTex), phospho-Src (1:100, Santa Cruz Biotechnology), and phospho-Met (1:100, Cell Signaling Technology). The number of apoptotic cells was determined using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore, Billerica, MA USA) as described (18). Slides were read and scored for the number of positive cells per five fields per sample. Results are reported as the mean ± SE.
Statistical analysis
Statistical analyses were performed using PRISM 4 software (GraphPad, La Jolla, CA), the P-values were obtained by the Wilcoxon–Mann–Whitney test or Student’s t-test. Results were considered as statistically significant with a P<0.05.
RESULTS
Overexpression of activated c-Src confers resistance to erlotinib in HNSCC cells in vitro
Activation of c-Src is associated with more aggressive tumor behavior in head and neck cancers (8). The contribution of c-Src activation to effectiveness of EGFR targeting in head and neck cancer has not been previously explored. We evaluated the expression of phosphorylated and total EGFR as well as phosphorylated and total c-Src in a panel of 8 HNSCC cell lines and found variable expression of activated EGFR and c-Src with no correlation in expression levels (Figure 1A). To investigate the consequences of c-Src activation on erlotinib sensitivity, we generated stable head and neck cancer clonal cell lines expressing activated c-Src (by ectopic expression of dominant active c-Src, DA-Src) in an invasive HNSCC cell line that readily forms tumors in xenografts, 686LN, with moderate endogenous c-Src expression and low endogenous activated c-Src (Figure 1A). These cells also have moderate EGFR and phospho-EGFR expression (Figure 1A). As shown in Figure 1B, the DA-Src clones (DA-Src-3 and DA-Src-5) were validated and confirmed by Western blotting to express greater than 3-fold higher levels of activated c-Src when compared to vector control clones, VC-1 and VC-2 (P<0.05), with unchanged level of total EGFR. Functional activation of c-Src was also confirmed by the increase in Tyr845 phospho-EGFR expression in the DA-Src cell lines, since activated c-Src is known to induce EGFR phosphorylation specifically at amino acid residue Tyr845 (19). A slight increase in EGFR phosphorylation at the Tyr1068 autophosphorylation site was also observed suggesting that overall levels of EGFR tyrosine phosphorylation are somewhat affected.
Figure 1.
Activated c-Src confers resistance to erlotinib in vitro (A) Expression of p-EGFR, total EGFR, p-Src, and total c-Src were examined by Western blotting in 8 representative HNSCC cells. β-Tubulin is shown as loading control. Relative densitometry quantitation of each protein normalized to β-tubulin is shown beneath each blot. The experiment was performed three times with similar results. (B) Validation of stable cells expressing activated c-Src (DA-Src-3, DA-Src-5) vs. vector control cells (VC-1 and VC-2) by Western blotting. Protein expression levels of p-Src, total c-Src, p-EGFR, and total EGFR were compared. Cumulative densitometry p-Src results from 3 independent experiments are shown in the graph. (C) VC and DA-Src clones were treated with various doses of erlotinib, followed by MTT assay at 48 hrs.
To determine the sensitivity of these cells to an EGFR TKI, VC stable clones and DA c-Src clones were subjected to treatment with erlotinib at increasing concentrations followed by a cell viability assay. A representative experiment is shown in Figure 1C. Erlotinib induced dose-dependent growth inhibition of the 686LN vector control clones, VC-1 and VC-2, with an average EC50 of 2.7μM. Expression of activated c-Src in DA-Src-3 and DA-Src-5 cells was found to confer marked resistance to erlotinib (shift of concentration-response curve to the right), when compared to the vector control clones, with an average EC50 of 12.5μM, a 4.6-fold increase. Cumulative data from multiple independent experiments showed that both the DA-Src-3 and DA-Src-5 clones were 4-fold more resistant to erlotinib compared to VC-1 and VC-2 cells (n=10, P=0.0004, data not shown). To determine the specificity of c-Src activation to erlotinib sensitivity, the same cells were treated with cisplatin, a common chemotherapeutic agent for the treatment of HNSCC. c-Src activation status did not alter the sensitivity of HNSCC cells to cisplatin (Supplemental Figure 1), indicating that increased c-Src activation does not generally contribute to drug resistance. EGFR TKIs have also been shown to inhibit cancer cell invasion (17), and c-Src is also known to mediate invasion. We have previously shown that HNSCC cells containing DA-Src are more invasive than vector control cells and the invasive behavior was resistant to treatment with the EGFR TKI gefitinib (17). We confirmed that the increased invasion seen with DA-Src transfection was also resistant to erlotinib compared to vector control cells (data not shown).
