Abstract
Aurora kinases play an important role in chromosome alignment, segregation, and cytokinesis during mitosis. In the present study, we used a ligand-docking method to explore the novel scaffold of potential Aurora B inhibitors. One thousand compounds from our in-house compound library were screened against the Aurora B structure and one compound, (E)-3-((E)-4-(benzo[d][1,3]dioxol-5-yl)-2-oxobut-3-en-1-ylidene)indolin-2-one (designated herein as HOI-07) was selected for further study. HOI-07 potently inhibited in vitro Aurora B kinase activity in a dose-dependent manner, without obvious inhibition of another 49 kinases, including Aurora A. This compound suppressed Aurora B kinase activity in lung cancer cells, evidenced by the inhibition of the phosphorylation of histone H3 on Ser10 in a dose- and time-dependent manner. This inhibition resulted in apoptosis induction, G2/M arrest, polyploidy cells, and attenuation of cancer cell anchorage-independent growth. Moreover, knocking down the expression of Aurora B effectively reduced the sensitivity of cancer cells to HOI-07. Results of an in vivo xenograft mouse study showed that HOI-07 treatment effectively suppressed the growth of A549 xenografts, without affecting the body weight of mice. The expression of phospho-histone H3, phospho-Aurora B, and Ki-67 was also suppressed in the HOI-07 treatment group. Taken together, we identified HOI-07 as a specific Aurora B inhibitor, which deserves further investigation.
Keywords: Aurora B, antitumor activity, lung cancer
Introduction
Targeting the progression of mitosis is a highly successful strategy for anticancer treatment [1]. A closely related subgroup of three serine/threonine kinases, the Aurora kinases, are believed to play a key role in protein phosphorylation in mitosis and have been shown to contribute in the development and progression of cancer. In mammals, the Aurora kinase family comprises three members: Aurora A, B and C [2]. They display distinct roles during mitosis, which are reflected in their subcellular locations and functions. Aurora A is localized at the centrosome from the time of centrosome duplication through mitotic exit. It has been implicated in several processes required for the generation of bipolar spindle apparatus, including centrosome maturation and separation [3, 4]. Small molecule inhibition of Aurora A kinase activity causes defects in centrosome separation with the formation of characteristic monopolar spindles [5]. Aurora B is localized to the centromeres from the prophase to the metaphase-anaphase transition. Thereafter, it is localized to midzone spindle microtubules during the telophase and subsequently to midbody during cytokinesis [2, 3]. Aurora B is a chromosomal passenger protein in complex with the inner centromere proteins (INCENP), survivin, and borealin. During mitosis, as the “equatorial-kinase”, Aurora B is required for histone H3 phosphorylation, chromosome bi-orientation, the spindle assembly checkpoint, and cytokinesis [6, 7]. Inhibition of Aurora B kinase activity with small molecules leads to failure in cytokinesis and abnormal exit from mitosis, resulting in endoreduplication, polyploidy cells, and ultimately apoptosis [8, 9]. Aurora C is also a chromosomal passenger protein considered to have a similar sub-cellular location as Aurora B. It has been described only in mammals, where it is expressed in testis and certain tumor cell lines and localizes to spindle poles during late mitosis [2, 10].
Inhibition of Aurora kinases had been shown to be an effective strategy for anticancer therapy, and several Aurora inhibitors have been described, including VX-680 [11], Hesperadin [8], AZD1152 [12], and MLN8237 [13]. More than 30 small molecule Aurora kinase inhibitors are currently in different stages of preclinical and clinical development [14]; however, none have yet been approved by the FDA for clinical use.
Herein, we used a ligand-docking computational method to identify (E)-3-((E)-4-(benzo[d][1,3]dioxol-5-yl)-2-oxobut-3-en-1-ylidene)indolin-2-one (i.e., HOI-07) as a novel Aurora B kinase inhibitor. Biological testing further confirmed that HOI-07 selectively and potently inhibited Aurora B activity and exhibited anti-tumor activity in vitro and in vivo.
Materials and Methods
Reagents and materials
Compound HOI-07 was synthesized in-house following the reported protocol of similar compounds but with some modifications [15]. All cell lines were purchased from American Type Culture Collection (ATCC) and were cultured in monolayers at 37°C in a 5% CO2 incubator according to ATCC protocols. Cells were cytogenetically tested and authenticated before the cells were frozen. Each vial of frozen cells was thawed and maintained for about two months (10 passages). For transfection experiments, the jetPEI (Qbiogene, Inc., Montreal, Canada) transfection reagent was used following the manufacturer’s instructions.
Anchorage-independent cell transformation assay
Tumor cells were suspended in Basal Medium Eagle (BME) media and added to 0.6% agar, with different concentrations of HOI-07 in a base layer and a top layer of 0.3 % agar. The cultures were maintained at 37°C in a 5% CO2 incubator for 1 to 2 wk and then colonies were counted under a microscope using the Image-Pro Plus software (v.4) program (Media Cybernetics, Silver Spring, MD).
Cell cycle and apoptosis analyses
Cells were plated in 60-mm plates and treated or not treated with HOI-07 for the indicated time. At each time point, cells were fixed in 70% ethanol and stored at −20 °C for 24 h. After staining, cell cycle distribution or apoptosis was determined using a BD FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA).
MTS assay
To estimate the cytotoxicity of HOI-07, cells were seeded (8×103 cells per well) in 96-well plates and cultured overnight. Cells were then fed with fresh medium and treated with different doses of HOI-07. After culturing for various times, the cytotoxicity of HOI-07 was measured using an MTS assay kit (Promega, Madison, WI) according to the manufacturer’s instructions.
