Abstract
The cardiac homeobox gene Nkx2.5 plays a key and dosage-sensitive role in the differentiation of outflow tract and right ventricle from progenitors of the second heart field (SHF) and Nkx2.5 mutation is strongly associated with human outflow tract congenital heart disease (OFT CHD). Therefore defining the regulatory mechanisms controlling Nkx2.5 expression in SHF populations serves an important function in understanding the etiology of complex CHD. Through a comparative analysis of regulatory elements controlling SHF expression of Nkx2.5 in the chicken and mouse, we have found evidence that Nkx2.5 autoregulation is important for maintaining Nkx2.5 expression during SHF differentiation in both species. However the mechanism of Nkx2.5 maintenance differs between placental mammals and non-mammalian vertebrates: In chick Nkx2.5 binds directly to a genomic enhancer element that is required to maintain Nkx2.5 expression in the SHF. In addition, it is likely that this is true in other non-mammalian vertebrates given that they possess a similar genomic organization. By contrast, in placental mammals, Nkx2.5 autoregulation in the SHF functions indirectly through Mef2c. These data underscore a tight relationship in mammals between Nkx2.5 and Mef2c in SHF transcriptional regulation, and highlight the potential for evolutionary cis-regulatory analysis to identify core, conserved components of the gene networks controlling heart development.
Keywords: Nkx2.5, Mef2c, Outflow Tract, Second Heart Field, transcription, congenital heart disease, enhancer, transgenic, progenitor, myocyte
Introduction
Second heart field development and outflow tract congenital heart disease
Outflow tract (OFT)-associated malformations of the aorta and pulmonary artery comprise a significant proportion of all congenital heart disease (CHD) with an overall prevalence of 200–400,000 in the US population alone (Lloyd-Jones et al., 2010). A principle source of myocardial, smooth muscle and endothelial progenitors that contribute to the OFT is the second heart field (SHF) and misspecification of the SHF plays a critical role in the genesis of CHD in animal models. Because of this, factors regulating the development of the SHF are the topic of intense study in the field of cardiac embryology. The SHF is defined as a pharyngeal mesoderm population whose differentiation and contribution to the developing heart is distinct from that of the cardiac crescent or first heart field (FHF) that primarily contributes to the left ventricle. The SHF, together with the more spatially restricted secondary heart field (Waldo et al., 2001), gives rise to OFT and right ventricle (Cai et al., 2003; Kelly, 2012; Kelly et al., 2001; Meilhac et al., 2004; Zaffran et al., 2004).
Nkx2.5 in OFT CHD
A gene high in the hierachy of SHF regulation and one frequently associated with OFT CHD in humans is the cardiac homeobox gene Nkx2.5. It is estimated that Nkx2.5 mutations account for approximately 4% of human cardiovascular malformations (Benson, 2010). Point mutations that create hypomorphic alleles of the Nkx2.5 gene have been implicated in familial, sporadic, syndromic and non-syndromic CHD. In particular, both Tetralogy of Fallot (TOF), a cardiac looping defect leading to pulmonary artery stenosis with secondary right ventricular hypertrophy, ventricular septal defect, and overriding aortic valve; and secundum atrial septal defect without atrio-ventricular conduction delay are associated with Nkx2.5 mutations (Goldmuntz et al., 2001; McElhinney et al., 2003). Loss of function experiments in mice, reveal that Nkx2.5 regulation is critical for normal SHF development: OFT malformations of increasing severity are observed with progressively lower levels of Nkx2.5 expression from heterozygous, null or hypomorphic alleles (Lints et al., 1993; Prall et al., 2007; Tanaka et al., 1999) likely due to altered regulation of direct or indirect Nkx2.5 target genes (Barth et al., 2010; Kasahara et al., 2000; Kasahara et al., 2001; Prall et al., 2007). Given the strong relationship between Nkx2.5 expression levels in the SHF and normal development, an important aspect of OFT morphogenesis is the cis-regulation of Nkx2.5 in SHF progenitors and differentiating myocytes of the aortic pole. Nkx2.5 likely functions within a complex network of transcription factors, growth factors, matrix molecules and other determinants of cell phenotype collaboratively controlling heart formation. Unravelling the normal connections of Nkx2.5 to these other pathways is therefore an important step towards better understanding genetically complex CHD, and has the potential to help define new disease causing genes (Benson, 2010; Granados-Riveron et al., 2011).