c-Src activation contributes to erlotinib resistance in vivo
Next, we sought to examine if c-Src-mediated resistance to erlotinib was also observed in vivo. 686LN VC-2 and DA-Src-5 cells were injected s.c. into immunocompromised mice and were treated with vehicle (20% trappsol) or erlotinib (40mg/kg) by oral gavage after a 5 day period to establish the tumors. The initial pre-treatment tumor volumes of all groups were comparable with no statistical differences. Erlotinib treatment reduced the tumor volume established from the VC cells by 68.7% compared to vehicle treatment (average tumor volume= 1215 ±320 mm3 vs 382 ±32 mm3, n=14, P=0.034) (Figure 2A). However, tumors established from DA c-Src cells were completely insensitive to erlotinib compared to vehicle (average tumor volume=977±200 mm3 versus 910±140 mm3, n=14, P= 0.95). These results suggest that c-Src activation also contributes to erlotinib resistance in vivo. Differences in tumor volume between VC and DA c-Src cells are most likely due to a lower stromal content in DA-Src tumors.
Figure 2.
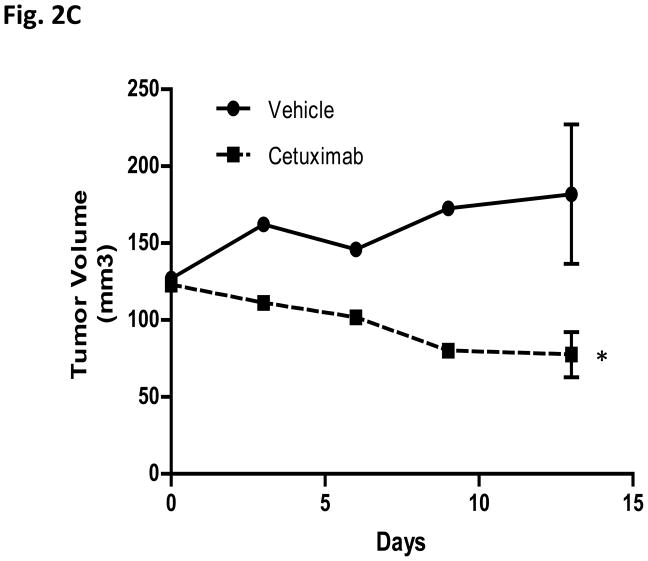
c-Src activation contributes to erlotinib resistance but not cetuximab resistance in vivo. (A) VC-2 and DA-Src-5 HNSCC cells were inoculated into the flanks of nude mice. Mice were treated 5 days per week with either vehicle control (20% trappsol) or 40mg/kg erlotinib by oral gavage for 4 weeks. Treatment began 5 days after tumor implantation. Results represent the mean tumor volumes ± S.E. of 14 tumors per treatment group. Unpaired Student’s t-test, *P<0.05. (B) Representative images and quantitative results of immunohistochemical staining of cell proliferating cells by Ki67 and apoptotic cells by Tunel assay. The results are presented as the mean number of apoptotic cells or Ki67 positive cells per area ± S.E. Unpaired Student’s t test, P<0.05*, P<0.0005***. Scale bar= 50μm. (C) 1 × 106 DA-Src-5 HNSCC cells were inoculated into the flanks of nude mice. Mice were treated 2 days per week with either vehicle control (saline) or 0.03mg cetuximab i.p. for 13 days. Treatment began 15 days after tumor implantation. Results represent the mean tumor volumes ± S.E. of 7 tumors per treatment group. Unpaired Student’s t-test, *P<0.05.
Extent of apoptosis and cell proliferation in tumor xenografts were examined in formalin-fixed excised tumors (Figure 2B); staining of apoptotic nuclei was increased, while Ki67 staining was decreased by erlotinib treatment in the VC but not in the DA-Src tumor xenografts. The number of apoptotic and proliferating cells was quantitated in sections from 4 tumors per treatment group showing a significant 4-fold increase in apoptotic cells with erlotinib treatment in the VC xenografts (mean of 177±11 apoptotic cells/field) compared to control treatment in VC xenografts (mean 43.7±9.2 apoptotic cells/field, P=0.0007). There was a statistically significant 52.7% decrease in cell proliferation with erlotinib treatment in the VC xenografts (mean 140.7±40.2 proliferating cells/field) compared to control (mean 298±19 proliferating cells/field, P=0.025). No significant differences in apoptosis or cell proliferation were observed in DA-Src xenografts treated with erlotinib compared to vehicle control. In addition, we observed that the DA-Src tumor xenografts expressed 5.2-fold more phospho-Src compared to VC xenografts (P<0.005), irrespective of erlotinib treatment (data not shown).
In parallel experiments, we found that DA-Src cells remained sensitive to the EGFR neutralizing antibody cetuximab (at the EC50 dose for parental cells) with a 57.4% inhibition in tumor volume (P<0.05) (Figure 2C) compared to a 63.4% inhibition in the VC xenografts. The 686LN parental cells have previously been shown to be sensitive to cetuximab in vivo (20). The in vivo cetuximab response in DA-Src xenografts was consistent with in vitro invasion assays in which cetuximab demonstrated a significant decrease of number of invading cells in the DA-Src cell line (data not shown).
