Abstract
Persistence of Human Papillomavirus (HPV) infection is necessary for the development of cervical cancer. Additionally, infection with HPV is implicated in the majority of cases of other genital tract malignancies including vulvar, penile, and vaginal cancer. HPV testing and vaccination are a routine part of OB/GYN clinical practice. With an enhanced public awareness of HPV infections, many patients turn to their OB/GYN with questions about transmission, testing and prevention. In this review, we will discuss the biology of HPV, epidemiology of disease, methods and indications for testing, and vaccination strategies.
Keywords: Cervical cancer screening, HPV biology, HPV testing, HPV vaccination, Human Papillomavirus
Introduction
Prior to the 1970s, the prevailing theory on the cause for genital tract malignancies was infection with the Herpes Simplex Virus. The scientific community initially doubted Dr Harald Zur Hausen, a German physician and virologist, when he suggested that the same viral particles noted in genital warts might be the culprit for genital tract malignancies. 1 Zur Hausen’s work evolved and eventually in 1983, his lab isolated Human Papillomavirus (HPV) 16 and implicated its role in the development of cervical cancer. 2 One year later, his lab isolated HPV 18, thus discovering the two HPV types that today are known to cause approximately 70% of cervical carcinoma and many other invasive and preinvasive lesions of the vulva, vagina, penis, anus, and head and neck. 3–7
Since the discoveries of Zur Hausen and his colleagues, our clinical and scientific knowledge of HPV has increased dramatically. In this review, we will discuss the biology of HPV, including transmission and viral lifecycle, as well as the epidemiology of disease, methods and indications for testing, and vaccination strategies.
The Biology of HPV
Transmission
HPV infections are almost exclusively acquired from sexual exposure. Areas of micro-trauma within the skin and mucosal surfaces are the proposed sites of infections. Early data confirm sexual transmission by noting that only sexually active women acquire HPV infections, and the rate of acquisition correlates with the number of sexual partners 8. For reasons that are poorly understood, not all sexual partnerships result in HPV transmission. Concordance of HPV infection between sexual partners is variable and ranges from 40–60%. 9–12 Risk factors for concordance are still being elucidated. Length of sexual relationship, frequency of intercourse, condom use and number of lifetime sexual partners may play a role. 9,12
HPV has been detected in multiple anatomic sites including the penis, cervix, anus and hands. 13,14 HPV has also been detected on areas of unprotected genital skin such as the vulva and scrotum, providing an explanation as to why condoms offer incomplete protection against HPV infection. 15 Although transmission has been documented to occur between many anatomic sites, the cervix is the most common site of transmission. In one study, transmission was almost 3 times as likely to occur from the cervix to penis than penis to cervix. 14 Circumcision does play a role in reduction of HPV transmission which has led some to suggest it as a method to reduce the disease burden in endemic communities where vaccination and screening are not yet feasible or practical. 16
Although the primary method of HPV transmission is through sexual exposure, transmission between mother and infant has also been documented. Transmission has been suggested via contact with vaginal and cervical mucosa during delivery, transplacental transmission, transmission via amniotic fluid and horizontal transmission during infancy. The rate of vertical transmission to newborns varies dramatically between studies and may be a result of older, less reliable HPV tests. Newer studies suggest a vertical transmission rate of approximately 20–30%. 17–19 The majority of neonatal infections are cleared by the first year of life, with one study showing a 100% clearance rate. 18 More recent prospective data suggest that children of HPV positive mothers are more likely to test HPV positive at the 6 week postpartum visit; however, a significant number of infants (~17%) born to HPV negative mothers will also test HPV positive at some point in their first two years of life, suggesting other modes of horizontal infection play a crucial role. 19
Lifecycle
Upon entry into the cells at areas of micro-trauma, HPV targets the actively proliferating basal cells of the epithelium. In normal squamous epithelium, the basal layer is the area of active cell division. After division, the daughter cells migrate away from the basal layer and no longer progress through the cell cycle. High molecular-weight keratins are produced by these terminally differentiated keratinocytes until the nuclear envelope breaks down and the cells become empty keratin-filled sacs.
