Summary
The frog Xenopus can normally regenerate its limbs at early developmental stages but loses the ability during metamorphosis. This behavior provides a potential gain-of-function model for measures that can enhance limb regeneration. Here we show that frog limbs can be caused to form multidigit regenerates after receiving transplants of larval limb progenitor cells. It is necessary to activate Wnt/β -catenin signaling in the cells, and to add Sonic hedgehog, FGF10 and thymosin β4. These factors promote survival and growth of the grafted cells and also provide pattern information. The eventual regenerates are not composed solely of donor tissue; the host cells also make a substantial contribution despite their lack of regeneration-competence. Cells from adult frog legs or from regenerating tadpole tails do not promote limb regeneration, demonstrating the necessity for limb progenitor cells. These findings have obvious implications for the development of a technology to promote limb regeneration in mammals.
Keywords: Xenopus, limb, regeneration, fibrin patch, Sonic hedgehog, Fibroblast growth factors, Wnt/β-Catenin, Thymosin β4
Introduction
Some vertebrate animals, mostly urodele amphibians, can regenerate limbs after amputation while others cannot (Brockes and Kumar, 2008; Nacu and Tanaka, 2011; Nye et al., 2003). Until now it has not been possible to impart regenerative capacity to animals that cannot do it. The anuran amphibian Xenopus can normally regenerate its limbs at early developmental stages but gradually loses the ability in late tadpole stages, such that post-metamorphic frogs can only produce an unsegmented cartilaginous spike after amputation (Dent, 1962). This behavior provides a potential gain-of-function model for measures that can enhance limb regeneration.
There have been many attempts and claims to enhance frog limb regeneration in the past. However all reports of stimulating regeneration in postmetamorphic frog limbs have proved irreproducible, although some may have worked in tadpole limbs (Carlson, 2007; Muller et al., 1999).
One key requirement for successful limb regeneration is the re-establishment of patterning information in the regenerating blastema. In urodele amphibian limb regeneration and frog tadpole limb regeneration, genes encoding patterning information signals, such as those encoding bone morphogenetic protein (BMP), Wnt, fibroblast growth factor (FGF) and Shh signaling pathways, as well as key transcription factors, are re-expressed in the regenerating blastema, mimicking the expression during development (Beck et al., 2006; Christen and Slack, 1997; Christen and Slack, 1998; Christensen et al., 2002; Endo et al., 1997; Han et al., 2001; Imokawa and Yoshizato, 1997; Zeller et al., 2009). In post-metamorphic frogs, however, expression of these factors is defective, resulting in formation of a single unsegmented cartilaginous spike after amputation (Yakushiji et al., 2009). It has been reported that application of Fgf10 in the late tadpole limbs can prolong its regeneration capacity (Yokoyama et al., 2001), but this is not seen in postmetamorphic frogs.
Recent studies have indicated the potential for using tissue progenitor cells for replacement therapy. For example, transplantation of muscle satellite cells has been shown to lead to functional recovery of dystrophic muscle (Zammit et al., 2006), and pancreatic precursors made from embryonic stem cells can cure diabetic animals (Kroon et al., 2008). Early studies on Xenopus limb regeneration demonstrated that regeneration capacity is an intrinsic property of the developing limb rather than depending on the physiological state of the host (Muneoka et al., 1986; Sessions and Bryant, 1988). As a first step in the investigation of possible cell transplantation therapies for limb regeneration we were interested to see whether larval progenitor cells, when transplanted to the limb amputation surface, could participate in and stimulate regeneration.
Here we show that larval limb progenitor cells can indeed promote frog limb regeneration, but that success requires a number of conditions without which no regeneration is obtained. First the cells must be applied in a manner enabling survival and for this purpose we have employed a “patch” of fibrin matrix, which can be attached to the cut surface. Secondly, the activation of Wnt/β-catenin signaling is necessary, and for this we have used transgenic animals containing an inducible gene for stabilized β-catenin. Finally exogenous factors are needed to promote growth and survival and to provide patterning information. We have used Shh and FGF10 delivered from Affi-Gel beads, together with thymosin β4 incorporated into the fibrin matrix. With all these conditions satisfied, grafted larval limb cells will support regeneration of postmetamorphic frog limbs, generating at least some segmented digits. Remarkably the eventual regenerates are not composed solely of donor tissue; we find that the host cells also make a substantial contribution despite their lack of regeneration-competence. Neither the stimulation of Wnt/β-catenin, nor the application of Shh and FGF10, is sufficient to enable frog limbs to regenerate. The additional presence of larval limb cells is essential. Cells from adult frog legs or from regenerating tadpole tails do not promote limb regeneration, demonstrating the necessity for actual limb progenitor cells.
Results
Forced expression of Shh and Fgf fails to promote limb regeneration
We have forced expression in frog limbs of Shh and FGF10 by administration of protein on Affi-Gel beads, both in wild type limbs and in those expressing activated β-catenin (βcat*), but fail to observe any enhanced limb regeneration (Table S1, row 1, and data not shown). These observations show that the cells of frog limbs are unable to produce a regeneration blastema in response to these factors.
A larval limb bud graft improves limb regeneration in thymectomized frogs, and both donor and host cells contribute
We have extended the previous studies on limb buds grafted to postmetamorphic hosts to see whether regeneration as well as growth is possible. We used a transgenic GFP label to monitor both cell survival and the composition of the eventual regenerates. Although the construct contains a nuclear localization sequence, the GFP protein is found in both nucleus and cytoplasm, possibly due to the high expression level. Stage 53 developing limb buds were grafted to either the forelimb or the hindlimb muscle of postmetamorphic hosts at the zeugopod level, just proximal to the wrist or ankle, and then the limb was amputated through the graft, leaving some graft tissue in place. In order to enable the grafts to survive long term we found it was necessary to thymectomize the host frogs, a method known to impair the immune response after allografts (Horton and Manning, 1972). This was done when the future hosts were stage 48 tadpoles, using an electrocautery apparatus (Fig. 1). In immunocompetent hosts, we observed that GFP expression disappeared soon after transplantation, and most frogs regrew just the usual spikes (19/22, Fig. 1A–C, Fig. S1A, B). In sections, a severe immune reaction to the grafted limb bud, with lymphocyte infiltration, is obvious (Fig. S1C,G). By contrast, in thymectomized hosts, we found that an implanted limb bud can survive and retain its GFP expression for at least long enough for the limb to regenerate (Fig. 1F, Fig. S1E, F). A high proportion of these cases did regenerate multidigit limbs (13/20, Fig. 1F, Fig. S1D,H, Table 1 row 1–2). These regenerates are somewhat disorganized, and in many cases only cartilages are formed (Fig. S1D). Nevertheless, this shows that regeneration of larval buds does occur in postmetamorphic frogs.
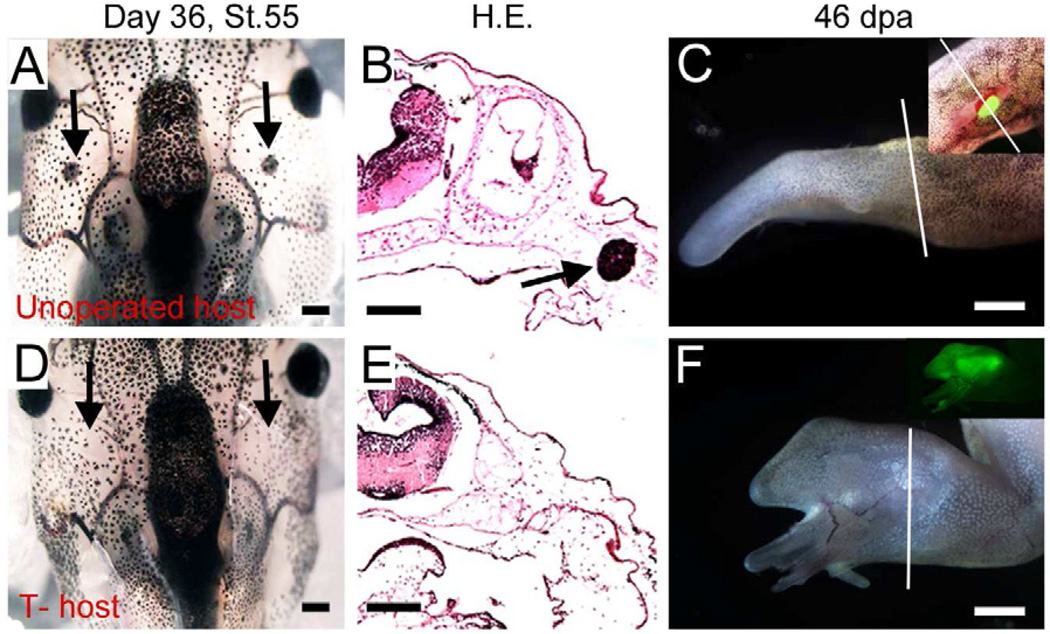
Fig. 1. Thymectomy is necessary for transplantation experiments in Xenopus frogs.