Activated c-Src induces c-Met phosphorylationin HNSCC cells
Amplification and overexpression of c-Met has been associated with acquired erlotinib resistance in NSCLC (11). To determine the role of c-Met in c-Src-mediated erlotinib resistance, we assessed the expression of total and phosphorylated forms of c-Met in HNSCC cells expressing DA-Src. As shown in Figure 3A, both DA-Src-3 and DA-Src-5 demonstrated a greater than 25-fold increase in phospho-Met, with a very moderate increase in total c-Met expression, when compared to controls. Consistent with our previous findings that HGF is a paracrine factor in HNSCC (10), we confirmed by ELISA that the increase in c-Met phosphorylation in the DA-Src cells was independent of the c-Met ligand, HGF, because no HGF production was detected (Supplemental Figure 2). This suggests activation by phosphorylated c-Src is responsible for the observed c-Met activation, as has been reported (21). We also confirmed that in vivo, phospho-Met expression was up-regulated in tumor xenografts from DA-Src cells. A 20-fold increase was detected by IHC in DA-Src cells compared to VC cells (Supplemental Figure 3).
Figure 3.
Activated c-Src induces marked c-Met phosphorylation in HNSCC cells. (A) Expression levels of p-c-Met and total c-Met in VC-1 and VC-2 cells and DA-Src-3 and DA-Src-5 cells. Densitometry quantification of the representative blot shown in the bottom panel. Similar results were observed in at least 3 independent experiments. (B) DA-Src-5 cells were treated with 200nM dasatanib or 1μM erlotinib for 24 hrs. Cell lysates were prepared and analyzed for p-EGFR, p-c-Met, and p-Src expression. A representative Western blot is shown and the quantitation of p-c-Met expression normalized to β-tubulin from 3 independent experiments. P<0.0001***, ns= non-significant. (C) A panel of 8 HNSCC cell lines (CAL-33, UM-SCC-22A, HN-5, PCI-15B, VC-1, VC-2, DA-Src-3, DA-Src-5) with varying erlotinib EC50s (1.56–13.14 μM) were evaluated for phospho-Met expression following dasatinib or erlotinib treatment as in (B). The densitometric ratio of residual phospho-Met expression observed in dasatinib treatment cells to that observed in erlotinib-treated cells [P-Met expression (Dasatinib/Erlotinib)] was calculated and analyzed for correlation to erlotinib EC50.
Since ligand-independent activation of c-Met has been reported for both EGFR (12) and c-Src (21), we examined the extent to which EGFR or c-Src inhibitors modulated phospho-Met in the presence of activated c-Src. In DA c-Src cells, erlotinib treatment resulted in a non-significant minimal reduction (10–15%) of phospho-Met, while dasatinib treatment achieved almost a complete reduction of phospho-Met levels (Figure 3B). Both dasatinib and erlotinib inhibited their respective targets, phospho-Src and phospho-EGFR. To confirm this finding, we examined extent of inhibition of phospho-Met in four HNSCC cell lines from Fig. 1A (CAL-33, UM-SCC-22A, HN5 and PCI-15B) with widely varying erlotinib sensitivity (Ref. 20 and Supplemental Table 1), along with the two 686LN DA-Src clones and the two VC clones. We found a significant correlation (P=0.045) between erlotinib EC50 and the extent to which phospho-Met was inhibited in the presence of dasatinib versus erlotinib, using a concentration that produced complete reduction in phosphorylation of the respective target (c-Src or EGFR). For this determination, the ratio of residual phospho-Met seen in dasatinib-treated cells compared to residual phospho-Met observed in erlotinib-treated cells was calculated (Figure 3C). The more resistant the cells were to erlotinib, the lower this ratio was. This suggests that in DA c-Src cells and other HNSCC cell lines that are erlotinib resistant, ligand-independent c-Met activation is coupled to c-Src more so than to EGFR, because it is more sensitive to dasatinib than to erlotinib. This can provide an alternative pathway for tyrosine kinase signaling in the presence of an EGFR tyrosine kinase inhibitor.
Knockdown of c-Met enhances sensitivity to erlotinib
We next used c-Met siRNA to determine if knockdown of c-Met in DA-Src cells would increase erlotinib sensitivity. Figure 4A shows that knockdown of c-Met resulted in a 2- to 3-fold increase in erlotinib sensitivity (P<0.05). Control siRNA transfected cells showed a 7.6±5.0% and 23.9±2.9% inhibition of cell growth with 0.1μM and 0.5μM erlotinib, respectively, while c-Met knockdown resulted in 24.9±0.4% and 40.7±0.5% inhibition, respectively, at these erlotinib concentrations (c-Met versus control siRNA growth inhibition at 0.1μM erlotinib P=0.040, 0.5μM erlotinib P=0.016). The inset shows an 83.8% inhibition of c-Met expression with siRNA treatment.