Materials and Methods
Vectors and Plasmids
Chicken Nkx2.5 reporters
Cloning of wild-type chicken Nkx2.5 reporter constructs, including: Nkx2.5-lux-CAR3, Nkx2.5-lacZ-CAR3, Nkx2.5-lux-BMPRE have been previously described (Lee et al., 2004). BMPRE linker scanning mutant m5 was created by PCR mutagenesis substituting bps 35–45 of a 200 bp BMPRE cassette with a modified NotI restriction site using the oligonucleotide and its complement: 3’-CAG TCA AAA CAT CGC GGC CGC GTC TGA GAT TGT CC-5’ (IDTDNA, Coralville, IA) and the QuikChange site-specific mutagenesis kit (Stratagene, LaJolla, CA). Similarly, the Nkx2.5 binding consensus mutation (nm) was created in 200 bp BMPRE and 2 kb CAR3 enhancer cassettes using the oligonucleotide 5’-CAG TCA AAA CAT CCT CCGAGT CTG AGA TTG TCC-3’ and its complement. Mutagenized BMPRE and CAR3 cassettes were ligated 3’ of the Nkx2.5 promoter-reporter constructs expressing lacZ, or luciferase coding region derived from the pGL3 basic plasmid (Promega, Madison, WI) or NLS cre recombinase (a kind gift of F. Alt) fused in-frame with the first 10 amino acids of the chicken Nkx2.5 coding region. The CNkx2.5-SHF-lacZ nm reporter additionally contained a 5’ HS4 insulator sequence from the chicken β-globin locus (Chung et al., 1993) (a kind gift of G. Felsenfeld).
Mouse Nkx2.5 reporter genes
Mouse Nkx2.5 lacZ and lux reporter genes were created by PCR amplifying the 494 bp conserved 5’ mouse Nkx2.5 enhancer (representing nt (−9109) to (−9603) relative to transcriptional initiation site of Nkx2.5, accession #NM008700.2) from C57BL6 genomic DNA using the oligonucleotides 5’-GGC CTC GAG CCT CGC TCC AGT CAA ACT TC-3’ and 5’-GGC CTC GAG TTG GCT GTT CCT TGT GTT-3’ and cloning the obtained fragment into a 5’ XhoI site of the chicken Nkx2.5 endogenous minimal promoter-reporter (above and (Lee et al., 2004). Mutation of the consensus Mef2 site at nt (−9218) to (−9226) was accomplished using PCR-mediated site specific mutagenesis as above using the oligonucleotide 5’-AAT CGA TAG GGC CCT TTCGAA TAG CTC CGA GTT TCC TGT CGG-3’ and its complement. Similarly, conversion of the Mef2 CArG site to an Nkx2.5-binding NKE element was accomplished using the oligonucleotide: 5’- GGG AAG ATA AAG TAA TCG ATA GGA GAC ACA CTC AGA GGC CGA GTT TCC TGT CGG GCC AGG -3' and its complement.
Mouse lines and generation of knockout embryos
Nkx2.5 wild-type, heterozygous and null embryos were generated through timed mating, maternal sacrifice and embryo collection from Nkx2.5-lacZ knock-in heterozygous mice (a kind gift of W.T. Pu) and genotyped according to previously described methods (Tanaka et al., 1999). Mef2c knockout mice were generated from mice bearing a conditional null allele of Mef2c (Arnold et al., 2007) (a kind gift of E. Olson) by germ line deletion through mating to EIIa-Cre driver mice (Jackson Labs, Bar Harbor, ME). Mef2c wild-type, heterozygous and null embryos were generated as above and genotyped as previously described (Arnold et al., 2007).