In HPV infected cells, keratinocytes do not undergo terminal differentiation. Once inside the basal epithelial cell, the viral genome begins to replicate. These HPV infected cells migrate away from the basal layer and are characterized by active viral replication. In the uppermost epithelial layer, HPV DNA is assembled into infectious virions. Typical HPV-associated cytopathic changes such as koilocytosis, multinucleation, and nuclear enlargement are due to the assembly of the viral particles in the upper epithelial layers. The epithelium is then shed and HPV particles are released which can then infect a new host.
Not all HPV infections lead to dysplasia or invasive carcinoma. The oncogenic potential of the HPV type is mediated primarily by the behavior of the E6 an E7 proteins. These proteins are transcribed early in the viral life cycle, hence abbreviated ‘E’. Their activity is complex and multifaceted. E6 binds to and degrades tumor-suppressor protein p53 in the host cell - inhibiting expression of genes involved in apoptosis and cell cycle arrest. Thus less apoptosis and growth-arrest occurs, leading to cellular proliferation 20.
The E7 protein in high-risk HPV infection is involved with cell immortalization through the retinoblastoma (Rb) protein family. Rb proteins regulate the cell cycle by controlling the transition at the G1/S phase. When E7 binds Rb, the Rb-E7 complex is degraded and the cell proceeds unregulated through S phase21.
The Prevalence of HPV
Infection with HPV is the most common of all sexually transmitted diseases. Over 40 HPV types specifically infect the anogenital tract 22 and these types can further be separated based on their oncogenic potential. Currently, 12 HPV types are designated by the International Agency for Research on Cancer (IARC) as being carcinogenic and 8 additional types are designated as “probably” or “possibly” carcinogenic (Table 1). 23 Commercially available HPV tests are able to identify 14 of these designated “high-risk” HPV types.
Table 1.
HPV classification
| High Risk HPV types | |
| Carcinogenic* | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 |
| Probably Carcinogenic* | 68 |
| Possibly Carcinogenic* | 26, 53, 66, 67, 70, 73, 82 |
| Tested for in commercially available detection systems |
16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 |
| Low Risk HPV types | 6, 11, 40, 42, 43, 44, 54, 61, 72, 81, 89 |
Data adapted from Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. Apr 2009;10(4):321–322.
Although race, geographic region, and other modifiable risk factors all correlate with HPV prevalence, age is the strongest predictor of HPV prevalence. As part of the 2011 National Health and Nutrition Examination Survey (NHANES) in the US, over 4,000 females collected cervicovaginal specimens for HPV testing. The overall prevalence of 37 different types of HPV was 42.5%, which represents approximately 40 million infections. Specifically, the prevalence of high-risk HPV types was 29%. The prevalence of HPV was lowest among females 14–19 years of age (32.9%) and highest among women 20–24 years of age (53.8%). HPV prevalence varied by race with non-Hispanic blacks having the highest prevalence (59.2%), followed by Mexican Americans (44.2%) and non-Hispanic whites (39.2%) (Figure 1). 24
Figure 1. Prevalence of HPV infection in US Females.
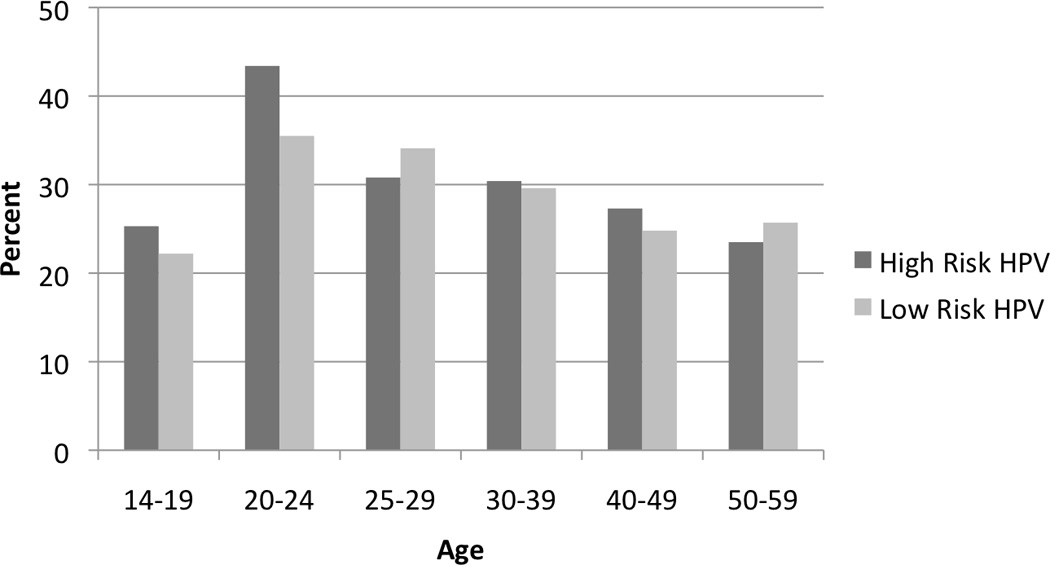
Data adapted from: Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. Aug 15 2011;204(4):566–573. Reprinted with permission.