Figure 4.
Specific knockdown of c-Met by siRNA in DA-Src cells restores sensitivity to erlotinib. 686LN DA-Src-5 cells were transiently transfected with c-Met or control siRNA (300pmoles) for 48 hrs followed by treatment with erlotinib at the indicated doses. Cell viability was assessed by MTT assay after 48 hrs. The results from three independent experiments are shown. Student’s t-test, *P<0.05. Knockdown of c-Met was confirmed by Western blotting (inset). (B) 686LN DA-Src-5 cells were treated with 0.1μM and 0.5μM erlotinib alone or in combination with PF04217903 (1μM) or dasatinib (EC50, 380nM) for 48hrs followed by MTT assay. Results from three independent experiments are shown. Student’s t-test, *P=0.010, **P=0.003, ***P<0.0001. (C) DA-Src-5 tumor bearing mice received the following treatments 5 days per week for 4 weeks by oral gavage: vehicle control, erlotinib (40mg/kg), PF04217903 (15mg/kg), or combination. Treatment began 6 days following tumor implantation. Results represent the mean tumor volumes ± S.E. of 12 tumors per treatment group. Unpaired Student’s t-test, *P=0.0068 compared to control. (D) Representative images and quantitative results of immunohistochemical staining of cell proliferating cells by Ki67 and apoptotic cells by Tunel assay. The results are presented as the mean number of apoptotic cells or Ki67 positive cells per area ± S.E. Unpaired Student’s t test, P<0.05*, P<0.0005***. Scale bar= 50μm.
We next combined a c-Src or a c-Met inhibitor with erlotinib in the DA c-Src cells and examined cell viability to determine if the erlotinib response could be enhanced by dual treatment (Figure 4B). In this experiment, erlotinib alone resulted in a 10.6±3.7% and 14.7±5.6% inhibition of cell growth at 0.1μM and 0.5μM, respectively. The c-Met TKI PF04217903 (13), demonstrated minimal inhibition (10%) of cell proliferation as a single agent up to 5μM. However, addition of 1μM PF04217903 resulted in 21.9±3.1% and 31.4±9.0% inhibition in combination with 0.1μM and 0.5μM erlotinib, respectively. This represents a 2-fold increase in sensitivity. Similarly, addition of dasatinib at its EC50 (380 nM) to erlotinib at 0.1μM and 0.5μM in the DA-Src cells resulted in 81.1±0.7% and 81.7±0.3% inhibition of cell growth, respectively, enhancing the effect of erlotinib over 6-fold. A similar trend was observed with addition of dasatinib at its EC25 (30nM) to 0.1μM and 0.5μM erlotinib with 43.8±1.5% and 58.8±1.9% inhibition of cell growth, respectively (data not shown). These results suggest that erlotinib resistance due to over-expression of activated c-Src can be overcome by addition of a selective c-Met inhibitor to erlotinib, or by combining erlotinib with a Src inhibitor.
c-Met inhibition sensitizes DA-Src tumors to erlotinib in vivo
If erlotinib resistance is due to activation of c-Src, this suggests the alternate use of a c-Src inhibitor in patients who show high levels of c-Src activation. Results of a recent phase 2 trial in HNSCC patients showed that dasatinib as a single agent lacks clinical activity and was associated with toxicity, even at a dose that was inhibiting c-Src (22). Increased activity of dasatinib against HNSCC in a preclinical model has been previously observed when combined with a Met inhibitor in vivo (21), consistent with our in vitro observation that c-Met activation is coupled to over-active c-Src. Since combination treatments with dasatinib (including adding dasatinib to either erlotinib or a c-Met inhibitor) might show unacceptable toxicities in patients, we investigated whether the marked c-Met activation conferred by DA-Src could serve as an alternate therapeutic target in vivo. We used a tumor xenograft model with the DA-Src-5 cells to test if the addition of a c-Met inhibitor could improve response to erlotinib. Inhibitors of c-Met have been successfully and safely combined with erlotinib in clinical trials of lung cancer patients (23). The c-Met inhibitor PF04217903, at the published dose of 15mg/ml (24), was used alone and combined with erlotinib. Immunocompromised mice bearing DA-Src-5 xenograft tumors were treated with either vehicle control, erlotinib (40mg/kg), PF04217903 (15mg/kg) or erlotinib plus PF04217903 for 5 days per week for 4 weeks. Initial tumor volumes were similar between all treatment groups. Both erlotinib and PF04217903 alone resulted in no significant difference in tumor volume compared to control (P>0.05). The combination treatment, however, resulted in a 56.2% decrease in tumor volume compared to control (P=0.0068) (Figure 4C), comparing favorably to the 68.7% inhibition in tumor volumes seen with erlotinib-sensitive VC controls cells treated with erlotinib alone (Figure 2A). This result shows that in vivo erlotinib resistance in c-Src activated HNSCC cells can be about 80% overcome by the addition of a c-Met inhibitor.