Western blot analysis
Proteins were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA), which were blocked with nonfat milk and hybridized with specific primary antibodies. The protein bands were visualized using an enhanced chemiluminescence reagent (GE Healthcare, Pittsburgh, PA) after hybridization with a horseradish peroxidase-conjugated secondary antibody.
Aurora B and Aurora A in vitro kinase assays
Inactive histone 3 proteins (1 µg) were used as the substrate for an in vitro kinase assay with 100 ng of active Aurora B or Aurora A kinase. Reactions were carried out in1 × kinase buffer (25 mM Tris-HCl pH 7.5, 5 mM beta-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4, 10 mM MgCl2 and 5 mM MnCl2) containing 100 µM ATP at 30°C for 30 min. Reactions were stopped and proteins detected by Western blotting.
Immunofluorescence microscopy
A549 cells were seeded in 4-chamber slides and cultured overnight. The cells were then treated with DMSO or HOI-07 (1 µM) for 48 h at 37°C. After treatment, the cells were washed with PBS and fixed with methanol for 12 h, followed by blocking with 3% PBS for 1 h. The cells were then incubated with an α-tubulin antibody (1:100) overnight and DNA was stained with 4’-6-diamidino-2-phenylindole (DAPI, Pierce, Rockford, IL) for 30 min at room temperature. The cells were evaluated by fluorescent microscopy.
Hematoxylin–eosin staining and immunohistochemistry
Tumor tissues from mice were embedded in a paraffin block and subjected to hematoxylin and eosin (H&E) staining and immunohistochemistry. Tumor tissues were deparaffinized and hydrated, then permeabilized with 0.5% Triton X-100/1 PBS for 10 min, hybridized with phospho-histone H3 (1:50), phospho-Aurora B (1:50), and Ki-67 (1:500) as the primary antibodies and an HRP-conjugated goat anti-rabbit antibody was used as the secondary antibody. After developing with 3,30-diaminobenzidine, the sections were counterstained with hematoxylin. All sections were observed by microscope (400X magnification) and the Image-Pro Plus software (v.4) program (Media Cybernetics).
Xenograft mouse model
Athymic nude mice [Cr:NIH (S), NIH Swiss nude, 6-wk old] were obtained from Harlan Laboratories and maintained under “specific pathogen-free” conditions based on the guidelines established by the University of Minnesota Institutional Animal Care and Use Committee. Mice were divided into different groups (n = 10 in each group). A549 lung cancer cells (3 × 106/0.1 mL) were injected subcutaneously into the right flank of each mouse. HOI-07 was prepared once a week and protected from light and kept at 4°C. Compound or vehicle control was administered by i.p. injection twice a week. Tumor volumes and body weights were measured.
Statistical analysis
All quantitative data are expressed as mean values ± S.D. or S.E. and significant differences were determined by Student’s t test or by one-way ANOVA. A probability value of p < 0.05 was used as the criterion for statistical significance.
Results
The predicted binding mode of HOI-07 with Aurora B and cytotoxicity
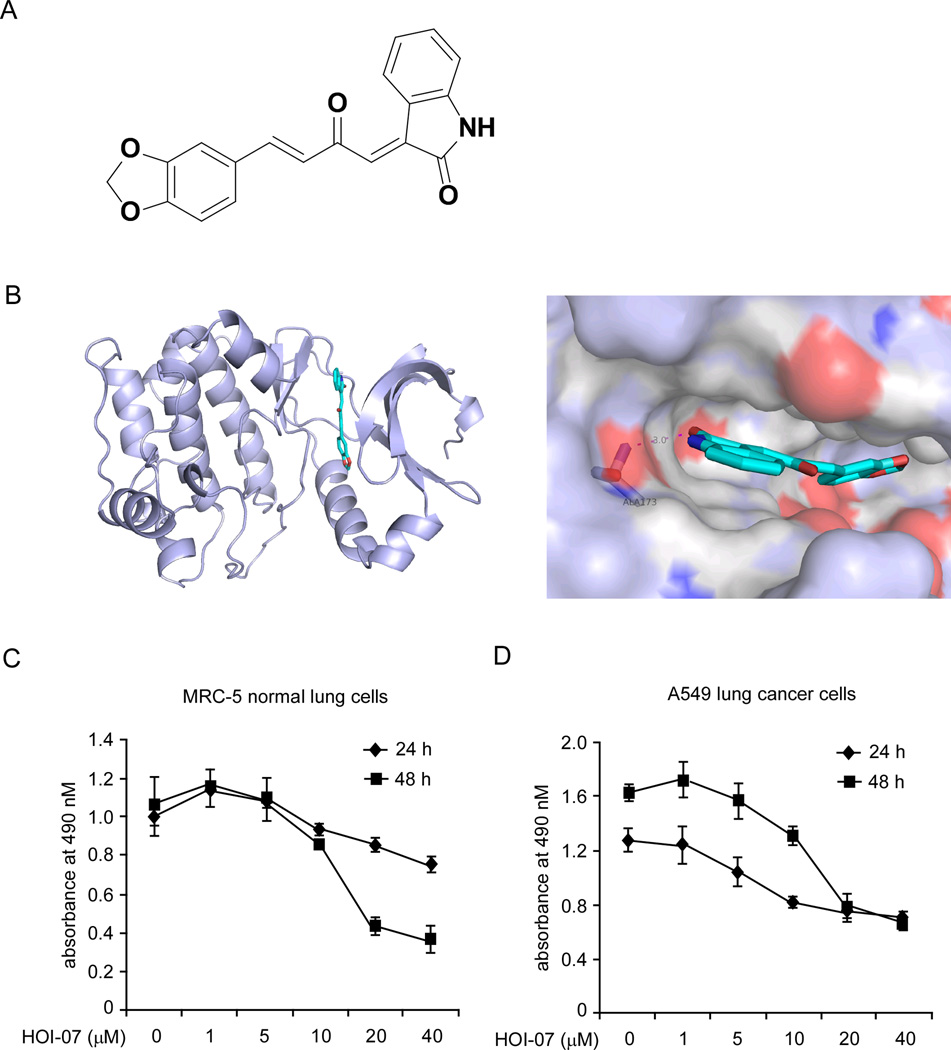
With the purpose of identifying a novel Aurora B kinase inhibitor, we performed an intensive molecular docking analysis using Glide v5.7 [16] to screen our in-house library of compounds against the structure of Aurora B. HOI-07 (Fig. 1A) was identified as a potential Aurora B inhibitor based on its high docking score. HOI-07 is a novel compound synthesized in our laboratory. The predicted binding mode of HOI-07 and Aurora B showed that HOI-07 occupies the ATP-binding site and forms a hydrogen bond with amino acid Ala173 in the hinge linker region, which is quite similar to the binding of other Aurora B kinase inhibitors (Fig. 1B). We then examined the toxicity of HOI-07 on both MRC-5 normal lung cells (Fig. 1C) and A549 lung cancer cells (Fig. 1D). The result showed that HOI-07 possessed substantial toxicity to both cell types at concentrations greater than 10 µM. At a concentration of 1 µM or less, no obvious cytotoxic effects were observed in either cell line. At 5 µM, HOI-07 treatment for 48 h resulted in a weak toxicity toward A549 cancer cells, but not to MRC-5 normal cells.