In some studies, there appears to be a second peak of HPV prevalence among post-menopausal women. 25 Women can acquire new HPV infections later in life and, similar to trends seen in young women, HPV infection in older women is strongly associated with number of new sexual partners .26 Additionally, this second peak can also be explained by a reduced immune response associated with post-menopausal immunosenescence. 27
Because it is a sexually transmitted infection, it is important to understand the epidemiology of HPV infections in men. The largest US population-based study demonstrated the prevalence of all HPV types in men to be 61%. The prevalence of high-risk HPV infections was 23%. In contrast to trends seen in women, the prevalence of HPV infection was not affected by age. 28
Risk Factors for HPV Infection
Even before the link between HPV infection and genital tract malignancies was established, it was well recognized that the components of a woman’s sexual history put her at risk for cervical cancer. Now that the link between HPV and malignancy is well defined, sexual history has specifically been correlated with risk of acquiring HPV infection. The number of both recent and lifetime male sexual partners increases the rate of HPV infection, particularly high-risk HPV infection. 29 The most recent data from the NHANES show that number of lifetime partners is an independent risk factor for all races except non-Hispanic black women, where no independent correlation is seen. 24 Early onset of sexual activity is an independent risk factor in some but not all studies. 24,29
Co-infection with other sexually transmitted diseases and vaginal infections is associated with increased susceptibility to HPV infection. Bacterial vaginosis, trichomoniasis, herpes simplex virus infection and vulvar warts all increase the risk of HPV infection, although the correlation with development of cervical intraepithelial neoplasia (CIN) is not as strong. 30–32 Chlamydial infection has long been implicated in the development of invasive squamous cell cervical cancer. 33 More recent cohort studies have found that chlamydial infection promotes both the acquisition and persistence of HPV infection. 34,35
In 2004, cervical cancer was added to the list of cancers causally related to smoking according to the International Agency for Research on Cancer. 36 However, since then, conflicting data have emerged regarding the association between cigarette smoking and HPV acquisition, persistence and progression. Some studies have shown that women who smoke are less likely to be compliant with screening guidelines, thereby confounding the strength of association between smoking and development of cervical cancer. 37,38 Most studies have shown that current (but not past) smoking increases the prevalence of HPV infection. 39,40 Current smokers have greater high-risk HPV viral loads than non-smokers and former smokers. 41 Additionally, delayed clearance of HPV infection and progression to CIN 2/3 is more common in women who smoke. 42,43
Human Immunodeficiency Virus (HIV) infection is strongly associated with HPV incidence, persistence, and progression. 44 CD4 counts and HIV RNA levels correlate with the incidence of high-risk HPV infections. 45 Although highly active antiretroviral therapy (HAART) has many positive effects on HIV positive women including increased life expectancy, it has not been shown to definitively affect the incidence or persistence of HPV infections. 46
The Natural History of HPV Infection and Burden of Disease
Despite their high prevalence, the majority of HPV infections are cleared, and only a minority persist and progress to cervical intraepithelial neoplasia (CIN) or invasive cancer. The 1-year clearance rate of incident HPV infections in women ranges from 40–70% depending on the population studied. Clearance rates are as high as 70–100% in young women at 2–5 years. 32,47–50 Young women are more likely to clear infections than older women, and low-risk HPV infections clear more quickly than high-risk HPV infections. 47 Men have higher rates of HPV clearance with almost 75% of HPV infection cleared within 1 year. 28
Among women who do not clear their infection, studies report variable rates of progression to CIN 2/3 ranging from 8–28%. 51–53 Additionally, an estimated 3–5% will eventually develop cervical cancer without any intervention. 54,55
There is limited information on interventions that may increase rates of clearance and decrease rates of progression. Preliminary data suggest diet may play a role. Increased intake of riboflavin, thiamine, vitamin B12 and folate through diet or supplementation is associated with a lower rate of CIN. 56
