Abstract
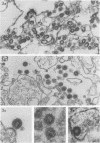
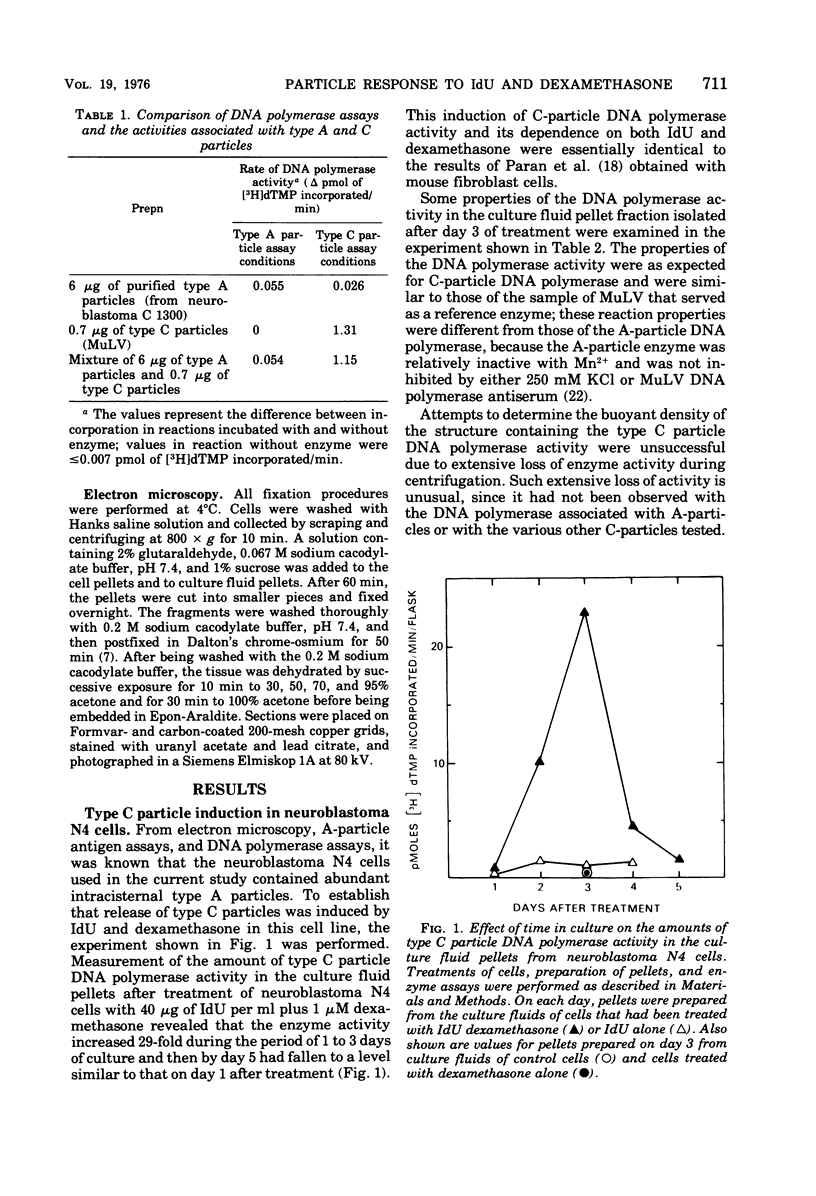
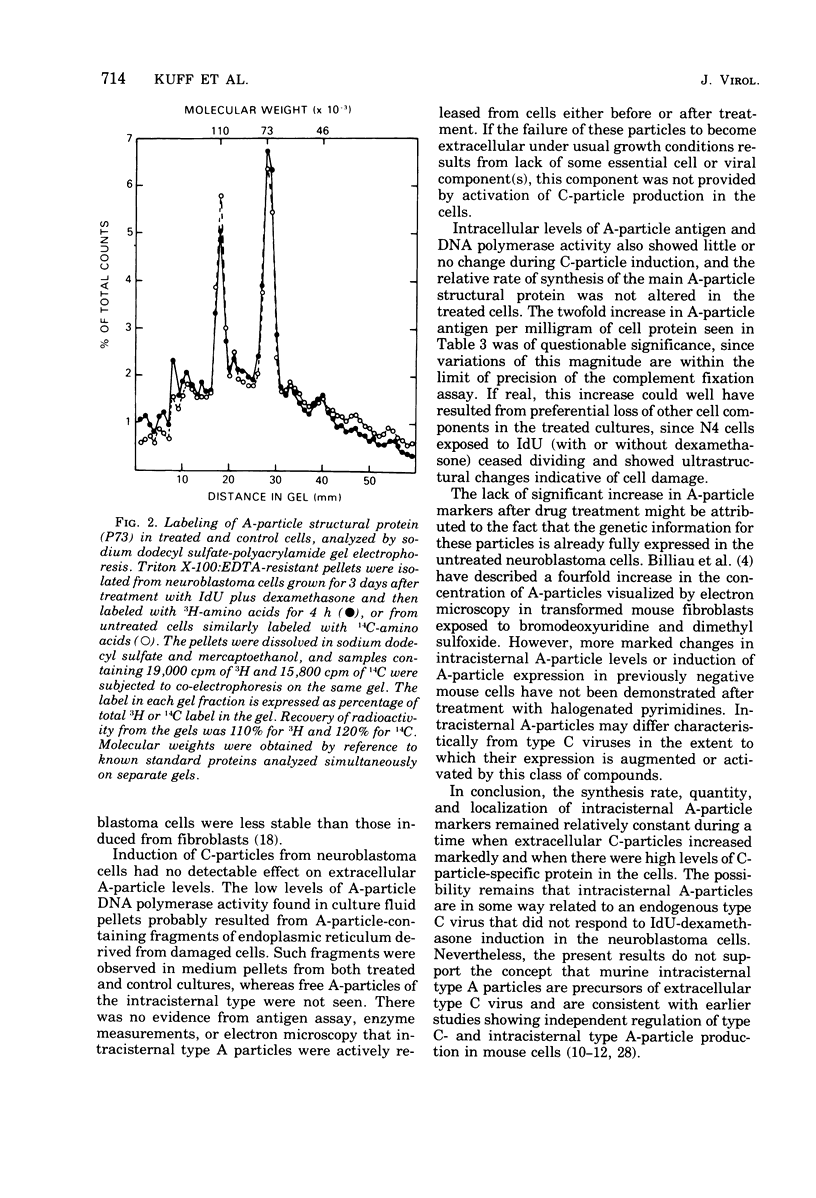
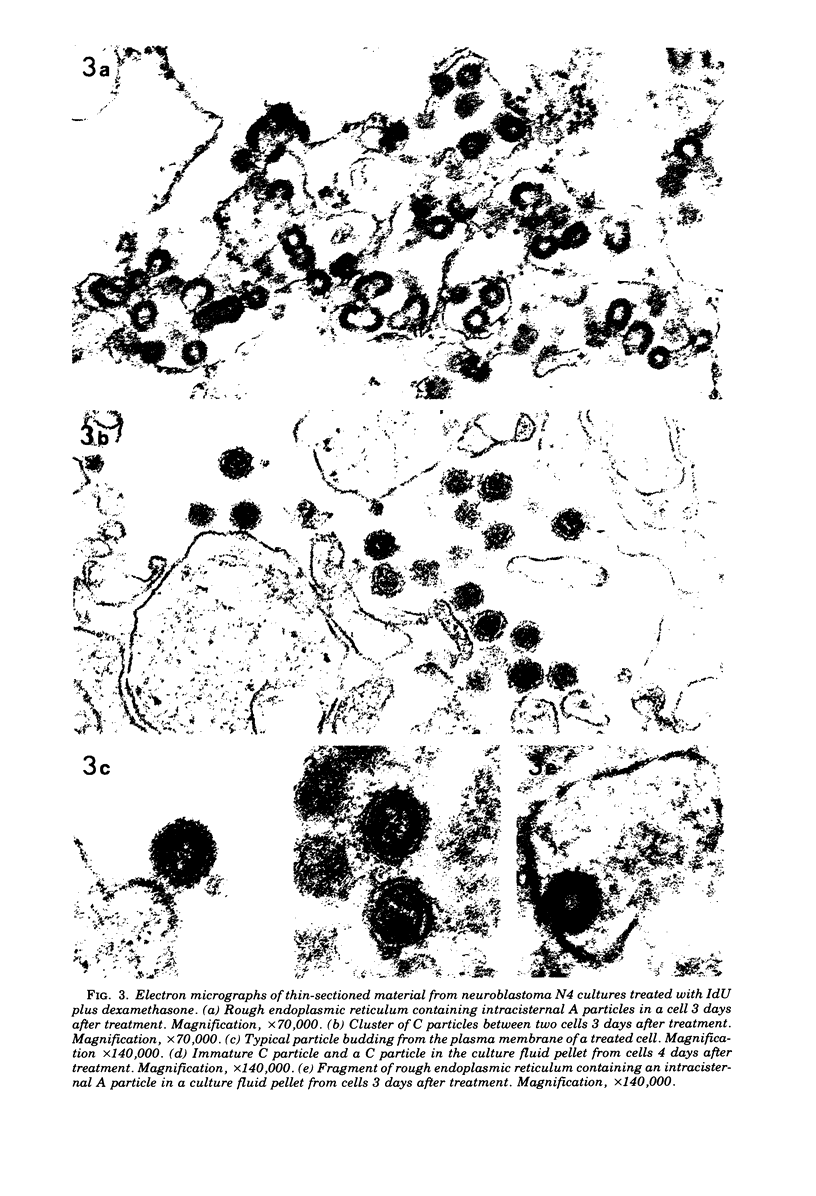
Mouse neuroblastoma cells containing intracisternal type A particles were treated with iododeoxyuridine and dexamethasone to induce the release of type C oncornavirus particles. For 5 days after treatment, antigenic markers and DNA polymerase activities specific to particles of each of the two types were assayed in the cells and in pellets obtained by high-speed centrifugation of the culture fluid. There was a marked release of C-particle antigen (p30) and DNA polymerase activity in extracellular particulate form, reaching a maximum on day 3 after treatment and falling thereafter. In contrast, no extracellular A-particle antigen was detected, and A-particle-specific DNA polymerase activity in the medium pellets did not increase from the original very low level. Electron microscopy confirmed the presence of free type C virus particles, but not intracisternal type A particles, in the culture fluid. Although intracellular levels of C-particle antigen rose 20- to 30-fold per milligram of cell protein, intracellular A-particle antigen and DNA polymerase activity did not vary more than two-fold. The relative rate of A-particle synthesis in the treated cells, as judged by incorporation of radioactive amino acids into the major structural protein (P73), was also unchanged over the period of observation. Thus, the induction of type C virus particle formation in cultured neuroblastoma cells had no detectable effect on the quantity, synthesis rate, or location of intracisternal type A particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Biczysko W., Solter D., Graham C., Koprowski H. Synthesis of endogenous type-A virus particles in parthenogenetically stimulated mouse eggs. J Natl Cancer Inst. 1974 Feb;52(2):483–489. doi: 10.1093/jnci/52.2.483. [DOI] [PubMed] [Google Scholar]