Figure 1.
Computational predicted binding mode of HOI-07 with Aurora B and the cytotoxicity of HOI-07. A, chemical structure of HOI-07. B, computational predicted binding mode of HOI-07 with Aurora B. Left: HOI-07 is docked, using Glide, into the binding pocket of Aurora B. Aurora B is shown as light blue cartoon and HOI-07 is shown as sticks. Right: Interaction between HOI-07 and Aurora B. HOI-07 forms a hydrogen bond with Ala173. C and D, cytotoxicity of HOI07 on MRC-5 normal lung cells (C) and A549 lung cancer cells (D). No obvious cytotoxicity by HOI-07 was observed in eitherMRC-5 and A549 cells at concentrations below 1 µM.
HOI-07 inhibits anchorage-independent growth of human lung cancer cells
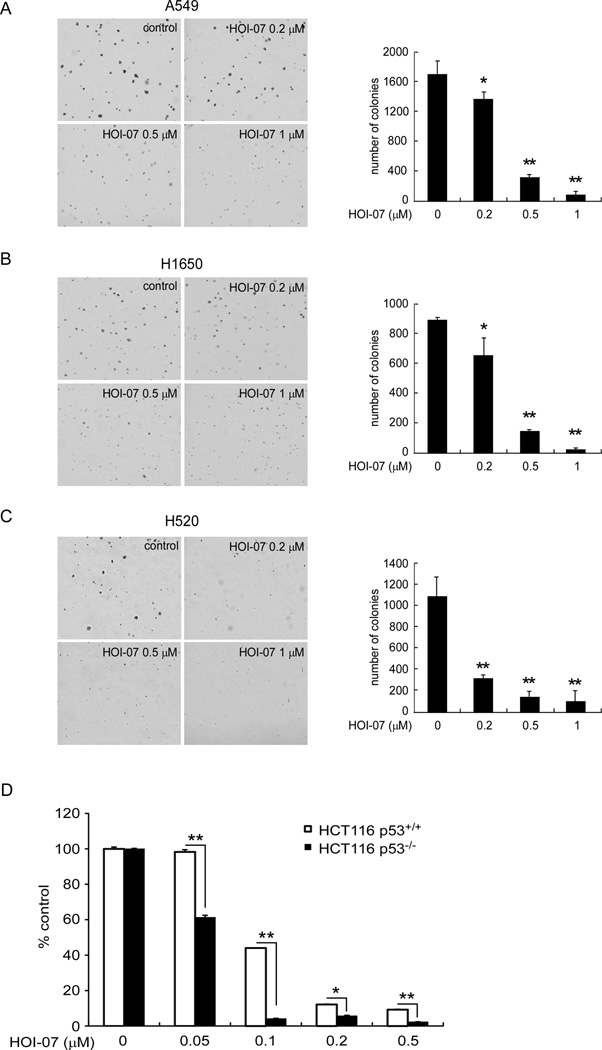
We then examined the effect of HOI-07 treatment on anchorage-independent growth of human lung cancer cells, including A549 (Fig. 2A), H1650 (Fig. 2B), and H520 (Fig. 2C) cells. Treatment of these cells with HOI-07 potently inhibited their anchorage-independent growth in a concentration-dependent manner. HOI-07 at 0.5 or 1 µM caused a decrease of more than 80 or 90% compared to control in all cell lines detected. The inhibition by HOI-07 was not due to cytotoxicity because no toxicity was observed at 1 µM HOI-07 (Fig. 1). Therefore, the results indicated that HOI-07 is a novel and very potent compound possessing anti-tumor activity and deserves further investigation. We also examined the effect of HOI-07 in a pair of colon cancer cell lines, p53 wildtype (HCT116 p53+/+) and p53-deficient (HCT116 p53−/−) cells to determine whether the sensitivity of cells to HOI-07 directly correlates with the status of p53 in cells. Soft agar assay results showed that HCT116 p53−/− cells are more sensitive to HOI-07 than HCT116 p53+/+ cells (Fig. 2D). HOI-07 at 0.05 µM caused a 40% inhibition of growth of HCT116 p53−/− cells, whereas no inhibition was observed in the wildtype p53 cells. Moreover, 0.1 µM HOI-07 inhibited growth by more than 95% in HCT116−/− cells but only 55% inhibition in HCT116 p53+/+ cells. These data are consistent with a previous report [17] showing that p53−/− cells are more sensitive to Aurora B inhibitors compared to p53+/+ cells.