HPV infection and persistence is necessary for the development of cervical cancer and its precursors. Among gynecologic malignancies worldwide, the burden of cervical cancer remains highest. It is the third most common cancer in females worldwide. The incidence of cervical cancer is much lower in nations that have adopted cervical cancer screening programs; however, there will still be an estimated 12,000 new cases diagnosed in the US this year. 57 HPV infection is also found in approximately 40% of squamous cell vulvar carcinomas, 65% of vaginal cancer and almost 50% of penile cancers. Among all HPV types, HPV 16 is the most common type found in vulvar (29%), vaginal (55%) and penile cancers (31%). 5,6
HPV Testing
The US FDA has approved 5 tests for detecting HPV (Table 2). Because of the high prevalence and clearance rates of HPV infection, particularly in young women, primary HPV testing is not recommended as an upfront screening strategy. However, because of its improved sensitivity compared to conventional cytology, HPV testing plays an important role in various screening algorithms.
Table 2.
FDA approved HPV tests.
| Test | HPV types | Uses |
|---|---|---|
| Digene Hybrid Capture 2 High-Risk HPV DNA Test1 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 |
|
| Cervista HPV HR2 | 16, 18, 31, 33, 35, 39, 45, 51, 52,56, 58, 59, 66, and 68 |
|
| Cervista HPV 16/182 | 16, 18 |
|
| Cobas HPV test3 | Specifically identifies 16 and 18 while concurrently testing for 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 |
|
| APTIMA HPV assay4 | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 |
|
QIAGEN (Germantown, MD)
Hologic (Bedford, MA)
Roche Molecular Systems (Pleasanton, CA)
Gen-Probe (San Diego, CA) ASCUS, Atypical Squamous Cells of Undetermined Significance.
The American Society for Colposcopy and Cervical Pathology (ASCCP) recommends HPV testing in a variety of specific situations including triage of women 21 years or older with an ASC-US Pap smear, co-testing with cytology in women greater than age 30, follow-up after excisional procedures or ablation of CIN2/3, management of post-menopausal women with LSIL, management of women with atypical glandular cells (AGC) on cytology, and follow-up for CIN1 when it was preceded by the following Pap smear categories- LSIL, ASC-US, and ASC-H (Table 3). 58,59
Table 3.
| Indication for HPV test | Follow up if HPV+ | Follow up if HPV− |
|---|---|---|
| ASC-US triage if ≥ 21 years old |
Colposcopy | Ages 21–29: Repeat cytology 3 years Ages 30–65: Repeat cytology 3 years or cytology/HPV co- testing in 5 years |
| Screening in low-risk women≥ 30 years (cytology/HPV co-testing) |
If ASC-US/HPV+: Colposcopy If cytology negative/HPV+: Option 1: 12-month follow-up with cytology/HPV co-testing Option 2: Test for HPV16 or HPV16/18 genotypes If HPV16+ or HPV16/18+: colposcopy If HPV16 or HPV16/18-: 12- month follow-up cytology/HPV co- testing |
Cytology/HPV co-testing in 5 years |
| Follow-up after excisional or ablative procedure for CIN 2/3 at 6 and 12 months (other follow-up options available) |
Colposcopy | Routine screening |
| Post-menopausal women with LSIL cytology |
Colposcopy | Routine screening |
| AGC cytology, negative colposcopy |
Follow-up cytology/HPV co testing at 6 months |
Follow up cytology/HPV co- testing at 12 months |
| Follow-up CIN1 when preceded by LSIL, ASC-US, or ASC-H cytology |
Colposcopy | Routine screening |
Moreover, the ASCCP recommends genotyping assays, which specifically detect HPV 16 and 18, as a way to further triage women over the age of 30 with normal cytology and positive pooled high risk HPV tests. Women with negative cytology who are HPV 16 or 18 positive should be referred for immediate colposcopy, whereas those who are high-risk HPV-positive but HPV 16 and 18 negative can be followed up in a year with repeat cytology and HPV testing. 59
Because there is no treatment for asymptomatic HPV infections in men, routine partner HPV testing is not recommended. 60 There is no FDA approved use of the HPV DNA test in men and there are no current guidelines for the management of partners of women who are HPV positive. Unlike the importance of partner notification in treatable infections such as HIV and syphilis, recommendations are less clear for HPV infections. Many practitioners still recommend that their patients notify their partners of HPV positivity as it may affect sexual activity and condom use, which may decrease rates of transmission.