(A–B) The developing thymus in a control stage 55 tadpole, shown as in whole mount animal (A) and on cross section after haematoxylin and eosin staining (B). Black arrows indicate thymus.
(C) Limb regeneration after transplantation of a GFP-labeled limb bud to a wild type frog host. GFP is undetectable in the single spike regenerate, 46 days post amputation (dpa). Inset shows a green limb bud immediately after transplantation. Note the relative size of the donor and host.
(D) A thymectomized tadpole after 36 days with no regeneration of the thymus (arrows), confirmed by histology (E).
(F) Limb regeneration after transplantation of a GFP limb bud to thymectomized (T-) host. The graft has survived long term and generated a multidigit regenerate. Inset shows GFP fluorescence.
White lines in (C) and (F) indicate amputation levels. Scale bars are 100µm for A, B, D, E and 250µm for C, F.
See also Fig. S1 and S2.
Table 1.
Xenopus limb regeneration after cell transplantations
| Graf (all are to thymectomized frog limbs except row 1) |
Total No. |
Experimental side: | Control side: | Notes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. digits/spikes | No. digits/spikes | ||||||||||||||
| 5 | 4 | 3 | 2 | 1 | Mean extra digits (p) |
5 | 4 | 3 | 2 | 1 | Mean extra digits |
||||
| 1. Limb bud grafted to T+ frog limb | 22 | 0 | 0 | 1 | 2 | 19 | 0.18 | <0.01 | 0 | 0 | 0 | 0 | 22 | 0 | Thymus intact |
| 2. Limb bud | 20 | 0 | 3 | 8 | 2 | 7 | 1.35 | 0 | 0 | 0 | 0 | 20 | 0 | ||
| 3. Limb bud cell patch | 25 | 0 | 0 | 0 | 0 | 25a | 0 | <0.01 | 0 | 0 | 0 | 0 | 25 | 0 | a 2 have some extra cartilage |
| 4. βcat* limb bud cell patch +Shh +FGF10 |
30 | 0 | 3b | 1 | 10 | 16 | 0.70 | 0 | 0 | 0 | 0 | 30 | 0 | b1 has calcium deposition | |
| 5. βcat* limb bud cell patch +Shh +FGF10 + Tβ4 |
57 | 3d | 5c | 10 | 11 | 28 | 1.0 | 0 | 0 | 0 | 0 | 57 | 0 | c,dCalcium deposition | |
| 6. Regenerating βcat* tail cell patch +Shh +Fgf10+ Tβ4 |
20 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 20 | 0 | ||
Calculation of “mean extra digits” is done by counting all the digits above one and dividing by the number of cases.
Numbers are pooled from at least three independent experiments. The t-test is used to compare rows 1 and 2; and one-way analysis of variance (ANOVA) to compare rows 3–6.
As shown in Fig. 1F, we observed GFP expression in the majority of the regenerated limb tissues, despite the fact that the original donor limb bud is relatively tiny compared to the host limb (around 1/60 in section area). We studied the relative contribution of graft and host cells to these regenerates, using transgenic GFP-labeled donors and wild type hosts, and scoring the different tissue types for the presence of GFP-positive cells. Unexpectedly, the percentage of GFP-positive cells in the regenerating cartilage and muscle is quite low, showing substantial host participation in the regenerates (Fig. S2A–D). Our previous work using pCMVnGFP transgenic tadpoles indicated that GFP is not always present in all the cells (Daughters et al., 2011). To exclude the possibility that the low percentage of donor cells is an artifact due to silencing of GFP expression, we repeated the experiment by transplanting wild type limb buds to pCMVnGFP frog hosts. In un-amputated host limbs we found some silencing or low levels of GFP expression, particularly in cartilage (Fig. S3A–F). But when we checked the GFP expression in the regenerates formed after limb bud grafting, we observed that, despite the presence of some silencing, about half of the non-epidermal cells are GFP-positive (Fig. S2E–H). These observations confirm that many host cells are recruited to participate in the limb regenerate, even though they do not on their own have the ability to form anything other than a cartilage spike.
Enhanced regeneration by limb progenitor cells with active Wnt/β-catenin signaling and provision of growth factors
We then examined whether dissociated larval limb progenitor cells can also promote regeneration. To deliver the cells into the frog limbs, we tested several methods including direct injection and application of cells embedded in hydrogel or fibrin gel patches. We found that a fibrin gel patch applied to the amputation surface is an effective delivery method. This is prepared by suspending the dissociated larval limb cells in medium containing fibrinogen and then adding thrombin to polymerize the fibrinogen to fibrin (Zhang et al., 2008). The fibrin patch increases cell survival and migration after transplantation, and provides a platform for the application of slow release beads (Fig. 2A–C). Since the fibrin patch is applied directly onto the amputation surface, it occupies the position of a blastema and slows the covering of the wound surface by the host epidermis (Fig. 2D–E). However, unlike whole limb bud grafting, transplantation of dissociated limb bud cells in a fibrin patch did not promote limb regeneration, the best results consisting only of slight extra cartilage formation near the amputation surface (Fig. 3A–C, Table 1 row 3).
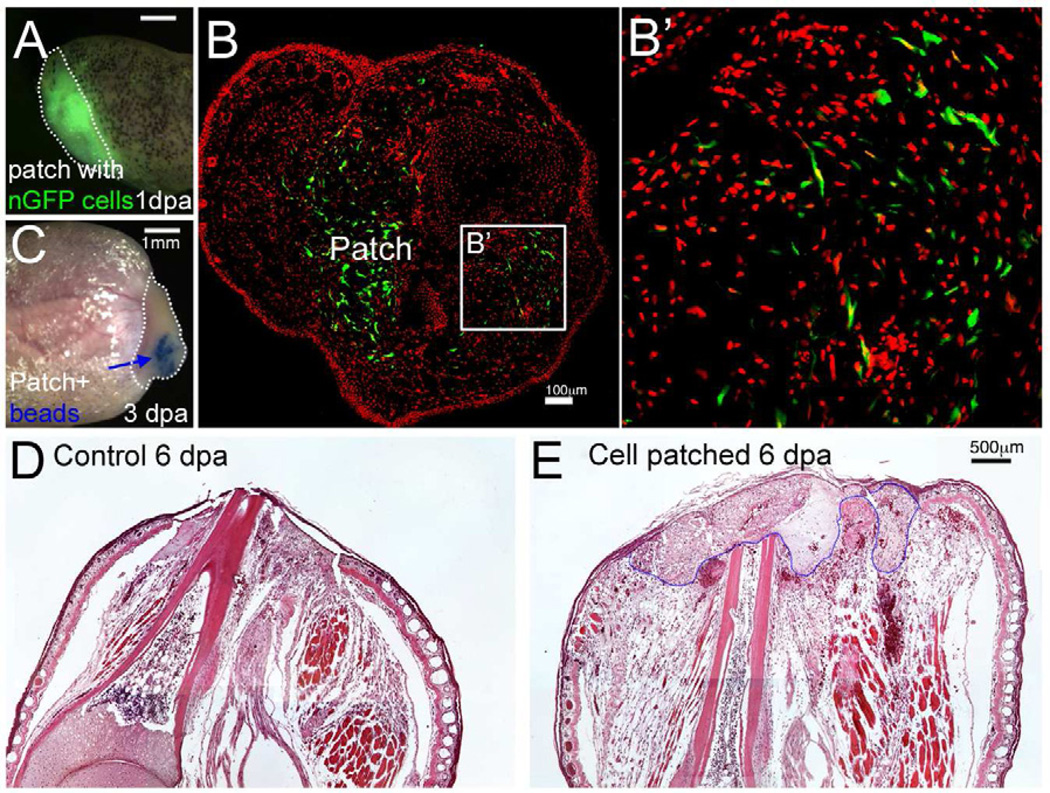
Fig. 2. Delivery of cells to the limb stump using a fibrin patch.
(A) A frog limb stump covered with a fibrin patch containing GFP labeled cells, 1 dpa. The white dotted line indicates the patch. Scale bar 1 mm.
(B) Migration of nGFP (green) cells out of the patch in an early regenerate, 3 dpa. Nuclei stained with propidium iodide (PI) are shown in red. An area far away from the patch (outlined in B) is shown in (B’). Scale bars, 100 µm.
(C) The patch allows application of growth factor beads to the limb stump. The white dotted line outlines the patch, and the blue arrow indicates Affi-Gel beads. Scale bar 1 mm.
(D–E) Sagittal sections of limb stump with (E) or without (D) fibrin patch. The blue dotted line indicates the boundary of the patch. Scale bar 500 µm.