Figure 2.
HOI-07 inhibits the anchorage-independent growth of a panel of NSCLC cell lines, including A549 cells (A), H1650 cells (B), and H520 cells (C). The asterisk indicates a significant (* p < 0.05, **p < 0.01) decrease in colony formation in cells treated with HOI-07 compared with the DMSO-treated group. D, HCT116 p53−/− cells are more sensitive to the effects of HOI-07 compared to wildtype HCT116 p53+/+ cells. The asterisk indicates a significant (* p < 0.05, **p < 0.01) decrease in anchorage dependent growth in HCT116 p53+/+ colon cancer cells compared to HCT p53−/− cells.
HOI-07 is a potent inhibitor of Aurora B, but not Aurora A
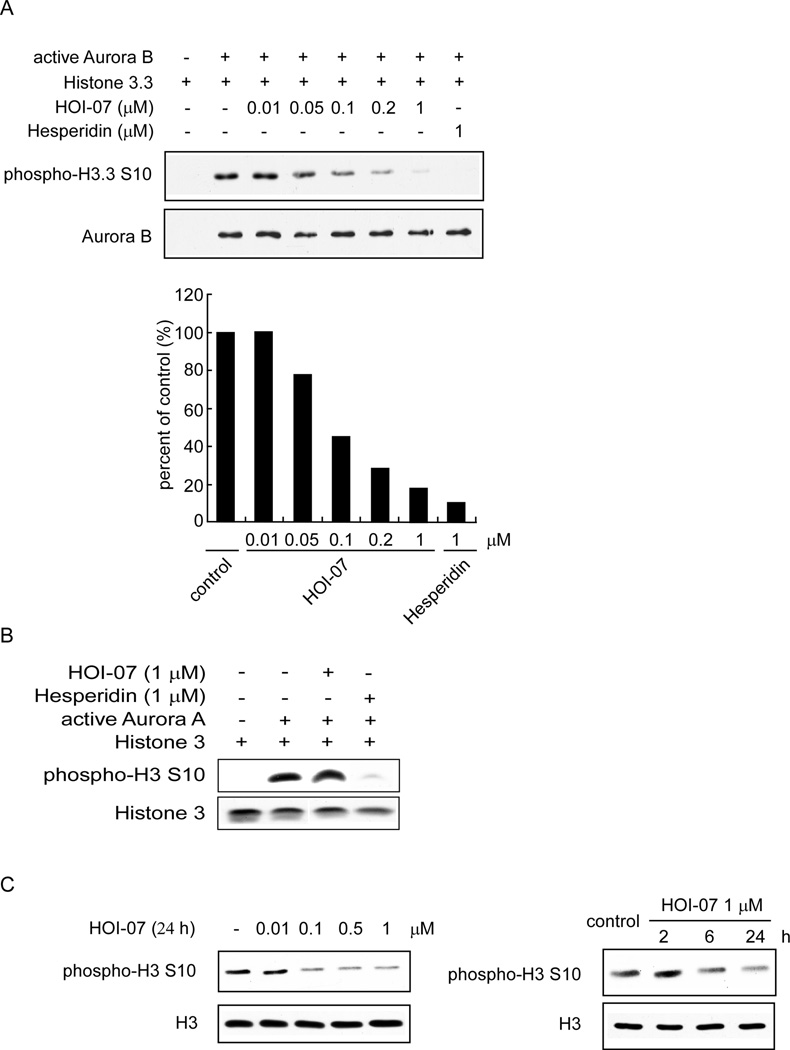
We then used an in vitro kinase assay with a recombinant Aurora B protein and various concentrations of HOI-07 to determine whether HOI-07 could inhibit Aurora B kinase activity. Results indicated that the phosphorylation of histone H3 on Ser10, an Aurora B substrate, was strongly inhibited by HOI-07 in a concentration-dependent manner (Fig. 3A). For example, 0.05 µM HOI-07 caused a 22% inhibition of Aurora B kinase activity and 0.1 µM HOI-07 resulted in a 50% inhibition. At a concentration of 1 µM, only a weak histone H3 (Ser10) band was observed. In addition, we also examined the effect of HOI-07 on Aurora A kinase activity using an in vitro kinase assay and no effect on histone H3 (Ser10) phosphorylation was observed at 1 µM HOI-07 compared with control (Fig. 3B). These results continued to support HOI-07 as a potent inhibitor of Aurora B kinase activity.
Figure 3.
HOI-07 inhibits Aurora B kinase activity both in vitro and ex vivo. A, HOI-07 inhibits Aurora B in vitro kinase activity in a concentration-dependent manner. An inactive histone 3.3 protein was used as the substrate for an in vitro kinase assay with active Aurora B and 100 µM ATP. Proteins were resolved by SDS-PAGE and detected by Western blotting. B, HOI-07 has no effect on Aurora A in vitro kinase activity. C, HOI-07 inhibits phosphorylation of the Aurora B downstream target, histone H3 (Ser10) in human lung cancer cells in a time- (left) and dose-dependent (right) manner. A549 cells were treated with HOI-07 at the indicated concentration for 24 h or at 1 µM and harvested at the indicated time points. Cells were harvested and histone proteins were extracted and subjected to Western blot analysis.
HOI-07 blocks phosphorylation of histone H3 on Ser10 in lung cancer cells
To provide evidence showing that HOI-07 is acting by inhibiting Aurora B in cancer cells, we examined its effects on Aurora B downstream signaling. Evidence has shown that histone H3 is a direct downstream target of the Aurora kinases. Phosphorylation of a highly conserved serine residue (Ser10) in histone H3 is thought to be crucial for entry into mitosis. Our results showed that HOI-07 suppresses histone H3 phosphorylation on Ser10 in cancer cells in a dose- and time-dependent manner (Fig. 3C), suggesting that the Aurora B kinases are involved in the antitumor activity of HOI-07.