HPV testing as the primary screening test has also been proposed as a way to improve the sensitivity of screening, and improve access to screening services. In a pooled analysis of studies from Europe, Canada, and the US, HPV testing was compared to conventional cytology for detecting cervical dysplasia. Notably, HPV testing was substantially more sensitive than cytology in detecting high grade lesions (96% vs. 53%), but was less specific (90% vs. 96%). 61
This improvement in sensitivity has prompted large population-based trials throughout the world, which have showed promising results for HPV testing in the primary setting. 62–64 Primary HPV testing appears to be especially effective in women over age 35, especially when cervical cytology is used as a triage test following a positive HPV test. 64 Subgroup analysis from a large US based trial showed that HPV testing was more sensitive than cytology for detecting CIN3+ (92.0% vs. 53%). Adding cytology to HPV testing increased the sensitivity to 96.7%, though it increased the number of colposcopies performed by 35% compared with HPV testing alone. 65
In addition to its improved sensitivity, primary high-risk HPV testing has unique features, which may help target high-risk populations that are less likely to participate in the current screening programs. 66 In an attempt to make high-risk HPV testing more efficient, less invasive, and less costly, self-collection of samples for HPV testing has developed. This screening method also has the advantage of reaching patients in remote locations or settings with limited resources. 67 Thus far, self-collection has shown good concordance with physician collected samples. 68
Despite these advantages, currently the American Cancer Society and the ASCCP do not recommend primary HPV screening. 59 They acknowledge its promise as an effective screening tool but cite lack of well studied management strategies for handling positive test results. Colposcopy of all HPV-positive patients may lead to many unnecessary diagnostic and therapeutic treatments. Additionally, cost effectiveness and adherence to a new screening strategy are areas that need to be better studied prior a large scale change in screening paradigms. 59
HPV Vaccination
With an improved understanding of the role of HPV infection in genital tract malignancies, vaccination has emerged as an important element in cancer prevention. The primary target for prophylactic HPV vaccination is the L1 capsid protein, which is one of two viral capsid proteins of HPV. Vaccines are made with recombinant L1 proteins to form virus-like particles (VLPs), which induce both humoral and cellular immune responses. Following vaccination, antibody titers are 20–80 times higher than seen with natural infection.
Currently, two vaccines against HPV are approved in the US (Table 4). The quadrivalent vaccine Gardasil (Merck & Co., Inc., Whitehouse Station, NJ USA) protects against infection from HPV 6, 11, 16, and 18. It was initially approved in 2006 for women ages 9 to 26 for the prevention of cervical cancer caused by HPV 16 and 18, and genital warts caused by HPV 6 and 11. Its use has also been expanded to the prevention of vaginal, vulvar, and anal cancer, as well as the prevention of genital warts in boys and men ages 9 to 26.
Table 4.