Transient and stable transgenic mouse assays
Mouse and chicken Nkx2.5-SHF wild-type and mutant reporter constructs were purified from their p Blue script backbones by restriction digestion, gel electrophoresis and extraction using QIAEX bead affinity purification (Qiagen, Valencia, CA). Linear DNA was introduced by pronuclear injection into a one-cell staged FVB mouse embryos according to standard methods. F0 embryos were collected at 7.5–10.5 days post-injection following maternal sacrifice, fixed and stained for β-galactosidase activity according to previously described methods (Zimmerman et al., 1994). Transgenic status of individual embryos was determined by PCR from DNA derived from yolk sacs and embryo fragments using oligonucleotides for lacZ: 5’-CGG CCA GGA CAG TCG TTT GCC GTC TG-3’ and 5’-CCT GAC CAT GCA GAG GAT GAT GCT CG-3’; and, for the constitutive gene Prx1: 5’-CCT GAG TTA CCT GCA CTC TG’3’ and 5’-AGG ACT GAG GAG GAT TCT TG-3’. Stable lines were obtained by mating fully-grown F0 to wild-type FVB mice, and screening progeny by PCR of genomic DNA derived from tail preparations as above, and for Cre sequences using oligonucleotides 5’-CCT GGA AAA TGC TTC TGT CCT-3’ and 5’-CAG GGT GTT ATA AGC AAT CCC-3’. Lineage tracing of CNkx2.5-SHF nm-cre bearing lines was accomplished using timed matings of Cre lines to the conditionally activated LacZ cre reporter mouse line R26R Rosa-LacZ (Jackson Labs, Bar Harbor, MN), followed by embryo harvest, genotyping and LacZ staining as above. For section analysis, whole-mount stained embryos were dehydrated through a graded alcohol series, equilibrated in HistoClear (National Diagnostics, Atlanta, GA) and paraplast-embedded prior to sectioning, de-paraffinization in xylene, counter stain with eosin and mounting in Permount (Sigma-Aldrich, St. Louis MO).
In vivo cellular reporter assays
BMP and overexpression reporter gene assays were performed in P19CL6 cells as previously described (Lee et al., 2004). All assay results are presented as fold induction of duplicate unstimulated versus BMP stimulated activities; all transfections are representative of 3 independent experiments. pCS2 Nkx2.5 and pCS2 Nkx2.5 (Ile183→Pro) were a kind gift from S. Izumo and H. Kasahara. pCS2 MT-Smad1, pCS2 MT/Flag Smad4 and pCS2 Alk3* were a kind gift from M. Whitman. pcDNA rat GATA-4 was derived from pCG-GATA-4, a kind gift of M. Nemer. pcDNA mouse GATA-6 was a kind gift of T. Collins. pcDNA SRF was a kind gift of E. Olson. pCMV-SPORT6 Mef2c (based on cDNA accession number BC026841) was purchased from Thermo Scientific (OpenBiosystems, Huntsville, AL). Recombinant BMP4 and Noggin was purchased from R&D Systems (Minneapolis, MN). Statistical significance of reporter gene activation was calculated using Student’s t-test with significance threshold of p≤ 0.01.