HOI07 induces polyploidy, cell cycle arrest, and apoptosis in lung cancer cells
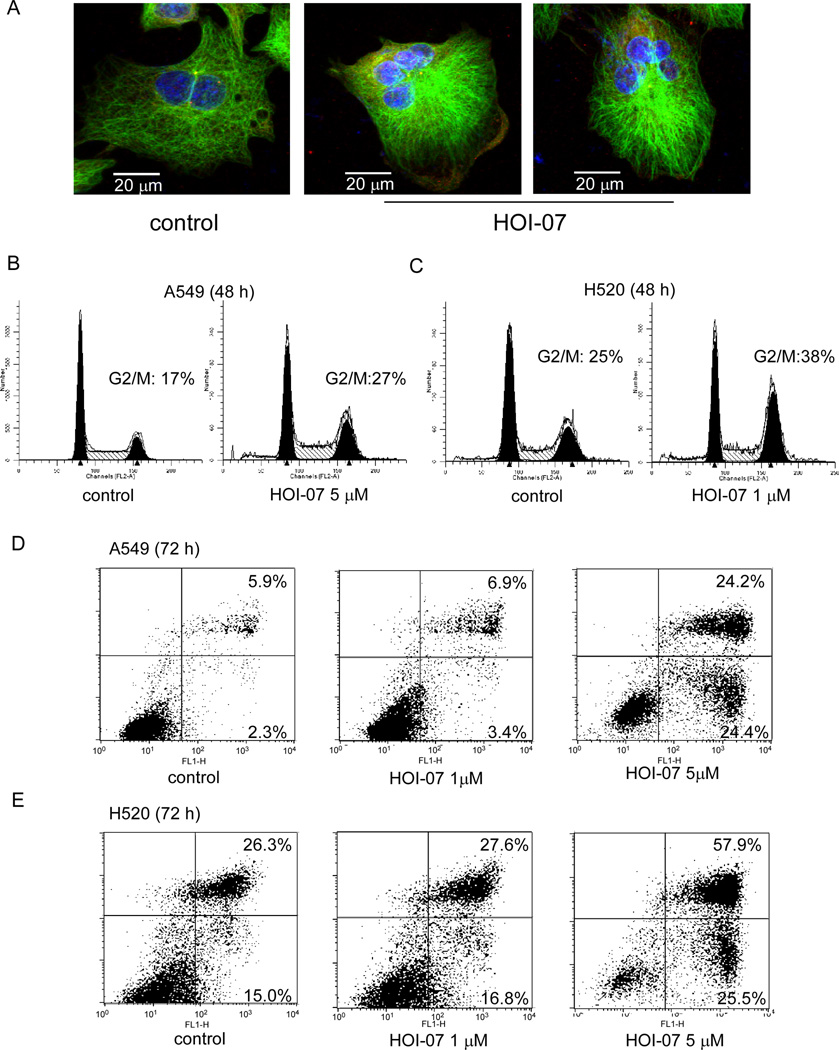
Aurora B inhibition leads to failure in cytokinesis and abnormal exit from mitosis, which could result in polyploidy cells, cell cycle arrest, and ultimately, apoptosis. The ability to induce polyploidy cells was further examined in A549 cells treated or not treated with HOI-07. Immunofluorescence results showed that treatment of A549 cells with 1 µM HOI-07 caused the induction of polyploidy cells, whereas no polyploidy cells were observed in control cells (Fig. 4A). In addition, HOI-07 treatment for 48 h caused an increase in the number of A549 (Fig. 4B) and H520 cells (Fig. 4C) occupying the G2/M phase. Moreover, exposure of these cells to HOI-07 for 72 h induced apoptosis as measured by Annexin V/PI staining (Fig. 4D–E). For example, exposure to 5 µM HOI-07 induced 48 or 82% apoptosis in A549 cells and H520 cells, compared to 8 or 38% in untreated control cells, respectively. These results demonstrated that HOI-07, as an Aurora B inhibitor, induces polyploidy, apoptosis and G2/M phase arrest in cancer cells and thus inhibits the growth of cancer cells.
Figure 4.
HOI-07 induces polyploidy, apoptosis and G2/M phase cell cycle arrest in lung cancer cells. A, HOI-07 induces polyploidy in A549 cells. Cells were treated with HOI-07 (1 µM) for 48 h and then were evaluated by immunofluorescence assay. Scale bar indicates 20 µm (600X). B and C, HOI-07 induces G2/M arrest in lung cancer cells. Cell cycle analysis by flow cytometry of A549 cells (B) and H520 cells (C) treated with HOI-07. D and E, HOI-07 induces apoptosis in lung cancer cells. Flow cytometry analysis of apoptosis. A549 cells (D) and H520 cells (E) were incubated with the indicated concentration of HOI-07 for 72 h, then collected and apoptosis was detected by Annexin V and PI staining.