The mean number of apoptotic and proliferating cells in the tumors at the end of the experiment were assessed by Tunel assay and Ki67 immunostaining, respectively (Figure 4D). The maximum number of apoptotic cells and the least amount of proliferating cells were found in the combination treatment group. Apoptotic cells were increased by all treatment groups (erlotinib: 18.0 ± 1.6, PF04217903: 28.2 ± 4.3, combination: 40.7 ± 2.4) compared to the control group (15.2 ± 1.8). The mean number of apoptotic cells in the combination treatment group was significantly higher compared to the mean number of apoptotic cells in the control (P<0.0001), erlotinib only (P<0.0001) and PF04217903 only (P=0.033) treatment groups. Similar results were observed with the number of proliferating cells. Erlotinib and PF04217903 treatment alone resulted in an 8.5% (P=0.26) and 26% (P=0.007) decreases in the number of proliferating cells compared to control. Combination treatment resulted in a 58.5% decrease compared to control (P<0.0001) and was also significantly lower compared to both single agent treatment groups. This effect on labeling of proliferative cells compares favorably to that observed with erlotinib alone in VC cell xenografts that are sensitive to erlotinib (52.7% decrease, Figure 2B).
Knockdown of c-Src in parental cells reduces c-Met activation and enhances sensitivity to erlotinib, while HGF treatment results in erlotinib resistance
To determine the contribution of endogenous c-Src to c-Met phosphorylation in the absence of an exogenously added c-Src vector, expression of total and phosphorylated c-Met in HNSCC parental cells treated with c-Src siRNA was examined. Transient transfection of c-Src siRNA in two representative HNSCC cell lines with moderate endogenous expression of c-Src, 686LN and FaDu, resulted in a significant but partial knockdown of c-Src protein at 48 hrs (63% in 686LN and 51% in FaDu) (Figure 5A). The expression of phosphorylated c-Met was almost completely abolished even with this partial knockdown of endogenous c-Src in both 686LN and FaDu cells. This result suggests that endogenous c-Src expression in HNSCC cells is also associated with c-Met activation in HNSCC cells. The activation of c-Met is most likely through a ligand-independent mechanism directly mediated by c-Src (21), especially since no HGF is detected in HNSCC cell lines (10).
We also tested whether downregulation of endogenous c-Src, with its accompanying reduction in activated c-Met, would enhance the sensitivity of HNSCC parental cells to erlotinib. As shown in Figures 5B & C, even with the partial knockdown of endogenous c-Src by siRNA, the erlotinib sensitivity of both 686LN and FaDu cells was enhanced at their respective EC50 and sub-EC50 doses of erlotinib. At 48 hrs of 0.1μM erlotinib treatment, transient transfection of c-Src siRNA increased erlotinib sensitivity of 686LN cells by 1.8-fold (63±1.7% growth inhibition versus 35.0±0.18% growth inhibition with control siRNA; n=3 experiments, P=0.003), and a consistent enhancement was also observed with 0.5μM erlotinib at 48 hrs (Figure 5B). In FaDu cells, which have higher endogenous c-Src and phospho-c-Src, as well as a higher EC50 for erlotinib (2.0μM) than 686LN cells (0.5μM), specific knockdown of c-Src resulted in 1.6-fold and 2.8-fold increases in erlotinib sensitivity at the EC50 (2.0μM) and sub-EC50 (0.5μM) concentrations of erlotinib, respectively (Figure 5C). These results indicate that endogenous c-Src contributes to erlotinib sensitivity in HNSCC cells.
To determine if the parental erlotinib sensitive 686LN cells could also become resistant to erlotinib by activating c-Met directly through HGF exposure, we treated these cells with exogenous human recombinant HGF (Figure 5D inset). A shift to the left in the erlotinib concentration response at 48 hr was observed (Figure 5D) demonstrating that parental cells became over 2-fold resistant to erlotinib following HGF treatment (EC50= 2.45μM without HGF treatment versus EC50=5.98μM with HGF treatment, P<0.0001). Together these observations support a role for c-Met activation in erlotinib resistance in HNSSC, which could occur through ligand stimulation as well as through ligand-independent activation.
Endogneous activated c-Src is a predictor of erlotinib resistance
Using the erlotinib EC50 values (ref. 20 and Supplemental Table I) for the panel of eight cell lines shown in Figure 1A, a significant correlation (P=0.019) was found between endogenous phospho-c-Src expression and erlotinib EC50 (Figure 6). Cells with higher basal expression of phospho-c-Src have higher erlotinib EC50 values and thus phospho-Src may represent a novel therapeutic biomarker for erlotinib response. In this panel of cell lines, we did not observe any significant correlation between total EGFR, phospho-EGFR, or total c-Src and erlotinib response, and combined consideration of phospho-EGFR with phospho-c-Src did not improve the correlation with erlotinib response seen with phospho-c-Src alone (all P>0.05, data not shown). There was also no relationship between erlotinib sensitivity and the extent of phosphorylation of EGFR residue Tyr845 (the c-Src-dependent residue). Relationship of phospho-Met to erlotinib sensitivity was also not significant, alone or combined with phospho-c-Src measurement (data not shown), suggesting that extent of c-Src activation is the most important variable in predicting erlotinib sensitivity.