Gel shift assays
Gel shift assays were performed as previously described (Lee et al., 2004) using nuclear extracts prepared from HEK293 cells programmed to express Nkx2.5, SRF or Mef2c using 0.5–1.0 ug dI/dC (Pharmacia), resolved on 5% acrylamide/1X TBE (Invitrogen, Carlsbad, CA) at 4°C, dried and autoradiographed on Kodak XAR X-ray film for digitization. Gel shifts were also performed using GST- Smad4 MH1 domain fusion proteins and control GST proteins as previously described (Lee et al., 2004). Gel shifts were performed using the following end-P32 labeled double stranded oligonucleotides and their complements: Nkx2.5 binding site: 5’-ACA TCC TCT GAG TGT CTG AG-3’; Nkx2.5 (NKE mut) 5’-ACA TCC TCT GCG AGT CTG AG-3’; mNkx2.5 Mef2 binding site: 5’-TAA TCG ATA GGG CCC TTT TAA ATA GCT CCG AGT TTC CTG TCG G-3’; mNkx2.5 Mef2 binding site mutant: 5’-AAT CGA TAG GGC CCT TTCGAA TAG CTC CGA GTT TCC TGT CG-3’; control Mef2c binding site: 5’-CGC TCT AAA AAT AAC CCT-3’; control Mef2c binding site (mutant): 5’-CGC TCT AAG GCT ACC CT-3’ (Yu et al., 1992). Gel shift quantification of Smad4 binding was calculated from digitized autoradiogram images using ImageQuantTL (v7.0) software (GE, Fairfield, CT)
Chromatin Immunoprecipitation Assays (ChIP)
Chromatin extracted from wild-type FVB E10.5 mouse hearts were assayed by ChIP according to previously described methods (Barth et al., 2010) using anti-Mef2c antibody (Sigma-Aldrich, #HPA005533) or antiserum for Nkx2.5 (a kind gift of C. Carlson and A. Ansari) and primers 5’ CTC TGC TGT GTG GCC TTG TA-3’ and 5’-CGA CAG GAA ACT CGG AGC TA-3’ (rev) (mNkx2.5 SHF region); 5’-CTC CTG CAA GGA GAT TGC TC-3’ (for) and 5’-CCT CAC CAG CCC ATT TAG TG-3’ (rev) (mNkx2.5 promoter region); and, 5’-CTA CAA GTG CAA GCG ACA GC-3’ (for) and 5’-GCG TTG TAG CCA TAG GCA TT-3’ (rev) (mNkx2.5 exon 2). Results are expressed as fold enrichment based upon ΔC(t) calculations compared to control antiserum or control Ig. For ChIP assay of chick Nkx2.5-SHF reporters, P19CL6 cells were seeded in 60 mM dishes as described above transfected after overnight incubation with 0.4 ug each CNkx2.5-SHF-GFP reporter and pCS2 Nkx2.5 expression vector. Cells were then formaldehyde fixed and harvested chromatin subject to overnight immunoprecipitation with either control IgG or anti-Nkx2.5 antibody (H-114, sc-14033, Santa Cruz Biotechnology, Santa Cruz, CA). Precipitated chromatin was assayed by qPCR using primers 5’-TAC AGT AGT CAC TAT TAA ATG-3’ (for) and 5’-TGT GCG GTT GGT CCC TTC GGG-3’ (rev) (CNkx2.5 BMPRE region); and 5’-CTG CTG CCC GAC AAC CAC-3’ (for) and 5’ GCT CGT CCA TGC CGA GAG TGA-3’ (rev) (GFP reporter region). Results are expressed as fold enrichment based upon ΔC(t) calculations compared to control antiserum or control Ig as above.
Results
Nkx2.5 enhancer analysis identifies an autoregulatory role for Nkx2.5 in the SHF
In previous studies of the chicken Nkx2.5 gene we identified a BMP-responsive enhancer region that controls Nkx2.5 expression in SHF cardiac progenitors and in the SHF (Lee et al., 2004). A lacZ reporter transgene driven by this enhancer (CNkx2.5-SHF-lacZ) is activated at mouse E8.5 in pharyngeal arch mesoderm and ectoderm, and continues to express lacZ activity in pharyngeal arch (PA) mesoderm, OFT and RV through E10.5. A linker scanning mutagenesis of the central 200 bp BMP response element (BMPRE) within the SHF enhancer identified several transcription factor (TF) binding sites critical for enhancer activation in vitro and for cardiac reporter gene expression in vivo. These included consensus binding sites for the cardiac TFs Gata4 and Gata6, for the BMP/TGFβ signal transducing Smad4 protein, and for the Smad complex-binding activator/repressor YY1 ((Lee et al., 2004) and Lee and Lassar, unpublished).