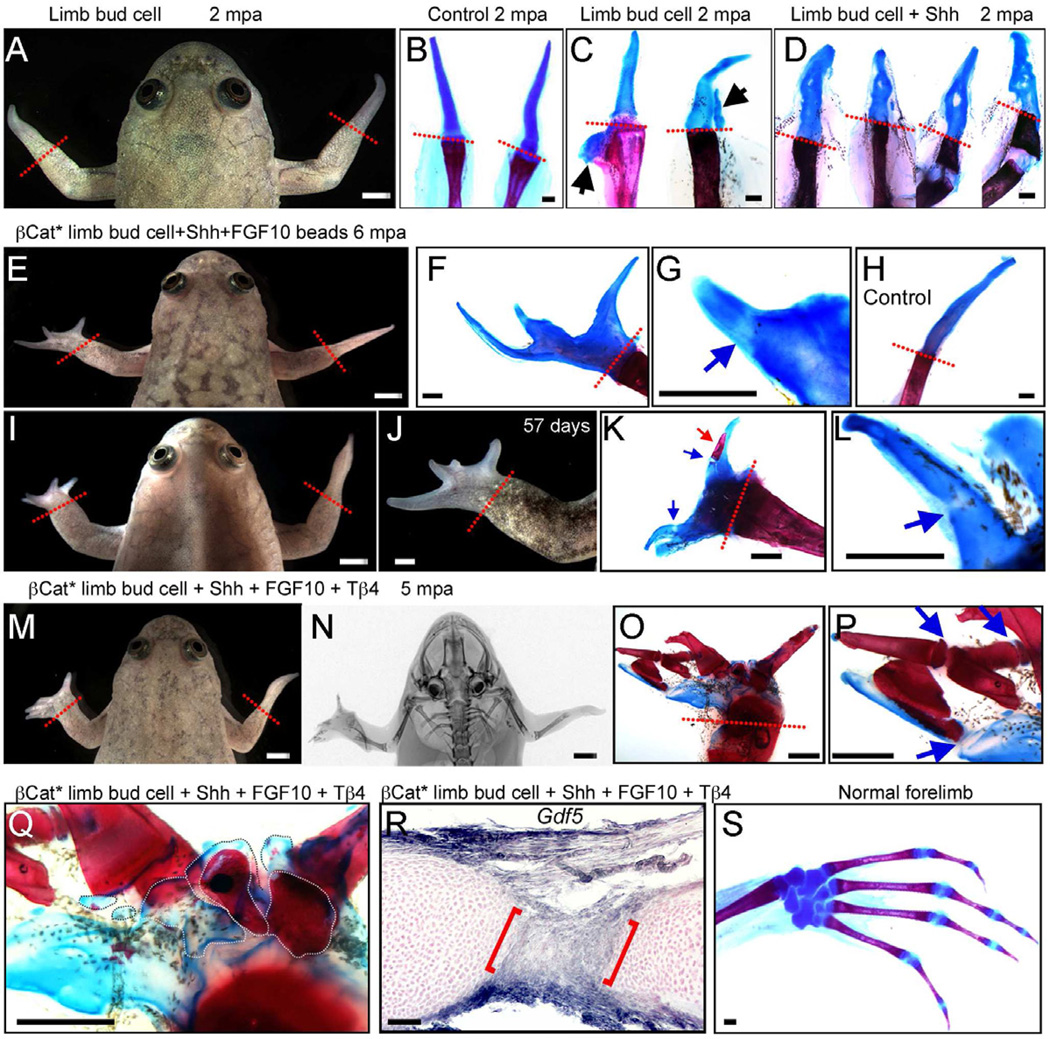
Fig. 3. Xenopus frog forelimb regeneration after cell transplantation.
Left limbs are transplanted and right limbs are controls with amputation only.
(A –C) Limb regeneration in a frog with a GFP limb bud cell patch, 2 months post amputation (mpa). Skeletal staining shows that regenerates are still simple spikes (A, C) similar to controls (B), although 2 cases gave slight extra cartilage (arrows in C).
(D) Skeletal preparations of 4 forelimbs treated with limb bud cell patches and Shh beads, showing slightly disturbed cartilages, 2 mpa.
(E–L) Frogs treated with a βcat* limb bud cell patch and Shh + FGF10 beads (BSF), 6 mpa. Multidigit regenerates are formed (E, F, I, J) and some proximodistal segmentation of cartilage is evident (blue arrows in G, L). One digit also has a small patch of calcium deposition (red arrow in K). All panels except (H) are for BSF treatment. (H) shows skeletal preparation of a typical spike as control, 6 mpa.
(M–Q) A frog treated with a βcat* limb bud cell patch, Shh + FGF10+ thymosin β4 (BSFT), 5 mpa. Multiple digits have regenerated (M) and X-ray shows the presence of extensive ossification in the regenerate, as confirmed by skeletal staining (O, P). Some proximodistal segmentation is also clear (blue arrows in P). (Q) Regeneration of intermediate skeletal elements in frog shown in (M). White dotted lines indicate individual metacarpal-like structures.
(R) Detection of Gdf5 by in situ hybridization in regenerate from frog treated with BSFT. Cross section of a digit (2 mpa) shows joint-like structure, with Gdf5 expression (between red brackets).
(S) A skeletal preparation of a normal forelimb, for comparison.
Red dotted lines indicate amputation levels. Scale bars in (A, E, I, J, M, N), 500 µm; Scale bars in (B-D, F-H, K, L, O–S), 100 µm.
See also Fig. S3 and S4.
Normal limb development involves the provision of several extracellular signals to control regional determination, especially FGFs from the distal epidermis and Shh from the posterior mesoderm (Poss et al., 2003; Tickle, 2006). Both these factors have been shown to be associated with Xenopus regeneration and development (Endo et al., 2000; Lin and Slack, 2008; Yokoyama et al., 2001). The essential spatial organization of the early limb is lost during the dissociation process (Hardy et al., 1995; Yokoyama et al., 1998), so we provided the limb bud cells with Shh and FGF10, loaded onto Affi-Gel beads embedded in the fibrin solution before polymerization. The Shh and FGF10 slow release beads themselves applied to an amputated frog limb did not provoke any regeneration (Table S1 row 1), nor did the limb bud cell patch supplemented with Shh alone (Fig. 3D). Just a few multidigit regenerates were obtained when the limb bud cell patch was supplemented with both types of bead (Table S1 row 2–3).
Previous studies from our own and other labs have shown that activated Wnt/β-catenin signaling can promote appendage regeneration in Xenopus tadpoles (Kawakami et al., 2006; Lin and Slack, 2008). We had previously made a transgenic line (pHsβ-Catenin-GFP) expressing a stabilized, and therefore constitutively active, form of β-catenin, controlled by a temperature inducible promoter (denoted here as βcat*). Heat shock treatment induces high βcat*GFP expression in the regenerating tissues and increases nuclear localization of activated form of βCatenin proteins (Fig. S3G–J). The heat shock induction of βcat* also leads to the activation of Wnt/β-catenin target genes (not shown). We used cells from limbs of these tadpoles to test whether combination of βcat* limb cells with Shh and FGF10 can stimulate limb regeneration more effectively. They did indeed produce a higher percentage of multi-digit limb regenerates (Fig. 3E–L, Table 1). The digits mainly contain cartilage structures but in some cases, there is mineralization at the distal tip (Fig. 3K) and evidence of proximal-distal segmentation (Fig. 3L).
Thymosin β4 further improves the quality of limb regenerates
Thymosin β4 has been reported to aid wound healing, mobilize progenitor cells, inhibit inflammatory responses, and promote bone formation (Huff et al., 2001; Matsuo et al., 2012; Qiu et al., 2011; Smart et al., 2007; Sosne et al., 2002).The addition of thymosin β4 to our cell transplants did not greatly increase the percentage of multiple digit regeneration, but does provide more complete regeneration (Table 1; Fig. 3M–Q). This has several aspects. There is more bone formation, as visualized by X-ray imaging (Fig. 3N) and alizarin staining (Fig. 3O). There is some clear proximal-distal segmentation in the regenerated digits (Fig. 3P), and some metacarpal-like structures at the proximal end of the digits (Fig. 3Q). To check whether there is joint formation we examined the expression of Gdf5, a marker for joint development (Satoh et al., 2005; Storm et al., 1994; Storm and Kingsley, 1996, 1999). We found that Gdf5 is expressed in our regenerates but is absent from control spikes (Fig. S4A–C). On sections, some digits (5/22) also showed localized expression of Gdf5 in the cartilage (Fig. 3R). Thus, we consider these structures to be digits rather than spikes. In addition, we found that there is abundant innervation of the regenerates, as shown both by electron microscopy and immunostaining for neuronal specific β-tubulin III (Fig. S4D–K). There are some small nerve bundles in control spikes, but they are much larger and more numerous in the regenerates.