- Billiau A., Sobis H., De Somer P. Influence of interferon on virus particle formation in different oncornavirus carrier cell lines. Int J Cancer. 1973 Nov 15;12(3):646–653. doi: 10.1002/ijc.2910120313. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- DALTON A. J., POTTER M., MERWIN R. M. Some ultrastructural characteristics of a series of primary and transplanted plasma-cell tumors of the mouse. J Natl Cancer Inst. 1961 May;26:1221–1267. [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Rongey R. W., Estes J. D., Turner H. C., Huebner R. J. C-type RNA tumour virus genome expression in wild house mice. Nature. 1971 Aug 27;232(5313):617–620. doi: 10.1038/232617a0. [DOI] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakefuda T., Roberts E., Suntzeff V. Electron microscopic study of methylcholanthrene-induced epidermal carcinogenesis in mice: mitochondrial dense bodies and intracisternal A-particles. Cancer Res. 1970 Apr;30(4):1011–1019. [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Synthesis and turnover of intracisternal A-particle structural protein in cultured neuroblastoma cells. J Biol Chem. 1975 Jul 10;250(13):5192–5199. [PubMed] [Google Scholar]
- Minna J. D., Gazdar A. F., Iverson G. M., Marshall T. H., STORMBERG K., Wilson S. H. Oncornavirus expression in human x mouse hybrid cells segregating mouse chromosomes. Proc Natl Acad Sci U S A. 1974 May;71(5):1695–1700. doi: 10.1073/pnas.71.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J. D., Lueders K. K., Kuff E. L. Expression of genes for intracisternal A-particle antigen in somatic cell hybrids. J Natl Cancer Inst. 1974 Apr;52(4):1211–1217. doi: 10.1093/jnci/52.4.1211. [DOI] [PubMed] [Google Scholar]