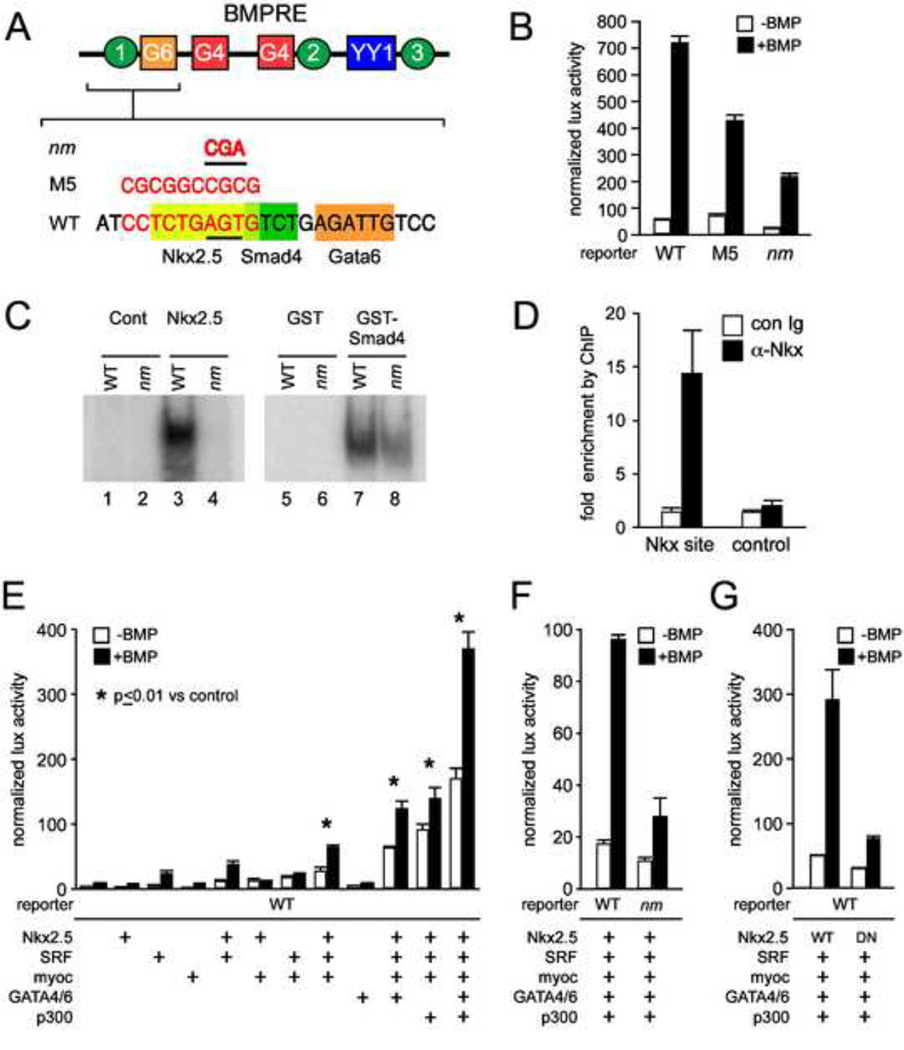
As an extension of these studies we characterized an additional BMPRE linker-scanning mutant that overlaps a potential consensus binding site for Nkx2.5 (M5; Fig. 1A) and which reduces the activation of a corresponding Nkx2.5 luciferase reporter, CNkx2.5-BMPRE-lux M5 (Fig. 1B). Gel shift and EMSA experiments confirm that this enhancer region binds Nkx2.5 in vitro (Fig. 1C, left panel), and chromatin immunoprecipitation (ChIP) experiments in P19 cells transfected with a CNkx2.5-SHF reporter and an Nkx2.5 expression plasmid demonstrate interaction of Nkx2.5 with the BMPRE region containing the Nkx2.5 binding site in vivo (Fig. 1D). Both the 10 bp linker scanning M5 mutation and a more specific 2 bp mutation (nm) of the consensus NKE abrogate Nkx2.5 gel shift binding, and the nm mutation also reduces reporter gene activation (Figs. 1B, C). Perhaps due to its proximity to the Nkx binding site, Smad4 interaction at an adjacent Smad binding site is also somewhat affected (reduced by 23%)in gel shifts of double stranded DNA oligos bearing either the M5 or the nm mutation (Fig. 1C, right panel). While targeted mutation of the Smad4 binding site leads to complete loss of BMP response in cell assays and reporter gene expression in vivo (Lee et al., 2004), both M5 and nm mutant reporters are still activated by BMP (Fig. 1B).