Exogenous factors improve cell survival and growth in cell patch transplant
What is the mechanism underlying the success of this cell-factor preparation? One obvious consideration is donor cell number, which can be affected by cell death and/or cell proliferation. When cells are simply injected into the limb we observed that there is considerable cell death, so that GFP-labeled cells are no longer detectable after a week. With the fibrin patch transplantation, we observed that GFP-positive cells could survive many weeks. The addition of Shh/Fgf10 beads and the activation of βcat* in the cells do not significantly reduce the percentage of donor cells undergoing apoptosis. But thymosin β4 in the patch does reduce apoptosis (Fig. 4A–F).
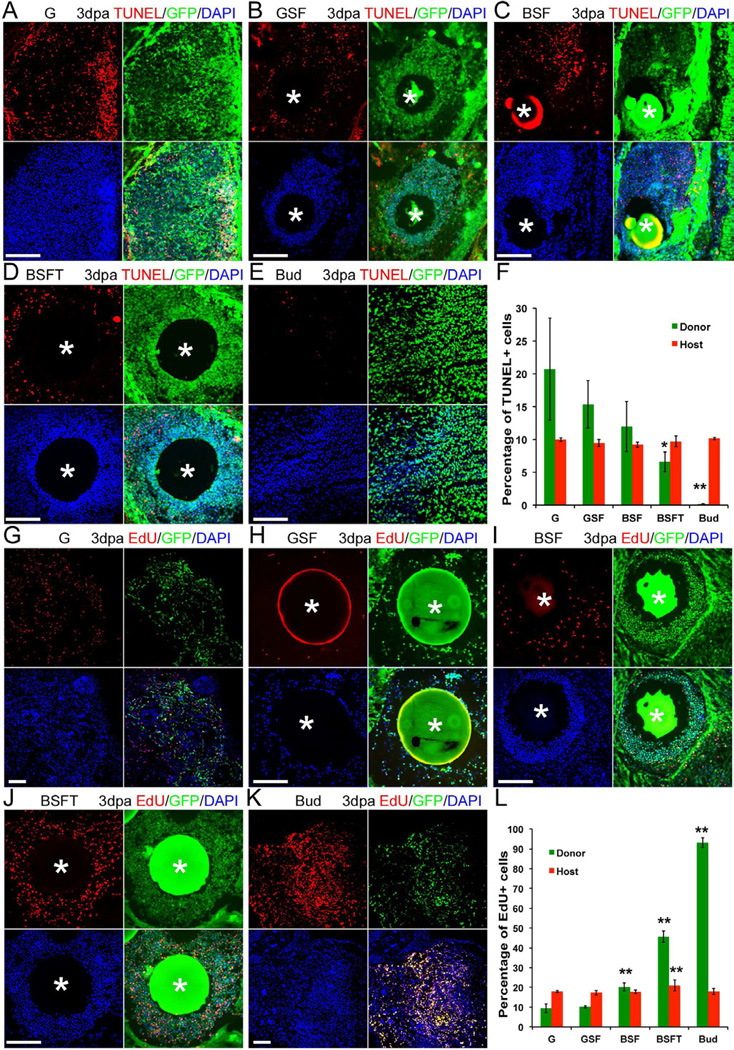
Fig. 4. Cell death and cell proliferation analysis in cell transplants.
(A–E) Cell death detection by TUNEL in 3 day regenerates after GFP-labeled limb cell patch or bud graft. (A) Cells with GFP label alone. (B) GFP limb cell patch with Shh and FGF10. (C) βcat* limb cell patch with Shh and FGF10. (D) βcat* limb cell patch with Shh+FGF10+thymosin β4. (E) Whole limb bud transplantation.Sections are through the cell patch or the transplanted bud. GFP shown in green indicates the nGFP or pHsβcat*GFP donor cells. The TUNEL signal is shown in red, and nuclei are shown in blue with 4',6-diamidino-2-phenylindole (DAPI) stain. White * in (B–D) indicate positions of Affi-Gel beads. Scale bars 100 µm.
(F) Quantification of cell death in the transplants (G=GFP label only; S=Sonic hedgehog; F=FGF10; B=activated β-catenin; T=thymosin β4). Error bars are standard deviations; n=4; Single factor ANOVA analysis shows a significant difference in groups; * (p<0.05), BSFT vs. BSF; ** (p<0.01), Bud vs. BSFT, as determined by t-test.
(G–K) Cell proliferation determined by EdU incorporation. EdU is shown in red, and nuclei are shown in blue. White * in (H–J) indicate positions of Affi-Gel beads. Scale bars 100 µm.
(L) Quantification of cell proliferation in the transplants, labels as above. Error bars are standard deviations; n=4; ANOVA and t-test analyses show significant differences between groups. ** indicates p<0.01, as determined by t-test.
The other key factor is the cell division rate. We examined cell proliferation by injecting EdU into the hosts and analyzing the proportion of donor and host cells undergoing DNA synthesis. The labeling of donor cells is low and is not significantly increased by Shh and FGF10. With βcat* activation it increases and is further increased by the presence of the thymosin β4 (Fig. 4G–L). The labeling index correlates well with the eventual mean extra digit count and so it is likely to be an important variable. Interestingly, while there is no significant difference of cell apoptosis in the host cells between different transplantation groups, addition of thymosin β4 in the cell patch does slightly, but significantly, increase the percentage of EdU-positive cells in the host limb tissue (Fig. 4L).
Reactivation of genes for patterning factors in cell patch transplants
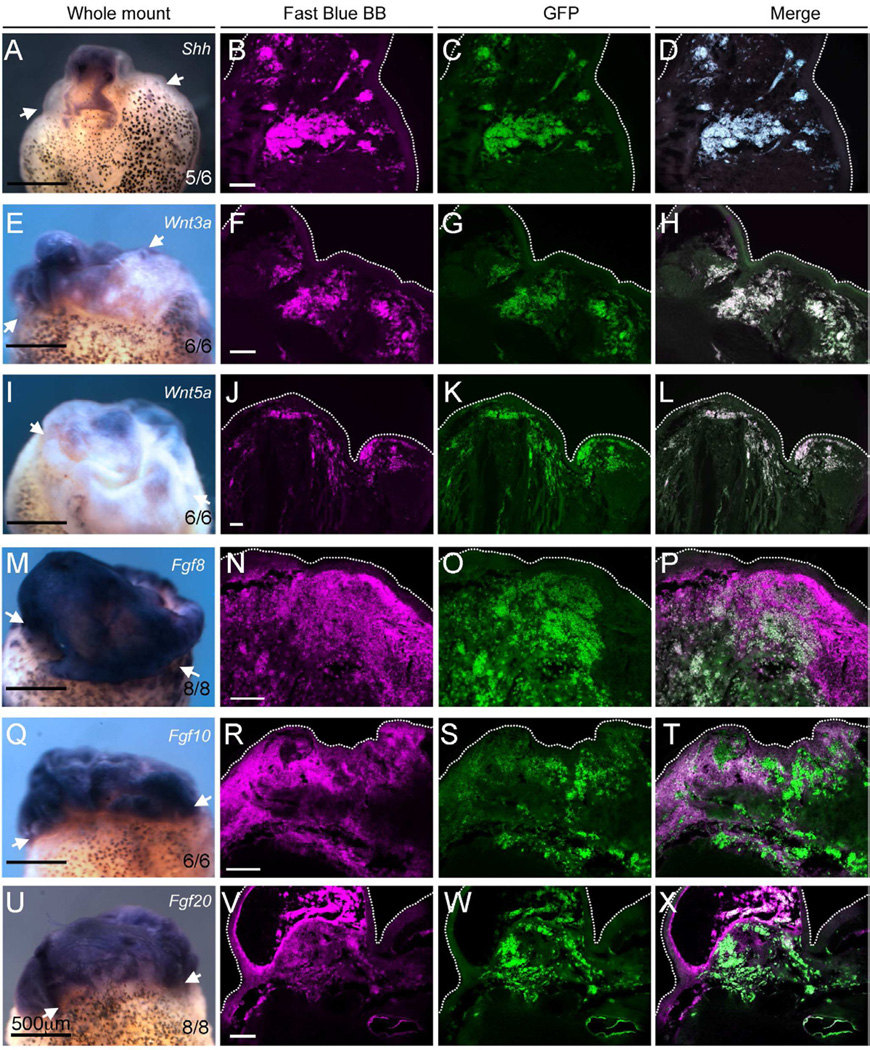
The other probable mechanism at work is the provision of pattern information by the Shh and FGF. It is likely that the proteins are lost from the Affigel beads after a few days but by this time expression of the endogenous genes in the graft should have become stabilized. Using in situ hybridization and RT-PCR we found that genes encoding FGFs, Wnts and Shh are expressed in the cell transplants (Fig. 5). By imaging the in situ signals with a far-red filter(700/±75nm emission) and GFP expression by a green filter (525/±50nm emission), we observed that Shh, Wnt3a, Wnt5a are exclusively expressed in the implanted cells, while there is expression of Fgf8, Fgf10 and Fgf20 both in the implanted cells and in the host cells (Fig. 5). Expression of Fgf20 is of particular interest, as it is thought to be regeneration-specific and essential for appendage regeneration (Whitehead et al., 2005). Consistent with its expression at the epithelium-mesenchyme boundary during appendage regeneration, we observed that Fgf20 is also expressed in GFP-negative cells in the deep layer of epithelium (Fig. 5V–X). This panel of genes is also expressed in regenerates from limb bud transplants, while, following the implantation of Shh/FGF10 beads alone, only Fgf8 and Fgf10 are slightly expressed (Fig. S5A–L).