- Paran M., Gallo R. C., Richardson L. S., Wu A. M. Adrenal corticosteroids enhance production of type-C virus induced by 5-iodo-2'-deoxyuridine from cultured mouse fibroblasts. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2391–2395. doi: 10.1073/pnas.70.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Baenziger N. L., Dobbertin D. C., Thach R. E. Characterization of DNA polymerase and RNA associated with A-type particles from murine myeloma cells. J Virol. 1975 Feb;15(2):407–415. doi: 10.1128/jvi.15.2.407-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. H., Bohn E. W., Matsukage A., Lueders K. K., Kuff E. L. Studies on the relationship between deoxyribonucleic acid polymerase activity and intracisternal A-type particles in mouse myeloma. Biochemistry. 1974 Mar 12;13(6):1087–1094. doi: 10.1021/bi00703a005. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wivel N. A., Smith G. H. Distribution of intracisternal A-particles in a variety of normal and neoplastic mouse tissues. Int J Cancer. 1971 Jan 15;7(1):167–175. doi: 10.1002/ijc.2910070119. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Trainor C. D., Gallo R. C. Murine intracisternal type A particles: a biochemical characterization. J Virol. 1975 Oct;16(4):887–896. doi: 10.1128/jvi.16.4.887-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Analysis of high-molecular-weight ribonucleic acid associated with intracisternal A particles. J Virol. 1973 Feb;11(2):287–298. doi: 10.1128/jvi.11.2.287-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Characterization of an endogenous RNA-dependent DNA polymerase associated with murine intracisternal A particles. J Virol. 1974 Mar;13(3):712–720. doi: 10.1128/jvi.13.3.712-720.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuyanagi Y., Ephrussi B. Behavior of three types of ribovirus-like particles in segregating hamster times mouse somatic hybrids. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4575–4578. doi: 10.1073/pnas.71.11.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]