HPV vaccines
| Gardasil* | Cervarix** | |
|---|---|---|
| HPV Types | HPV 6, 11, 16, 18 | HPV 16, 18 |
| Injection Schedule | 3 injections: 0, 2, 6 months | 3 injections: 0, 1, 6 months |
| FDA Approval | Females: prevention of vaginal, vulvar, and cervical cancer and their premalignant lesions (HPV 16, 18). Females and males: prevention of genital warts (HPV 6, 11), anal cancer and premalignant lesions (HPV 16, 18) |
Females: prevention of cervical cancer and premalignant cervical lesions |
| Additional Benefits | -Safe in lactation | -Safe in lactation -Efficacy against HPV 31,33, 45, and 51 |
Merck & Co., Inc. (Whitehouse Station, NJ)
GlaxoSmithKline (United Kingdom)
The bivalent vaccine Cervarix (GlaxoSmithKline, United Kingdom) protects against HPV 16 and 18 and was approved by the FDA in 2009 for the prevention of cervical cancer and precancerous cervical lesions in women from 10 to 25 years of age. Both of these vaccines are highly effective (>99%) in preventing CIN as well as adenocarcinoma in situ (AIS) due to HPV 16 and 18 in previously unexposed women. 69,70 Vaccination is much less efficacious in women with pre-existing high-risk HPV infection. 69
Although the mechanisms have not been clearly delineated, the bivalent HPV vaccine, with its unique adjuvant containing aluminum hydroxide and monophosphoryl lipid A, has been found to have a cross-protective effect against oncogenic HPV types 33, 31, 45, and 51. 71 In one large vaccine trial, the vaccination showed 94% efficacy against all CIN3, irrespective of HPV type, as well as 100% efficacy against all AIS. 70
Future Directions
Despite advances in screening, preinvasive and invasive disease of the lower genital tract continues to cause significant morbidity and mortality in the US and worldwide. With improved knowledge regarding the biology of HPV, detection systems and prophylactic vaccination have emerged in an effort to better understand and prevent disease. Future directions include continued research in the best use of HPV testing in screening algorithms (including screening within a vaccinated cohort), optimizing HPV vaccine uptake, and ensuring adequate adherence to screening guidelines in order to further reduce disease burden.
Acknowledgments
Financial Support or Funding: none
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors have a conflict of interest
References
- 1.Zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1974;36:794. [PubMed] [Google Scholar]
- 2.Durst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Guily JL, Jacquard AC, Pretet JL, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France--The EDiTH VI study. J Clin Virol. 2011 Jun;51(2):100–104. doi: 10.1016/j.jcv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009 Apr 1;101(7):475–487. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JS, Backes DM, Hoots BE, Kurman RJ, Pimenta JM. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet Gynecol. 2009 Apr;113(4):917–924. doi: 10.1097/AOG.0b013e31819bd6e0. [DOI] [PubMed] [Google Scholar]
- 6.Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009 May;20(4):449–457. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- 7.Boshart M, Gissmann L, Ikenberg H, Kleinheinz A, Scheurlen W, zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse) Cancer Epidemiol Biomarkers Prev. 2001 Feb;10(2):101–106. [PubMed] [Google Scholar]
- 9.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology. 2010 Jan;21(1):31–37. doi: 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker MC, Hogewoning CJ, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis. 2005 Sep 1;41(5):612–620. doi: 10.1086/431978. [DOI] [PubMed] [Google Scholar]
- 11.Benevolo M, Mottolese M, Marandino F, et al. HPV prevalence among healthy Italian male sexual partners of women with cervical HPV infection. J Med Virol. 2008 Jul;80(7):1275–1281. doi: 10.1002/jmv.21189. [DOI] [PubMed] [Google Scholar]
- 12.Nyitray AG, Menezes L, Lu B, et al. Genital Human Papillomavirus (HPV) Concordance in Heterosexual Couples. J Infect Dis. 2012 Apr 26; doi: 10.1093/infdis/jis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giuliano AR, Nielson CM, Flores R, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007 Oct 15;196(8):1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008 Jun;14(6):888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006 Jun 22;354(25):2645–2654. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 16.Albero G, Castellsague X, Giuliano AR, Bosch FX. Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sex Transm Dis. 2012 Feb;39(2):104–113. doi: 10.1097/OLQ.0b013e3182387abd. [DOI] [PubMed] [Google Scholar]