Fig. 5. Upregulation of Shh, Wnt and Fgf genes in limb regenerates.
(A, E, I, M, Q, U) Whole mount in situ hybridization shows expression of Shh, Wnt and Fgf genes in limbs following BSFT treatment, 6 days pa. Scale bars 500 µm. Numbers indicate frequency of observed expression in limb regenerates.
(B, F, J, N, R, V) Far-red (Magenta) image of in situ signals developed with Fast Blue BB, with Y5 filter cube.
(C, G, K, O, S, W) Detection of GFP-positive cells with anti-GFP antibody and AlexaFluor 488 conjugated secondary antibody, with GFP filter cube.
(D, H, L, P, T, X) Merges: the white color indicates gene expression in GFP-positive cells.
(A–D) Shh, (E–G) Wnt3a, (I–L) Wnt5a, (M–P) Fgf8, (Q–T) Fgf10, (U–X) Fgf20. White dotted lines indicate the edges of the regenerates. Black scale bars 500 µm; White scale bars 100 µm.
See also Fig. S5.
Consistent with the expression of Fgfs and Wnts in the cell transplant, we also detected increased expression of MPK3, Lgr5 and Axin2, which have been used to report FGF and Wnt signaling activities in transgenic animals (Fig. S5M)(Barker et al., 2007; Jho et al., 2002; Kawakami et al., 2003; Lustig et al., 2002; Molina et al., 2007). This suggests that both FGF and Wnt signaling pathways are active in the cell transplant.
Although we have attempted to localize the Shh beads to one side of the patch, this is difficult and the beads often spread out. For this reason we would not expect to see a normal digit pattern in these experiments but consider that local gradients of Shh and FGF are necessary to get some pattern. Fig. S5N-U shows in situ hybridization of hoxa13, which is associated with distal character, although this is also upregulated without regeneration (Ohgo et al., 2010); and of hand2 (=dHAND), which is associated with posterior character (Zeller et al., 2009). The localized expression of hand2 is evidence for some anteroposterior pattern in the cell patch and is dependent on the presence of limb cells in the patch, as it is not expressed in limb stumps with bead implantation alone.
Only limb progenitor cells promote limb regeneration
To investigate whether cells from other sources have a similar ability to promote regeneration, we transplanted cells isolated from pHsβcat*GFP transgenic tadpole tail regeneration buds and treated with Shh and Fgf10 beads, together with thymosin β4. Xenopus tails are able to regenerate throughout most of the tadpole lifespan and the mechanisms have been well studied (Beck et al., 2009; Slack et al., 2008). The presence of the βcat* transgene promoted more outgrowth of epidermis in this type of transplant, but the overall regeneration is similar to untreated controls. Only 3 out of 20 showed a little extra cartilage formation alongside the spike cartilage (Table 1 row 6, Table S1 row 4, Fig. S6). In addition, we found that fibroblasts cultured from frog limbs, with or without Shh/FGF10 beads, failed to produce any structures other than the usual spike (Table S1 row 5). These results strongly indicate that there is a specific requirement for regeneration-competent limb progenitor cells to enable regeneration of limbs.
Substantial contribution of host cells to limb regenerate
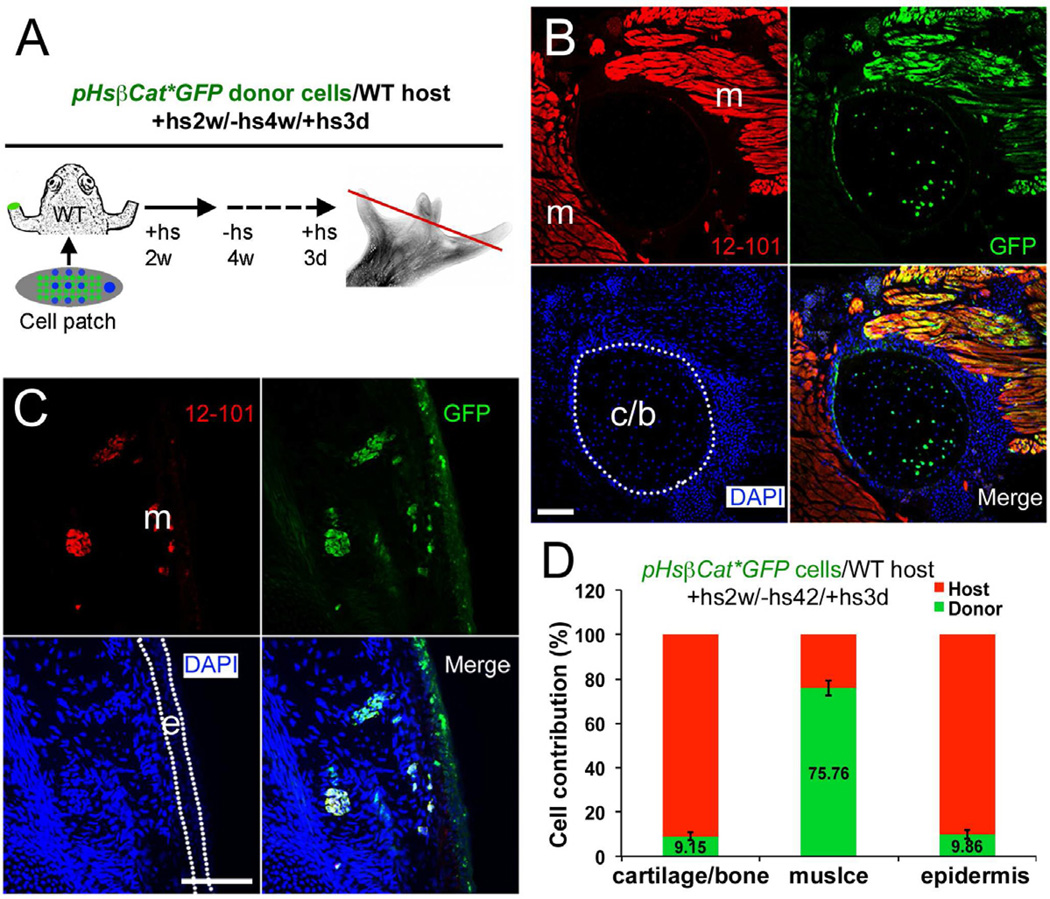
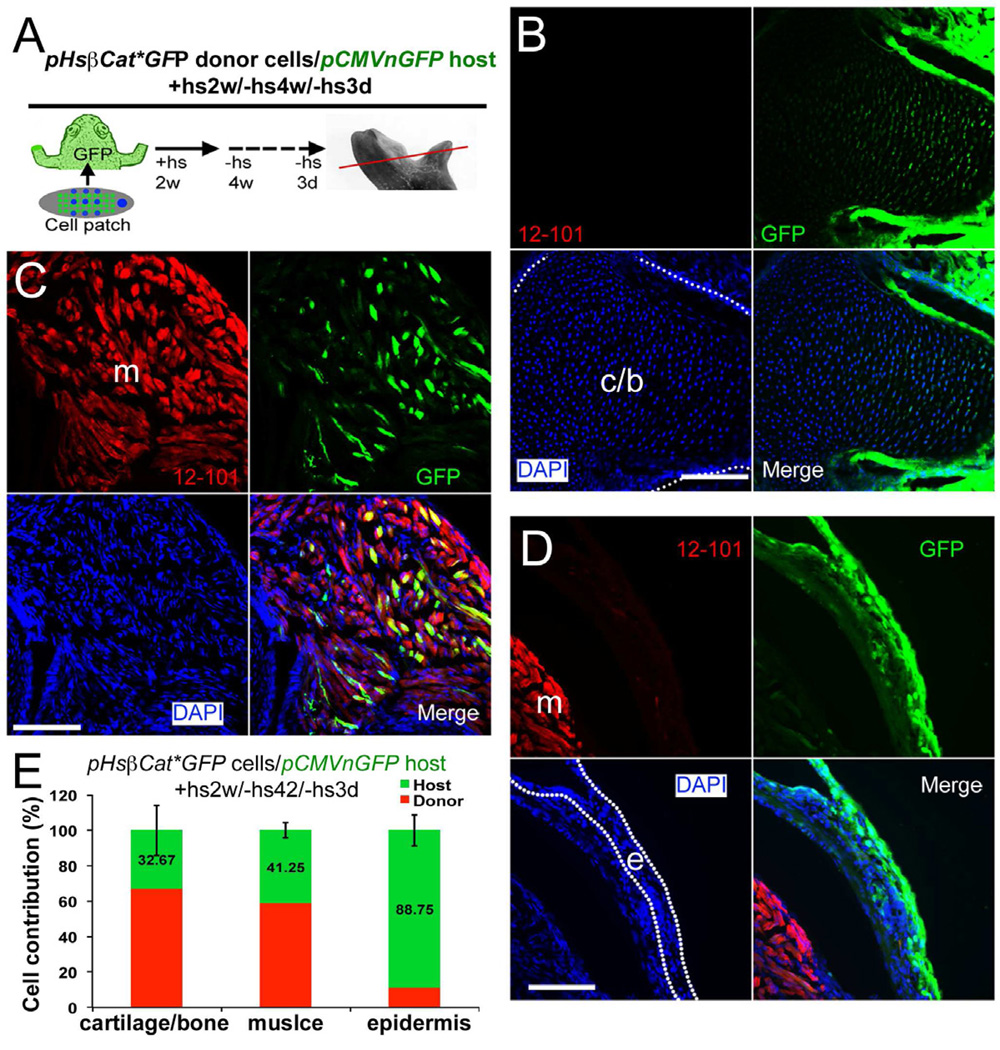
We had initially expected the regenerates to be composed entirely of donor cells, but this is not the case. To analyze cell composition we performed transplants using either GFP-labeled donors or hosts. Limb cells isolated from pHsβcat*GFP tadpole limb buds were used as donors in both types of experiments. After heat shock treatment for 3 days, GFP expression in pHsβcat*GFP limbs is activated in most, but not all, cells (Fig. S3K–P). This allows the detection of donor cells in experiments with wild type hosts (Fig. 6), but the presence of some unlabeled cells in the graft also raises the necessity for reciprocal experiments using labeled hosts. We found that GFP in pHsβcat*GFP tadpoles has ceased to be detectable by 4 weeks after the last heat shock. So for the pCMVnGFP host group, transplanted with pHsβcat*GFP cells, a period of at least 4 weeks without heat shocks was allowed before fixation (Fig. 7). Although there is some variation of cell contribution, both types of transplantation experiment showed that host cells contribute substantially to the regenerate in cartilage, muscle and connective tissues (Fig. 6 and 7). By contrast there is only limited contribution of donor cells to the epidermis of the regenerate.
Fig. 6. Analysis of donor cell contribution in limb regenerates after (pHsβcat*GFP donor)/ (wild type host) transplantations.
(A) Diagram of cell contribution analysis. Wild type host frogs were transplanted with a βcat* limb bud cell patch with Shh and FGF10 after amputation. Heat shock was given for the first two weeks to maintain βcat* expression. Frogs were left without heat shock for 4 weeks and heat shock was given 3 days before sample collection to activate GFP expression (fused to βcat*) in donor cells. The red line indicates the level of sections shown in (B,C)
(B,C) Detection of donor cells (green) in cartilage (B), muscle (red, with 12/101 antibody staining, B and C) and epidermis (C) in a regenerate illustrated as in (A), 45 dpa. m, muscle; c/b, cartilage or bone; e, epidermis. Muscle tissues are shown in red as revealed by immunostaining with 12/101, a specific muscle marker, and labeled as m in the red channel images; Cartilage and epidermis are outlined with white dotted lines in the blue channel images.