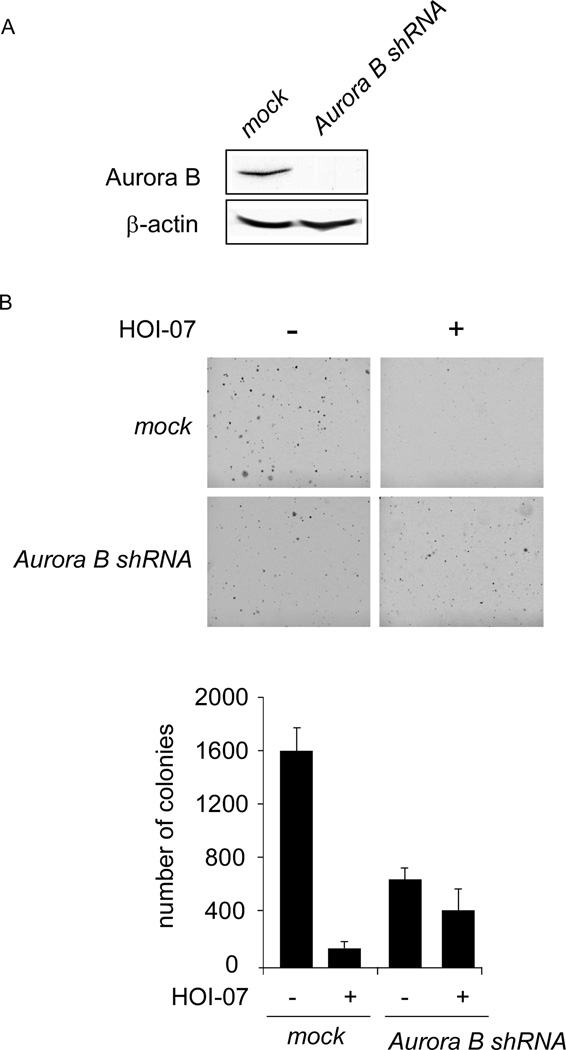
Knockdown of Aurora B decreases the sensitivity of cancer cells to HOI-07
We then examined whether knocking down Aurora B expression influences the sensitivity of A549 cancer cells to HOI-07. The efficiency of shRNA knockdown was examined and the expression of Aurora B was obviously decreased after shRNA transfection (Fig.5A). Moreover, the growth of cells in soft agar also decreased after transfection compared with the mock group (Fig. 5B). HOI-07 (0.5 µM) inhibited anchorage-independent growth of A549 cells transfected with mock shRNA by about 90%. In contrast, the inhibition was less than 40% in A549 cells transfected with Aurora B shRNA, indicating that A549 cells transfected with Aurora B shRNA were more resistant to HOI-07 treatment (Fig. 5B). These results suggested that Aurora B plays an important role in the sensitivity of A549 cells to the anti-proliferative effects of HOI-07.
Figure 5.
Knockdown of Aurora B in A549 cells decreases their sensitivity to HOI-07. A, efficiency of Aurora B shRNA in A549 cells. B, anchorage-independent growth of A549 cells transfected with mock shRNA or Aurora B shRNA, treated or not treated with HOI-07.
Kinase profile result
In order to identify other potential targets of HOI-07, a kinase profile assay was performed by Millipore, in which 49 kinases were examined. Only Akt2 activity was suppressed more than 50% after 5 µM HOI-07 treatment, compared with an untreated control (Supplementary Table 1). In addition, the kinase activity of Aurora A was decreased less than 25% at 5 µM HOI-07.
HOI-07 inhibits Akt1 and 2 kinase activities in vitro, but not in cells
According to the kinase profiler assay results, Akt2 also might be a potential target of HOI-07. We performed Akt1 and Akt2 in vitro kinase assays and results indicated that HOI-07 inhibited both Akt1 (Supplementary Fig. 1A) and Akt2 (Supplementary Fig. 1B) in vitro kinase activities in a concentration-dependent manner. Treatment with 5 µM HOI-07 caused a 32 or 52% inhibition of Akt1 or Akt2 activity, respectively, which is quite consistent with the kinase profiler assay result. However, Western blot analysis of A549 cells (Supplementary Fig. 1C) and H520 cells (Supplementary Fig. 1D) treated with HOI-07 showed that HOI-07 treatment had no effect on the phosphorylation of Akt or its downstream signaling molecules, including Akt (Ser473), GSK3β (Ser9), p70S6K (Thr389) or S6 (Ser235,236). These results indicated that although HOI-07 can inhibit Akt1 and Akt2 in vitro kinase activity, it has no effect on Akt activity in cancer cells, suggesting that Akt1 and Akt2 might not be major antitumor targets of HOI-07.
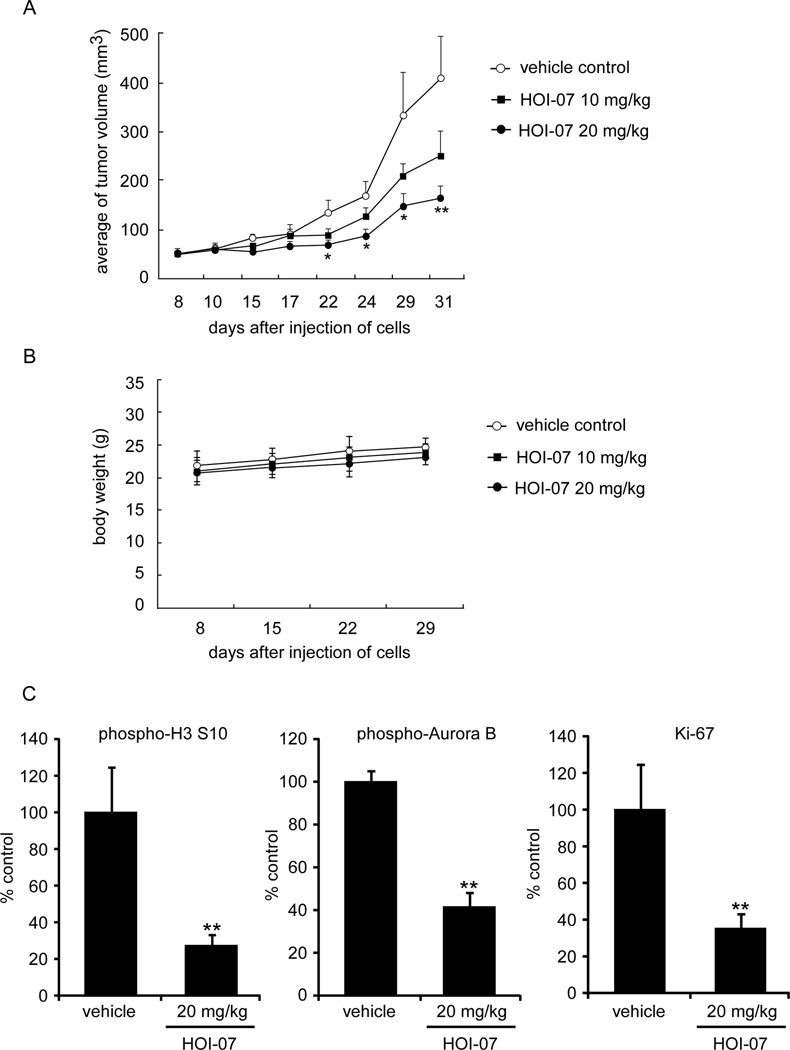
HOI-07 suppresses the growth of A549 xenografts in vivo
We then evaluated the ability of HOI-07 to inhibit the growth of human A549 lung cancer cell xenografts in athymic nude mice. Tumor volumes were measured twice a week and mouse weights were determined once a week. HOI-07 caused a marked reduction in tumor size in the human A549 xenograft model (Fig. 6A). In mice treated with HOI-07 at 20 mg/kg, twice a week intraperitoneally, mean tumor volumes were reduced to 164 mm3 in comparison with control group (408 mm3) (p < 0.01). In addition, no obvious loss of body weight was observed (Fig. 6B), indicating that HOI-07 is well tolerated by the mice. Moreover, the effects of HOI-07 on phosphorylation of histone H3 and Aurora B and a tumor proliferation marker were evaluated by immunohistochemistry and H&E staining of A549 tumor tissues after the 31 days of treatment. The expression of all three, including Ki-67, was markedly decreased by HOI-07 (Fig. 6C). These results indicated that HOI-07 suppressed tumor growth in vivo.
Figure 6.