- 17.Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Transplacental transmission of Human Papillomavirus. Virol J. 2008;5:106. doi: 10.1186/1743-422X-5-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP. Perinatal transmission of human papilomavirus DNA. Virol J. 2009;6:83. doi: 10.1186/1743-422X-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellsague X, Drudis T, Canadas MP, et al. Human Papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74. doi: 10.1186/1471-2334-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999 Dec 13;18(53):7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 21.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996 Oct 15;56(20):4620–4624. [PubMed] [Google Scholar]
- 22.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006 Aug 31;24(Suppl 3) doi: 10.1016/j.vaccine.2006.05.115. S3/1-10. [DOI] [PubMed] [Google Scholar]
- 23.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009 Apr;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 24.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011 Aug 15;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 25.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008 Oct;43(4 Suppl):S5–S25. e21–e41. doi: 10.1016/j.jadohealth.2008.07.009. S25. [DOI] [PubMed] [Google Scholar]
- 26.Trottier H, Ferreira S, Thomann P, et al. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010 Nov 1;70(21):8569–8577. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez P, Hildesheim A, Rodriguez AC, et al. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomarkers Prev. 2010 Dec;19(12):3044–3054. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008 Aug;17(8):2036–2043. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco EL, Villa LL, Ruiz A, Costa MC. Transmission of cervical human papillomavirus infection by sexual activity: differences between low and high oncogenic risk types. J Infect Dis. 1995 Sep;172(3):756–763. doi: 10.1093/infdis/172.3.756. [DOI] [PubMed] [Google Scholar]
- 30.King CC, Jamieson DJ, Wiener J, et al. Bacterial vaginosis and the natural history of human papillomavirus. Infect Dis Obstet Gynecol. 2011;2011:319460. doi: 10.1155/2011/319460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts DH, Fazzari M, Minkoff H, et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women. J Infect Dis. 2005 Apr 1;191(7):1129–1139. doi: 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- 32.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001 Jun 20;285(23):2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 33.Anttila T, Saikku P, Koskela P, et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001 Jan 3;285(1):47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- 34.Lehtinen M, Ault KA, Lyytikainen E, et al. Chlamydia trachomatis infection and risk of cervical intraepithelial neoplasia. Sex Transm Infect. 2011 Aug;87(5):372–376. doi: 10.1136/sti.2010.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silins I, Ryd W, Strand A, et al. Chlamydia trachomatis infection and persistence of human papillomavirus. Int J Cancer. 2005 Aug 10;116(1):110–115. doi: 10.1002/ijc.20970. [DOI] [PubMed] [Google Scholar]
- 36.Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 37.Byrne MM, Davila EP, Zhao W, et al. Cancer screening behaviors among smokers and non-smokers. Cancer Epidemiol. 2010 Oct;34(5):611–617. doi: 10.1016/j.canep.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Wilkerson JE, Bailey JM, Bieniasz ME, Murray SI, Ruffin MT. Psychosocial factors in risk of cervical intraepithelial lesions. J Womens Health (Larchmt) 2009 Apr;18(4):513–518. doi: 10.1089/jwh.2008.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Syrjanen K, Shabalova I, Petrovichev N, et al. Smoking is an independent risk factor for oncogenic human papillomavirus (HPV) infections but not for highgrade CIN. Eur J Epidemiol. 2007;22(10):723–735. doi: 10.1007/s10654-007-9180-8. [DOI] [PubMed] [Google Scholar]
- 40.Vaccarella S, Herrero R, Snijders PJ, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol. 2008 Jun;37(3):536–546. doi: 10.1093/ije/dyn033. [DOI] [PubMed] [Google Scholar]
- 41.Xi LF, Koutsky LA, Castle PE, et al. Relationship between cigarette smoking and human papilloma virus types 16 and 18 DNA load. Cancer Epidemiol Biomarkers Prev. 2009 Dec;18(12):3490–3496. doi: 10.1158/1055-9965.EPI-09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins S, Rollason TP, Young LS, Woodman CB. Cigarette smoking is an independent risk factor for cervical intraepithelial neoplasia in young women: a longitudinal study. Eur J Cancer. 2010 Jan;46(2):405–411. doi: 10.1016/j.ejca.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koshiol J, Schroeder J, Jamieson DJ, et al. Smoking and time to clearance of human papillomavirus infection in HIV-seropositive and HIV-seronegative women. Am J Epidemiol. 2006 Jul 15;164(2):176–183. doi: 10.1093/aje/kwj165. [DOI] [PubMed] [Google Scholar]