(D) Quantification of donor and host cell contributions in limb regenerates from (pHsβcat*GFP donor)/ (wild type host) transplantations. Error bars, standard deviation; n=9 animals.
See also Fig. S2, S3.
Fig. 7. Analysis of cell contribution in limb regenerates after (pHsβcat*GFP donor)/ (pCMVnGFP host) transplantations.
(A) Diagram of cell contribution analysis. pCMVnGFP host frogs were used, and GFP expression in pHsβcat*GFP donor cells was turned off by not giving heat shock treatments.Thus GFP+ cells shown in (B–D) are from the host. The red line indicates the level of sections shown in (B–D).
(B–D) Detection of host cells (green) in cartilage (B), muscle (C) and epidermis (D). Cartilage, muscle and epidermis are shown as above. Scale bars, 100 µm.
(E) Quantification of donor and host cell contributions in limb regenerates from (pHsβcat*GFP donor cells)/ (pCMVnGFP host) transplantations. Error bars, standard deviation; n=9 animals.
See also Fig. S2, S3.
Discussion
The combined total of rows 4 and 5 of Table 1 (i.e. regenerates with βcat* cell patch plus Shh, FGF, with or without thymosin β4) shows 43 multidigit regenerates out of 87 (49%), whereas there are none at all from control amputations, with or without applied factor-soaked beads. These regenerates are segmented, express the joint marker Gdf5 in some cases (Fig. 3), contain substantial muscle tissue (Fig. 6 and 7), may show ossification (Fig.3), and are innervated (Fig. S4). Though far from normal, they do seem to be functional. The frogs with the multidigit regenerates eat better than the spikebearing controls as their new limbs enable them to grab food. This means that they grow normally, while it is very common to see skinny control-amputated frogs. These results represent a remarkable stimulus of regeneration in an animal that does not normally do it.
The key requirements are the use of regeneration-competent limb progenitor cells, delivered in a manner enabling good cell survival, and supplemented with extracellular factors necessary for normal limb development. Both limb progenitor cells and growth factors are necessary for success (Fig. 3 and Table 1). Application of growth factors such as Fgf10 in tadpole limbs was reported to promote limb regeneration in late tadpole stages (Yokoyama et al., 2001), but we have failed to obtain similar results in either late stage tadpoles or post-metamorphic frogs (Table S1, Fig. 3 and data not shown). This suggests that the cells in post-metamorphic frog limbs have lost the competence to respond to growth factor signaling and highlights the potential of using progenitor cells in stimulating regeneration.
The fibrin patch method has been successfully used in delivery of bone marrow derived cells into the injured heart (Zhang et al., 2008), but it has not previously been adapted for appendage regeneration. Our results show that this delivery system is critical for success, which we believe is due to the ability to deliver a large number of cells to the amputation surface. In addition, the fibrin patch enables application of growth factors to the amputation surface (Fig. 2C). For example, thymosin β4 can bind directly to fibrin through the two glutamines (Q23, Q36) (Huff et al., 2001).
For long-term survival of transplanted cells the host need to be immunocompromized, such as by removal of the developing thymus, as reported here. One rationale for our use of thymosin β4 is the likelihood that there is some residual immune function following thymectomy at stage 48 (Horton and Manning, 1972), and regeneration might proceed better if this is locally suppressed. It has been proposed that loss of regeneration in vertebrate animals is correlated with development of an immune system that produce an inflammatory response in injured tissues (Mescher and Neff, 2005). The balance between inflammation and regeneration can be manipulated to favor one or the other. Suppression of immune responses with drugs has been shown to potentiate tail regeneration in Xenopus tadpoles (Fukazawa et al., 2009). It remains unclear whether immune suppression in adult frogs can also facilitate limb regeneration.
It is of great interest that non-regenerating host limb cells can contribute significantly to the multi-digit regenerates after cell patch transplantation and growth factor provision (Fig. 6 and 7). There are significant host contributions to cartilage, connective tissue and muscle fibers. The epidermis is almost entirely host-derived, but this is not surprising as the donor cells are taken from limb bud mesenchyme from which epidermis was excluded. The host contribution to internal tissues is surprising since the frog limb cannot normally regrow anything other than a simple cartilaginous spike. In other cell therapies such as treatment of muscular dystrophy by muscle stem cell transplantation (Zammit et al., 2006), it is expected that the transplanted progenitor cells should engraft and give rise to functional progeny, with restoration of normality of the injured or diseased tissue. But the diseased host cells remain defective. In our experiments, we believe that so long as regeneration-competent cells are present to define the pattern in the regenerating limb, the host cells can be mobilized and “fill in” the newly formed structures. This process may somewhat resemble limb myogenesis in normal development, where myoblasts from non-limb somite levels are able to populate the limb muscles, with the pattern determined by the mesenchyme of the limb bud (Chevallier et al., 1977; Rees et al., 2003).