Effect of HOI-07 on lung cancer growth in an A549 xenograft mouse model. A, HOI-07 significantly suppresses cancer growth in an A549 xenograft mouse model. The average tumor volume of vehicle-treated control mice (n = 10) and HOI-07-treated mice (n = 10 in each group) plotted over 31 days after tumor cell injection. The asterisks indicate a significant inhibition by HOI-07 on tumor growth (*, p < 0.05; **, p < 0.01). B, HOI-07 has no effect on mouse body weight. Body weights from treated or untreated groups of mice were measured once a week. C. H&E staining and immunohistochemical analysis of tumor tissues. Treated or untreated groups of mice were euthanized and tumors were extracted. Tumor tissue slides were prepared with paraffin sections after fixation with formalin and then stained with H&E or anti-Ki-67. Expression of Ki-67 was visualized with a light microscope. Stained cells were counted from 5 separate areas on the slide and an average of 3 samples was calculated per group. Data are expressed as mean percent of control ± S.D. The asterisk indicates a significant decrease in phosphorylated histone H3 (Ser10; left), phosphorylated Aurora B (middle) and Ki-67 expression (right).
Discussion
Cancer is a disease that is characterized by uncontrolled proliferation of abnormal cells. Modulation of atypical cell cycle regulation would therefore be a valuable therapeutic strategy for different types of tumors. Aurora kinases regulate many processes during cell division. Aurora B kinases are essential for chromosome condensation, kinetochore function, cytokinesis and the proper function of the spindle-assembly checkpoint when spindle tension is perturbed [2, 6, 9, 18]. Accumulating evidence has shown that Aurora B is implicated in cancer. For example, the expression of Aurora B is frequently elevated in various types of cancer, including NSCLC, colon, and prostate [19–21]. The evidence linking Aurora overexpression and malignancy has generated significant interest in the development of small molecule inhibitors against these proteins.
Oxindoles (indolin-2-ones) are an important class of molecules, which are known to possess a wide variety of biological properties, and in particular, as protein kinase inhibitors [22]. In the present study, HOI-07 was identified, using molecular docking methods, as an Aurora B kinase inhibitor. The structure of the molecule HOI-07 is oxindole based, however, it is structurally different from reported oxindole-containing Aurora inhibitors. Up until now, only one molecule, which is similar to HOI-07, has been reported in the literature, but without any known biological activity [15].
The results of an Aurora B kinase assay clearly showed that HOI-07 potently and dose-dependently inhibited Aurora B kinase in vitro activity, indicating that this compound is a potent and novel Aurora B inhibitor. In addition, HOI-07 had no effect on Aurora A kinase in vitro activity at the same concentration. A previous report showed that cells treated with an Aurora kinase inhibitor entered and exited mitosis without cell division, and then proceeded to a second S phase [9]. Therefore, we further examined the effect of HOI-07 on cancer cells and results showed that HOI-07 suppressed cell growth in a panel of NSCLC cell line. The inhibition was associated with induction of polyploidy cells, accumulation of G2/M cells, as well as apoptosis, which is consistent with Aurora B inhibition. Moreover, knocking down Aurora B expression in A549 cells decreased their sensitivity to HOI-07, indicating that Aurora B plays an important role in the antitumor activity of HOI-07. An in vivo xenograft study also indicated that HOI-07 effectively suppressed tumor growth without affecting mouse body weight and was accompanied with a decrease in Ki-67 expression, which is a marker of proliferation. HOI-07 treatment also decreased phosphorylation of histone H3 (Ser10) and Aurora B in tumor tissues.