Figure 6.
P-Src levels significantly correlate to erlotinib sensitivity. P-Src levels in a panel of 8 HNSCC cell lines were correlated to erlotinib EC50 value.
DISCUSSION
Constitutive activation of c-Src is observed in HNSCC, where expression of activated Src correlates with aggressive clinical features such as invasive tumor fronts, poor differentiation and lymph node metastasis (8). The implication of high c-Src activation on treatment selection for HNSCC has not been previously addressed. The findings in the present study demonstrate that increasing c-Src activation in HNSCC through either a dominant-active construct or via endogenous expression is associated with intrinsic resistance to erlotinib treatment. Expression of the dominant-active c-Src construct did not alter responsiveness to a common chemotherapy, cisplatin, suggesting the resistance to erlotinib is specific to dependence on its targeted pathway, EGFR, and not to a general change in tumor cell behavior. Ectopic expression of activated c-Src by HNSCC cells conferred resistance to erlotinib treatment in vivo as well as in vitro, implicating c-Src activation in erlotinib resistance in HNSCC patients. In addition, this resistance is specific to erlotinib as the EGFR monoclonal antibody cetuximab did not demonstrate the same resistance in vivo or in vitro suggesting that src activation does not contribute to cetuximab resistance. The connection between c-Src and erlotinib sensitivity was confirmed by specific knockdown of endogenous c-Src, which significantly enhanced erlotinib sensitivity in HNSCC cells that were not engineered to over-express c-Src. Higher constitutive c-Src activation, assessed by basal phospho-c-Src level, also was a significant indicator of erlotinib resistance in HNSCC cell lines, while level of basal phospho-EGFR was not, suggesting that c-Src signaling serves as a means of resistance to EGFR inhibitors. These findings suggest that c-Src activation directly contributes to erlotinib, but not cetuximab, resistance in EGFR-expressing HNSCC cancer cells. The mechanism by which increased c-Src activation occurs in HNSCC has not been fully elucidated. Our recent study of HNSCC mutation profiling in 74 patient tumors revealed the absence of any activating mutations of c-Src in HNSCC patients (25). Previous studies by us and others demonstrated that upstream signaling components, such as EGFR activation by autocrine ligands (26), other growth factors, or integrin signaling may also activate c-Src in HNSCC (27). Activation of c-Src may lead to an EGFR-independent means of stimulating cell proliferation or survival, mediated at least in part by activating c-Met.
Inhibiting c-Src directly is a possible strategy for erlotinib-resistant HNSCC tumors with high c-Src activation. However, results of a recent phase 2 trial in HNSCC patients showed that dasatinib lacked clinical efficacy, even though inhibition of c-Src was achieved in patients (22). Moreover, apparent toxicity of dasatinib has been observed in patients with recurrent and/or metastatic HNSCC after platinum-based therapy, illustrating the challenge of c-Src targeting in HNSCC (22). Further, treatment with Src inhibitors in laboratory studies often leads to decreased tumor cell migration and invasion, without affecting cell survival (28). Identifying targets downstream of c-Src that might mediate its responses and are more directly coupled with cell growth and survival could suggest alternative rational and clinically feasible (and possibly less toxic) strategies than dasatinib for HNSCC treatment.
EGFR/c-Met cross-talk signaling has been well-described in several cancer models including HNSCC (12). Extent of activation of c-Met by c-Src has recently been reported to be linked to dasatinib sensitivity in HNSCC cells, and alternate activation of c-Met by EGFR was found to be a factor in dasatinib resistance (21). This observation taken together with our data reported here indicate that the extent to which ligand-independent activation of c-Met is coupled to c-Src, as opposed to being coupled with EGFR, may determine whether sensitivity is linked to erlotinib or dasatinib. Our observations that: 1) erlotinib resistance associated with c-Src activation was accompanied by high ligand-independent activation of c-Met, and 2) erlotinib resistance could be overcome by down-regulating c-Met or by combining c-Met and EGFR inhibition demonstrate that the biologic coupling of c-Met activation to c-Src contributes to erlotinib resistance in HNSCC. This is consistent with the observation that c-Met phosphorylation was largely dependent on c-Src, but not on EGFR, in the DASrc cells. This was further supported by the association we observed between the degree of c-Met activation that was dependent on c-Src compared to EGFR and erlotinib resistance in the HNSCC cell lines.