Our procedure could in principle be used on the mammalian limb. The use of fetal limb cells to treat human limb amputations may cause ethical controversy, but equivalent cells could probably be produced with current induced pluripotent stem cell (iPSC) technology (Cohen and Melton, 2011; Stadtfeld and Hochedlinger, 2010). We can anticipate a procedure whereby iPSC are cultured from the patient, caused to differentiate to a state similar to normal limb bud cells, then be grafted onto an amputation surface together with the appropriate extracellular factors. This would provide a potential method for replacing large amounts of lost tissue without the need for immunosuppression.
Experimental Procedures
Thymectomy in Xenopus tadpoles
Xenopus laevis embryos were obtained by in vitro fertilization and staged according to the Nieuwkoop and Faber tables (Nieuwkoop and Faber, 1967). To prepare thymectomized hosts, the thymus was removed by coagulation with a Surgistat II electrosurgical generator (Valleylab). Stage 48–49 tadpoles were anesthetized in 0.02% MS222 (Sigma) and placed on a returning electrode. A tungsten needle electrode was inserted into the thymus and an electrical current was applied at a setting of 3 watts coagulation. Operated tadpoles were raised in 0.1× MMR to frogs 3–4 cm snout-vent length which were used as hosts for cell or tissue grafts.
Limb bud grafting and amputation
Both donor tadpoles and host frogs were anesthetized in 0.02% MS222. A 2–3 mm long incision was made in the left limb skin of the host proximal to the wrist or ankle and a piece of muscle tissue was removed. A donor limb bud was removed from a stage 53 tadpole and inserted into the site. The limb bud was positioned parallel to the host limb regarding the proximal-distal axis. The host was kept in MS222 solution for about an hour before returning back to frog water. Wounds usually close quickly. The day after grafting, both the host limb and the grafted limb bud were amputated at the same level. The right limb was amputated at the same level as control.
Transgenic animals
Transgenic animals were generated as described (Kroll and Amaya, 1996), except that the restriction enzyme was omitted. The heat shock inducible, GFP fused activated β-catenin plasmid (pCH85, renamed here as pHsβcat*GFP) was a gift from Arne Lekven (Texas A&M University). The N-terminus deletion of β-catenin stabilizes β-catenin and makes it constitutively active (Munemitsu et al., 1996). pCMVnGFP transgenic animals were generated by in vitro fertilization from founder animals that were previously created from pcDNA3nucGFP transgenic construct. Transgenic embryos were raised in 0.1×MMR and transferred to a circulating aquatic system before metamorphosis. Upon heat shock induction, pHsβCat*GFP animals express GFP in both the nuclei and cytoplasm. It is visible for a few days but becomes undetectable within 2 weeks. pCMVnGFP animals express GFP mainly in the nuclei, but cytoplasmic GFP is also apparent, especially in muscle fibers.
Limb progenitor cell dissociation and transplantation with fibrin patch
Thirty developing hindlimb buds from stage 53 tadpoles were isolated. The limb epidermis was removed by peeling with fine forceps after making an incision with a fine microsurgical knife. The mesenchyme tissue was minced as small as possible, collected into a 1.5 ml Eppendorf tube and washed twice in PBS (without Ca2+, Mg2+). The tissues were then incubated in 1 ml of TrypLE medium (Invitrogen) for 30 minutes at 37°C with rotation. The resulting single cell suspension was pelleted by mild centrifugation and washed three times in 70% Hank’s medium (Invitrogen). Dissociated cells were then resuspended in fibrinogen solution to prepare the fibrin gel for transplantation, according to the method described previously (Zhang et al., 2008). Immediately after limb amputation, fibrin gels containing limb progenitor cells were placed directly onto the limb stump. Affi-Gel beads previously soaked in growth factors (FGF10, Shh, R&D) were mixed into the fibrin gel or implanted into the fibrin patch shortly after cell transplantation. When pHsβcat*GFP cells were used, frogs were given a daily 30 minute heat shock in a 34°C water bath, starting from the second day, for 2 weeks.
Skeletal staining
This is as described previously (Inouye, 1976). Briefly, frogs were euthanized with overdose of MS222, skinned and eviscerated, washed briefly with water and then fixed in 95% of ethanol overnight. Cartilage was stained with 0.03% Alcian blue (Sigma, in 80% ethanol, 20% acetic acid solution). After washing in 95% ethanol the specimens were cleared in 1% KOH until the skeleton was clearly visible. Bone staining was carried out in 0.03% Alizarin Red (Sigma, in 1% KOH solution). After staining, specimens were washed in glycerol: 95% ethanol (1:1) solution for one to several days. Specimens were passed gradually through glycerol/ethanol solution (80% and 100%) and kept in glycerol.
Immunohistochemistry
Frog limb regenerates were fixed in Zamboni’s fixative (40 mM NaH2PO4, 120 mM Na2HPO4, 2% PFA, 0.1% saturated picric acid), washed in PBS, 15% sucrose/PBS and embedded in OCT medium for cryosectioning. The slides were dried overnight, permeabilized with 1% Triton in PBS, blocked with BM blocking reagent, and incubated with the muscle specific antibody 12–101 (Kintner and Brockes, 1984) or GFP antibodies for one to several hours. Slides were washed in PBS and secondary antibodies (Alexa Fluor dye conjugated goat anti-mouse, or goat anti-rabbit, antibodies, Invitrogen) were used at 1:500 dilution. Slides were counterstained with 4',6-diamidino- 2-phenylindole (DAPI) before mounted with Gel Mount medium.
Cell composition analysis in limb regenerates
To determine donor and host cell contributions in limb regenerate after whole limb bud transplantation (Fig. S2), either the donor limb bud or the host is transgenic for pCMVnGFP. GFP-positive cells were detected by immunofluorescence staining with anti-GFP antibody as described above. The percentage of GFP-positive cells in cartilage, muscle and epidermis were determined by counting at least three non-adjacent sections from each of 9 animals. Although the GFP construct contains a nuclear localization sequence there is also significant cytoplasmic expression in these transgenics. Labeled nuclei were counted for epidermis and cartilage, and labeled muscle fibers were counted by cytoplasmic GFP. The labeled muscle fibers may contain both donor and host nuclei due to myoblast fusion.
For cell contribution analysis after dissociated limb cell transplantation (Fig. 6, 7), either wild type or pCMVnGFP frogs were used as hosts, while the donor cells were from pHsβcat*GFP tadpole limb buds. In pHsβcat*GFP transgenic tadpoles, GFP expression can be upregulated in most of the limb cells with a 3-day (30 mins/day) heat shock (Fig. S3). It becomes undetectable if the tadpole is not subjected to heat shock treatments for 4 weeks. Thus GFP expression can be used to detect either donor cells in (pHsβcat*GFP donor)/(wild type host) transplantations, or host cells in (pHsβcat*GFP donor)/(pCMVnGFP host) transplantations. Cell counting was carried out as for the whole bud grafts.
Cell proliferation and cell death assays
A Click-iT EdU Assay kit (Invitrogen) was used for cell proliferation analysis according to the manufacturer’s instructions. Briefly, 0.1 ml of 1mM solution of EdU was injected into the frog I.P. Specimens were collected 48 hours later, fixed and cryosectioned, and EdU detection was performed after the antibody staining. For cell apoptosis analysis, an in situ cell death detection kit (Roche) was used after antibody staining, according to the manufacturer’s instructions. Proliferating and apoptotic cells were counted in two series of sections of regenerating tissues from three independent experiments. For calculation of the percentage of EdU-positive and TUNEL-positive donor cells, only GFP positive cells were counted. For host cells only GFP-negative cells were counted, in sections where few GFP-positive cells were present.
In situ hybridization
In situ hybridization on limb regenerates was performed essentially as described (Sive et al., 2000). For detection of GFP-positive donor cells in the limb regenerate after in situ hybridization, Fast Blue BB (Sigma) was used for color development. Stained specimens were embedded in Tissue Freezing Medium (Leica) and 10 µm sections were collected on poly-lysine-coated slides (Fisher). Sections were then immune stained with anti-GFP (1:500, Abcam) as first antibody and Alexa Fluor 488 goat anti-rabbit IgG (1:500, Invitrogen) as second antibody. Photomicrographs were taken on a Leica MDI6000 microscope. For fluorescence detection of in situ signals developed with Fast Blue, a Leica Y5 filter cube (excitation 620/60nm, emission 700/75nm) was used (Lauter et al., 2011). For GFP expression, a GFP filter cube (excitation 470/40nm, emission 525/50nm) was used. Images were processed with IPLab software (BioVision Technologies) and figures were prepared with Adobe Photoshop (Adobe).