According to our kinase profiling results, in which 49 kinases treated or not treated with HOI-07 were examined, Akt might also be a potential target of HOI-07. Further experimental results showed that HOI-07 inhibited Akt1 and Akt2 kinase activity in vitro at a higher concentration (1 µM or more) than that required for Aurora B inhibition (1 µM or lower). However, it had no effect on Akt downstream signaling in cancer cells, indicating that Akt might not be a major target of HOI-07. Together, these results indicated that HOI-07 is a potent and selective inhibitor of Aurora B. More than 30 Aurora kinase inhibitors are in different stages of preclinical and clinical development currently [23]. Most of the Aurora inhibitors are pan-Aurora inhibitors or Aurora A selective inhibitors, whereas only limited Aurora B selective inhibitors have been reported, including compound AZD1152, which also inhibits Aurora A at higher concentrations (IC50 of 0.37 vs 1368 nM for Aurora B and Aurora A kinases, respectively [23, 24]. Here, we identified HOI-07 as an Aurora kinase inhibitor that is specific for the Aurora B isoform. Previous reports showed that treatment of cells with dual inhibitors of Aurora A and B results in mitotic defect resembling inactivation of Aurora B. This might be caused by the potential mechanism by which Aurora B is responsible for mitotic arrest in the absence of Aurora A [18]. Meanwhile, several studies demonstrated that Aurora A and Aurora B should be distinguished as two distinctive therapeutic objectives that can be targeted independently [25, 26]. Thus, our identification of HOI-07 as a specific Aurora B inhibitor provides one more useful tool to study the phenomenon of Aurora B specific inhibition and to distinguish the potential differences between inactivation of Aurora A and Aurora B. Our work also presents a novel Aurora B selective inhibitor, which suppresses tumor growth both in vitro and in vivo and deserves further investigation and development.
Supplementary Material
Acknowledgments
This work was supported by The Hormel Foundation and National Institutes of Health grants R37 CA081064, CA120388, ES016548, CA0227501 and National Cancer Institute Contract No. HHSN-261200533001C-NO1-CN-53301. The authors thank Todd Schuster and Alyssa Langerud at The Hormel Institute for their technical support. We also thank Tonya M. Poorman at The Hormel Institute, University of Minnesota for help submitting our manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Jackson JR, et al. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7(2):107–117. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 2.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4(11):842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 3.Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24(32):5005–5015. doi: 10.1038/sj.onc.1208752. [DOI] [PubMed] [Google Scholar]
- 4.Barr AR, Gergely F. Aurora-A: the maker and breaker of spindle poles. J Cell Sci. 2007;120(Pt 17):2987–2996. doi: 10.1242/jcs.013136. [DOI] [PubMed] [Google Scholar]
- 5.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5(1):42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 6.Kallio MJ, et al. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol. 2002;12(11):900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 7.Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12(23):6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- 8.Hauf S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ditchfield C, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161(2):267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HL, et al. Overexpression of an Aurora-C kinase-deficient mutant disrupts the Aurora-B/INCENP complex and induces polyploidy. J Biomed Sci. 2005;12(2):297–310. doi: 10.1007/s11373-005-0980-0. [DOI] [PubMed] [Google Scholar]
- 11.Harrington EA, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10(3):262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, et al. AZD1152, a novel and selective aurora B kinase inhibitor, induces growth arrest, apoptosis, and sensitization for tubulin depolymerizing agent or topoisomerase II inhibitor in human acute leukemia cells in vitro and in vivo. Blood. 2007;110(6):2034–2040. doi: 10.1182/blood-2007-02-073700. [DOI] [PubMed] [Google Scholar]
- 13.Gorgun G, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115(25):5202–5213. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollareddy M, et al. Aurora kinase inhibitors: Progress towards the clinic. Invest New Drugs. 2012 doi: 10.1007/s10637-012-9798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beccalli EM, Pilati T MA. Synthesis of the carbazole alkaloids hyellazole and 6-chlorohyellazole and related derivatives. J. Chem. Soc., Perkin Trans. 1994;1:579–587. [Google Scholar]
- 16.Friesner RA, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 17.Ditchfield C, et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. The Journal of cell biology. 2003;161(2):267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H, et al. Mitotic requirement for aurora A kinase is bypassed in the absence of aurora B kinase. FEBS Lett. 2005;579(16):3385–3391. doi: 10.1016/j.febslet.2005.04.080. [DOI] [PubMed] [Google Scholar]
- 19.Tatsuka M, et al. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58(21):4811–4816. [PubMed] [Google Scholar]
- 20.Vischioni B, et al. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5(11):2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 21.Chieffi P, et al. Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate. 2006;66(3):326–333. doi: 10.1002/pros.20345. [DOI] [PubMed] [Google Scholar]
- 22.Millemaggi A TR. 3-Alkenyl-oxindoles: Natural Products, Pharmaceuticals, and Recent Synthetic Advances in Tandem/Telescoped Approaches. European Journal of Organic Chemistry. 2010;(24):4527–4547. [Google Scholar]
- 23.Schwartz GK, et al. Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients with advanced solid tumors. Invest New Drugs. 2012 doi: 10.1007/s10637-012-9825-7. [DOI] [PubMed] [Google Scholar]
- 24.Bates SM. The risks of informed decision-making. Blood. 2011;118(8):2030–2031. doi: 10.1182/blood-2011-06-361329. [DOI] [PubMed] [Google Scholar]
- 25.Girdler F, et al. Validating Aurora B as an anti-cancer drug target. J Cell Sci. 2006;119(Pt 17):3664–3675. doi: 10.1242/jcs.03145. [DOI] [PubMed] [Google Scholar]
- 26.Warner SL, et al. Comparing Aurora A and Aurora B as molecular targets for growth inhibition of pancreatic cancer cells. Mol Cancer Ther. 2006;5(10):2450–2458. doi: 10.1158/1535-7163.MCT-06-0202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.