Further evidence in favor of this hypothesis is the observation that in the absence of exogenously-induced c-Src activation, EGFR inhibition resistance could be produced by directly activating the c-Met pathway with its ligand, HGF. In this case, c-Met activation would also not depend upon the EGFR pathway. This is consistent with the idea that c-Met activation can prevent cell death in the presence of an EGFR inhibitor. We also found in lung cancer cells that ligand-independent activation of c-Met required c-Src (29). We did not find evidence for a direct interaction between c-Src and c-Met however, and both gene transcription and prolonged c-Src activation was involved in persistent c-Met activation (29), suggesting that an intermediary protein is involved. Integrins have been postulated to play a role in this mechanism (30).
The correlation we observed between endogenous phospho-c-Src expression and erlotinib sensitivity in HNSCC cell lines suggests that measurement of phosphorylated c-Src may serve as a clinical indicator to help identify erlotinib-resistant HNSCC patients. Such patients might benefit from dual EGFR and c-Met inhibitor treatment, or dual treatment with either erlotinib and a c-Src inhibitor, or a c-Met inhibitor combined with a c-Src inhibitor. Tolerability of combinations will be an important factor for consideration in clinical settings. In the HNSCC cell line panel, phospho-Met expression was generally higher as erlotinib sensitivity declined, but did not reach significance alone as a factor that correlated with erlotinib EC50, or when combined with phospho-c-Src expression. Total endogenous phospho-Met may not predict sensitivity to erlotinib because some phospho-Met may be driven by coupling with EGFR, and remains sensitive to EGFR inhibition. Although c-Met is likely not the only target that is phosphorylated by activated c-Src, and does not appear from our results to be solely responsible for erlotinib resistance mediated by activated c-Src, addition of a c-Met inhibitor to erlotinib reversed erlotinib resistance by approximately 2-fold in dominant-active c-Src cells in vitro, and restored about 80% of the activity of erlotinib in these cells in vivo, compared to the empty vector control cells. Reliable measurement of phospho-Src levels in patient tumor tissues may require routine freezing of tumor biopsies, rather than formalin fixation of tissues. The potential use of phospho-c-Src as a biomarker of erlotinib response in HNSCC patients will require future validation in clinical settings. Currently, tissues from responders and non-responders from an erlotinib clinical trial are not available for testing.
The addition of a c-Src inhibitor to erlotinib in the dominant-active Src cells enhanced the overall anti-growth effects of either agent alone, as would be expected. Given the aggressive behavior of HNSCC tumors with high endogenous c-Src activation (8) and the lack of clinical efficacy of the dasatinib single agent clinical trial in HNSCC patients (22), our findings may suggest an alternative therapeutic approach (combination EGFR and c-Met inhibition) for HNSCC patients with activation of c-Src. Combination c-Src and c-Met inhibitors or c-Src and EGFR inhibitors also would be logical choices. Several c-Met inhibitors are currently being tested as monotherapies in head and neck cancer, including foretinib (NCT00725764), which is a dual c-Met-VEGFR2 inhibitor. Cetuximab is another possible choice for patients with activation of c-Src because cells with constitutively activated c-Src remained sensitive to cetuximab. EGFR inhibitors with different mechanisms of action probably have different biomarkers of relative sensitivity. Cetuximab sensitivity may depend more on availability of EGFR for binding by the neutralizing antibody and competition for binding from EGFR ligands such as TGFα and epigen (31, 32). Our results suggest that extent of EGFR downstream signaling events through coupled Src and c-Met may be more predictive of erlotinib sensitivity in head and neck cancer, rather than state of the EGFR itself. Biomarker-guided use of targeted combination treatments is worthy of future clinical investigation in HNSCC patients.
Supplementary Material
Statement of Translational Relevance.
The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, erlotinib, has had limited clinical efficacy as monotherapy treatment to date in head and neck squamous cell carcinoma (HNSCC), but is still under investigation in many clinical trials of different HNSCC disease settings. Our results in HNSCC preclinical models suggest that c-Src activation contributes to erlotinib resistance, but not cetuximab resistance, through ligand-independent activation of c-Met in HNSCC. In the presence of elevated c-Src activation, c-Met activation is more dependent on c-Src than on EGFR, providing an alternate pathway for tyrosine kinase signaling. Expression of phosphorylated c-Src predicts erlotinib resistance in HNSCC cell lines. Dual treatment with erlotinib and either a c-Met or a Src inhibitor may be an effective therapeutic approach for HNSCC patients whose tumors have high levels of activated c-Src with EGFR expression. Activated c-Src in patient tumors may be a potential marker for erlotinib resistance.
Acknowledgments
Financial Support: This work was supported by NIH grants R01CA77308, P50CA097190, and the American Cancer Society (to JRG). VWYL was supported by Head and Neck SPORE Developmental Research Award, P50CA0970 and the Patricia L. Knebel Fund of the Pittsburgh Foundation. This project used the University of Pittsburgh Cancer Institute Animal Facility and was supported in part by award P30CA047904.