- 44.De Vuyst H, Lillo F, Broutet N, Smith JS. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur J Cancer Prev. 2008 Nov;17(6):545–554. doi: 10.1097/CEJ.0b013e3282f75ea1. [DOI] [PubMed] [Google Scholar]
- 45.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency viruspositive women. J Natl Cancer Inst. 2005 Apr 20;97(8):577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 46.Bratcher LF, Sahasrabuddhe VV. The impact of antiretroviral therapy on HPV and cervical intraepithelial neoplasia: current evidence and directions for future research. Infect Agent Cancer. 2010;5:8. doi: 10.1186/1750-9378-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003 Jun;12(6):485–490. [PubMed] [Google Scholar]
- 48.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998 Feb 12;338(7):423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 49.Dalstein V, Riethmuller D, Pretet JL, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003 Sep 1;106(3):396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 50.Ferreccio C, Van De Wyngard V, Olcay F, et al. High-risk HPV infection after five years in a population-based cohort of Chilean women. Infect Agent Cancer. 2011;6(1):21. doi: 10.1186/1750-9378-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- 52.Bae J, Seo SS, Park YS, et al. Natural history of persistent high-risk human papillomavirus infections in Korean women. Gynecol Oncol. 2009 Oct;115(1):75–80. doi: 10.1016/j.ygyno.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Bory JP, Cucherousset J, Lorenzato M, et al. Recurrent human papillomavirus infection detected with the hybrid capture II assay selects women with normal cervical smears at risk for developing high grade cervical lesions: a longitudinal study of 3,091 women. Int J Cancer. 2002 Dec 10;102(5):519–525. doi: 10.1002/ijc.10735. [DOI] [PubMed] [Google Scholar]
- 54.Castle PE, Wacholder S, Lorincz AT, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002 Sep 18;94(18):1406–1414. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 55.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008 Aug 19;26(Suppl 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez BY, McDuffie K, Wilkens LR, Kamemoto L, Goodman MT. Diet and premalignant lesions of the cervix: evidence of a protective role for folate, riboflavin, thiamin, and vitamin B12. Cancer Causes Control. 2003 Nov;14(9):859–870. doi: 10.1023/b:caco.0000003841.54413.98. [DOI] [PubMed] [Google Scholar]
- 57.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 58.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007 Oct;11(4):223–239. doi: 10.1097/LGT.0b013e318159408b. [DOI] [PubMed] [Google Scholar]
- 59.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. J Low Genit Tract Dis. 2012 Mar 13; doi: 10.1097/LGT.0b013e31824ca9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoover K, Friedman A, Montano D, Kasprzyk D, Greek A, Hogben M. What about the partners of women with abnormal Pap or positive HPV tests? Sex Transm Dis. 2009 Mar;36(3):141–146. doi: 10.1097/OLQ.0b013e31818eb765. [DOI] [PubMed] [Google Scholar]
- 61.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006 Sep 1;119(5):1095–1101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 62.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007 Oct 18;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 63.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010 Mar;11(3):249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 64.Leinonen M, Nieminen P, Kotaniemi-Talonen L, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009 Dec 2;101(23):1612–1623. doi: 10.1093/jnci/djp367. [DOI] [PubMed] [Google Scholar]
- 65.Castle PE, Stoler MH, Wright TC, Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011 Sep;12(9):880–890. doi: 10.1016/S1470-2045(11)70188-7. [DOI] [PubMed] [Google Scholar]
- 66.Gok M, Heideman DA, van Kemenade FJ, et al. HPV testing on self collected cervicovaginal lavage specimens as screening method for women who do not attend cervical screening: cohort study. BMJ. 2010;340:c1040. doi: 10.1136/bmj.c1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: new tools and old barriers. Cancer. 2010 Jun 1;116(11):2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petignat P, Faltin DL, Bruchim I, Tramer MR, Franco EL, Coutlee F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007 May;105(2):530–535. doi: 10.1016/j.ygyno.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 69.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007 Jun 2;369(9576):1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 70.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012 Jan;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 71.Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012 Jan;13(1):100–110. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]