Statistical analysis
Analysis of variance (ANOVA) and unpaired t-test were used for the analysis of the data shown Fig. 4. The Mann-Whitney Rank Sum test was used for analysis of the digit regeneration data shown in Tables 1 and S1. Differences were considered to be significant for P-values <0.05 (*), or P<0.01(**).
Supplementary Material
Highlights.
-
➢
Transplantation of limb progenitor cells promotes multiple digit regenerates
-
➢
Wnt/βCatenin activation is required to stimulate adult frog limb regeneration
-
➢
Growth factors promote cell survival and growth and give pattern information
-
➢
Regeneration- incompetent host limb cells contribute to the regenerated limbs
Acknowledgements
We thank Dr. Nobuaki Kikyo and members of our laboratory for valuable scientific discussions and comments, and Dr. Yasuhiko Kawakami for critical reading of the manuscript. We thank Fang Zhou (Characterization Facility, College of Science and Engineering, University of Minnesota) for assistance with electron microscopy. This work was supported by Eureka grant R01GM088500 from the National Institute of General Medical Sciences (NIGMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Barker D, Slack JMW. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech Dev. 2006;123:674–688. doi: 10.1016/j.mod.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Beck CW, Izpisúa Belmonte JC, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Comparative Aspects of Animal Regeneration. Annu Review Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Principles of Regenerative Biology. Burlington MA: Academic Press; 2007. [Google Scholar]
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol Eexp Morph. 1977;41:245–258. [PubMed] [Google Scholar]
- Christen B, Slack JM. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev Biol. 1997;192:455–466. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- Christen B, Slack JMW. All limbs are not the same. Nature. 1998;395:230–231. doi: 10.1038/26133. [DOI] [PubMed] [Google Scholar]
- Christensen RN, Weinstein M, Tassava RA. Expression of fibroblast growth factors 4, 8, and 10 in limbs, flanks, and blastemas of Ambystoma. Dev Dyn. 2002;223:193–203. doi: 10.1002/dvdy.10049. [DOI] [PubMed] [Google Scholar]
- Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- Daughters RS, Chen Y, Slack JM. Origin of muscle satellite cells in the Xenopus embryo. Development. 2011;138:821–830. doi: 10.1242/dev.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morph. 1962;110:61–77. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- Endo T, Tamura K, Ide H. Analysis of gene expressions during Xenopus forelimb regeneration. Dev Biol. 2000;220:296–306. doi: 10.1006/dbio.2000.9641. [DOI] [PubMed] [Google Scholar]
- Endo T, Yokoyama H, Tamura K, Ide H. Shh expression in developing and regenerating limb buds of Xenopus laevis. Dev Dyn. 1997;209:227–232. doi: 10.1002/(SICI)1097-0177(199706)209:2<227::AID-AJA8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. 2009;136:2323–2327. doi: 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- Han MJ, An JY, Kim WS. Expression patterns of Fgf-8 during development and limb regeneration of the axolotl. Dev Dyn. 2001;220:40–48. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1085>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hardy A, Richardson MK, Francis-West PH, Rodriguez C, Izpisua-Belmonte JC, Duprez D, Wolpert L. Gene expression, polarising activity and skeletal patterning in reaggregated hind limb mesenchyme. Development. 1995;121:4329–4337. doi: 10.1242/dev.121.12.4329. [DOI] [PubMed] [Google Scholar]
- Horton JD, Manning MJ. Response to skin allografts in Xenopus laevis following thymectomy at early stages of lymphoid organ maturation. Transplant. 1972;14:141–154. doi: 10.1097/00007890-197208000-00001. [DOI] [PubMed] [Google Scholar]
- Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. beta- Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Yoshizato K. Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds. Proc Natl Acad Sci U S A. 1997;94:9159–9164. doi: 10.1073/pnas.94.17.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Differential staining of cartilage and bone in fetal mouse skeleton by Alcian Blue and Alizarin Red. S. Congent Anom. 1976;16:171–173. [Google Scholar]
- Jho E-h, Zhang T, Domon C, Joo C-K, Freund J-N, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Martí M, Dubova I, Izpisúa Belmonte JC. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232–3237. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Rodríguez-León J, Koth CM, Büscher D, Itoh T, Raya A, Ng JK, Esteban CR, Takahashi S, Henrique D, et al. MKP3 mediates the cellular response to FGF8 signalling in the vertebrate limb. Nat Cell Biol. 2003;5:513–519. doi: 10.1038/ncb989. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulinsecreting cells in vivo. Nature Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Lauter G, Soll I, Hauptmann G. Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Dev Biol. 2011;11:43. doi: 10.1186/1471-213X-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Slack JMW. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Dev Biol. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Akasaki Y, Adachi K, Zhang M, Ishikawa A, Jimi E, Nishihara T, Hosokawa R. Promoting effects of thymosin beta4 on granulation tissue and new bone formation after tooth extraction in rats. Oral surgery, oral medicine, oral pathology and oral radiology. 2012;114:17–26. doi: 10.1016/j.tripleo.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Eng Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Molina GA, Watkins SC, Tsang M. Generation of FGF reporter transgenic zebrafish and their utility in chemical screens. BMC Dev Biol. 2007;7:62. doi: 10.1186/1471-213X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TL, Ngo-Muller V, Reginelli A, Taylor G, Anderson R, Muneoka K. Regeneration in higher vertebrates: Limb buds and digit tips. Semin Cell Dev Biol. 1999;10:405–413. doi: 10.1006/scdb.1999.0327. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Hollerdinsmore G, Bryant SV. Intrinsic Control of Regenerative Loss in Xenopus-Laevis Limbs. J Exp Zool. 1986;240:47–54. doi: 10.1002/jez.1402400107. [DOI] [PubMed] [Google Scholar]
- Nacu E, Tanaka EM. Limb Regeneration: A New Development? Annu Review Cell Dev Biol. 2011;27:409–440. doi: 10.1146/annurev-cellbio-092910-154115. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland; 1967. [Google Scholar]
- Nye HLD, A CJ, Chernoff EAG, Stocum DL. Regeneration of the urodele limb: a review. Dev Dyn. 2003;226:280–294. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- Ohgo S, Itoh A, Suzuki M, Satoh A, Yokoyama H, Tamura K. Analysis of hoxa11 and hoxa13 expression during patternless limb regeneration in Xenopus. Dev Biol. 2010;338:148–157. doi: 10.1016/j.ydbio.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dyn. 2003;226:202–210. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- Qiu P, Wheater MK, Qiu Y, Sosne G. Thymosin {beta}4 inhibits TNF- {alpha}-induced NF-{kappa}B activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J. 2011;25:1815–1826. doi: 10.1096/fj.10-167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, Young RD, Evans DJR. Spatial and temporal contribution of somitic myoblasts to avian hind limb muscles. Dev Biol. 2003;253:264–278. doi: 10.1016/s0012-1606(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Satoh A, Suzuki M, Amano T, Tamura K, Ide H. Joint development in Xenopus laevis and induction of segmentations in regenerating froglet limb (spike) Dev Dyn. 2005;233:1444–1453. doi: 10.1002/dvdy.20484. [DOI] [PubMed] [Google Scholar]
- Sessions SK, Bryant SV. Evidence that regenerative ability is an intrinsic property of limb cells in Xenopus. J Exp Zool. 1988;247:39–44. doi: 10.1002/jez.1402470106. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Slack JMW, Lin G, Chen Y. The Xenopus tadpole: a new model for regeneration research. Cell Mol Life Sci. 2008;65:54–63. doi: 10.1007/s00018-007-7431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AAD, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–299. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Development. 1996;122:3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- Storm EE, Kingsley DM. GDF5 coordinates bone and joint formation during digit development. Dev Biol. 1999;209:11–27. doi: 10.1006/dbio.1999.9241. [DOI] [PubMed] [Google Scholar]
- Tickle C. Developmental cell biology: Making digit patterns in the vertebrate limb. Nat Rev Mol Cell Biol. 2006;7:45–53. doi: 10.1038/nrm1830. [DOI] [PubMed] [Google Scholar]
- Whitehead GG, Makino S, Lien C-L, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- Yakushiji N, Yokoyama H, Tamura K. Repatterning in amphibian limb regeneration: A model for study of genetic and epigenetic control of organ regeneration. Semin Cell Dev Biol. 2009;20:565–574. doi: 10.1016/j.semcdb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Endo T, Tamura K, Yajima H, Ide H. Multiple digit formation in Xenopus limb bud recombinants. Dev Biol. 1998;196:1–10. doi: 10.1006/dbio.1998.8856. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Ide H, Tamura K. FGF-10 stimulates limb regeneration ability in Xenopus laevis. Dev Biol. 2001;233:72–79. doi: 10.1006/dbio.2001.0180. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Zeller R, López-Ríos J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.