We would like to acknowledge Jia-Ying Lee for technical support.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 2.Kalyankrishna S, Crandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 3.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Jänne PA, Lee JC, Tracy S, Greulick H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 6.Irby RB, Yeatman TJ. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 7.Masaki T, Igarashi K, Tokuda M, Yukimasa S, Han F, Jin YJ, et al. pp60c-src activation in lung adenocarcinoma. Eur J Cancer. 2003;39:1447–55. doi: 10.1016/s0959-8049(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 8.Mandal M, Myers JN, Lippman SM, Johnson FM, Williams MD, Rayala S, et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008;112:2088–100. doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Thomas SM, Xi S, Smithgall TE, Siegfried JM, Kamens J, et al. SRC family kinases mediate epidermal growth factor receptor ligand cleavage, proliferation, and invasion of head and neck cancer cells. Cancer Res. 2004;64:6166–73. doi: 10.1158/0008-5472.CAN-04-0504. [DOI] [PubMed] [Google Scholar]
- 10.Knowles LM, Stabile LP, Egloff AM, Rothstein ME, Thomas SM, Gubish CT, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–50. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Stabile LP, Gubish CT, Gooding WE, Grandis JR, Siegfried JM. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin Cancer Res. 201;17:4425–38. doi: 10.1158/1078-0432.CCR-10-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou HY, Li Q, Lee JH, Arango ME, Burgess K, Qiu M, et al. Sensitivity of selected human tumor models to PF-04217903, a novel selective c-Met kinase inhibitor. Mol Cancer Ther. 2012;11:1036–47. doi: 10.1158/1535-7163.MCT-11-0839. [DOI] [PubMed] [Google Scholar]
- 14.Sacks PG. Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metastasis Rev. 1996;15:27–51. doi: 10.1007/BF00049486. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res. 2011;17:7248–64. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lui VW, Boehm AL, Koppikar P, Leeman RJ, Johnson D, Ogagan M, et al. Antiproliferative mechanisms of a transcription factor decoy targeting signal transducer and activator of transcription (STAT) 3: the role of STAT1. Mol Pharmacol. 2007;71:1435–43. doi: 10.1124/mol.106.032284. [DOI] [PubMed] [Google Scholar]
- 17.Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, Freilino M, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284–91. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stabile LP, Rothstein ME, Keohavong P, Lenzner D, Land SR, Gaither-Davis AL, et al. Therapeutic targeting of human hepatocyte growth factor with a single neutralizing monoclonal antibody reduces lung tumorigenesis. Mol Cancer Ther. 2008;7:1913–22. doi: 10.1158/1535-7163.MCT-07-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 20.Sen B, Peng S, Saigal B, Williams MD, Johnson FM. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res. 2011;17:514–24. doi: 10.1158/1078-0432.CCR-10-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks HD, Glisson BS, Bekele BN, Ginsberg LE, El-Naggar A, Culotta KS, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011;117:2112–9. doi: 10.1002/cncr.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sequist LV, von Pawel J, Garmey EG, Akerley WL, Brugger W, Ferrari D, et al. Randomized Phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small cell lung cancer. J Clin Oncol. 2011;29:3307–15. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 23.Niswander LM, Guenther LM, Mendoza A, Khanna C, Christensen JG, Helman LJ, et al. Effect of modulation of MET with the small molecule inhibitor PF-04217903 on osteosarcoma metastasis in vivo. J Clin Oncol. 2010;28:9567. [Google Scholar]
- 24.Quesnelle K, Wheeler SM, Ratay M, Grandis JR. Preclinical Modeling of EGFR Inhibitor Resistance in Head and Neck Cancer. Cancer Biol Ther. 2012;13:935–945. doi: 10.4161/cbt.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–83. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 27.Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol. 2006;175:993–1003. doi: 10.1083/jcb.200605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 29.Dulak AM, Gubish CT, Stabile LP, Henry C, Siegfried JM. HGF-independent potentiation of EGFR action by c-Met. Oncogene. 2011;30:3625–3635. doi: 10.1038/onc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, et al. Ligand-independent activation of c-Met by fibronectin and a(5)b(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–1576. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jedlinski A, Ansell A, Johansson AC, Roberg K. EGFR status and EGFR ligand expression influence the treatment response of head and neck cancer cell lines. J Oral Pathol Med. 2012 doi: 10.1111/j.1600-0714.2012.01177.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Oshima G, Wennerberg J, Yamatodani T, Kjellen E, Mineta H, et al. Autocrine epidermal growth factor receptor ligand production and cetuximab response in head and neck squamous cell carcinoma cell lines. J Cancer Res Clin Oncol. 2012;138:491–499. doi: 10.1007/s00432-011-1127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.