Figure 1. Nkx2.5 directly regulates the CNkx2.5-SHF enhancer.
A. A schematic of the 200 bp BMPRE of the chick Nkx2.5 gene depicting previously characterized Smad4 (green circles), GATA4/6 (red and orange squares) and YY1 (blue rectangle) binding elements is shown over an expanded view of nt 35–50 (Lee et al., 2004) which contains an Nkx2.5 binding consensus (yellow rectangle). The 10 bp M5 linker scanning mutation of BMPRE (nt 37–47) overlapping Nkx2.5 and Smad4 binding consensus sites is shown over the affected nucleotides in red, as is the smaller 3 bp mutation (designated Nkx2.5 mutant or nm) specifically targeting the Nkx2.5 binding element. B. BMP mediated reporter gene activation of wild-type Nkx2.5-lux-BMPRE reporter or reporter bearing the M5 or 3 bp nm mutations of the Nkx2.5 consensus site. The M5 mutation results in an approximately 50% decrease in BMP induced Nkx2.5-lux-BMPRE driven luciferase activity while the nm mutation results in a 4-fold decrease in luciferase activity. C. Data are shown for gel shifts performed with control (lanes 1 and 2), or Nkx2.5 cell extracts (lanes 3 and 4), or purified control GST (lanes 5 and 6) and GST-Smad4-MH1 domain proteins (lanes 7 and 8). Gel shifts were performed using double stranded oligonucleotides representing the wild-type BMPRE Nkx2.5 consensus (lanes 1, 3, 5, and 7) or the Nkx2.5 consensus bearing the nm mutation (lanes 2, 4, 6, 8). D. ChIP assay of Nkx2.5 binding to CNkx2.5-SHF-GFP reporter detects specific and selective enrichment (approx. 15 x) of amplicons from the BMPRE surrounding the Nkx2.5 binding consensus (Nkx site) using α-Nkx2.5 antibody vs. control IgG. No appreciable enrichment is observed of control GFP coding regions of the reporter (control). E. While addition of Nkx2.5 alone does not lead to a significant increase in CNkx2.5-SHF-lux luciferase activity in response to BMP (second column set from left), combinatorial addition of SRF, SRF cofactor Myocardin and cardiac GATAs 4 and 6 increases BMP activation of Nkx2.5-SHF-lux approximately 20-fold. The BMP response is further amplified 50–100 fold by addition of p300. F. CNkx2.5-SHF-lux response to Nkx2.5 and SRF, GATA4/6, SRF, Myocardin and p300 co-expression and BMP stimulation is reduced approximately 70% by site-specific mutation (nm) of the Nkx2.5 binding site on the CNkx2.5-SHF enhancer as compared to wild-type enhancer (WT). G. Multi-TF and BMP activation of the wild-type CNkx2.5-SHF-lux reporter is similarly reduced approximately 75% by use of a mutated non-DNA binding Nkx2.5 isoform (DN Nkx2.5) as compared to wild-type (WT Nkx2.5).
Nkx2.5 overexpression alone has no significant effect on basal or BMP-mediated activation of the CNkx2.5-SHF-lux reporter (Fig. 1E, left columns). However, since Nkx2.5 can synergistically activate cardiac-specific genes when co-expressed with other early cardiac TFs (Chen et al., 1996; Lee et al., 1998) we investigated whether the CNkx2.5-SHF-lux reporter could be activated by a combination of Nkx2.5 and other TFs known to bind to Nkx2.5 through protein-protein interaction. As shown in Fig. 1E, co-expression of Nkx2.5 with serum response factor (SRF) (Chen and Schwartz, 1996) and its co-activator Myocardin (Wang et al., 2001) results in an approximate 10 fold activation of both basal and BMP-stimulated levels. Activation of the CNkx2.5-SHF-lux reporter by Nkx2.5, SRF and Myocardin is further increased (from 2 fold to approximately 20 fold) by the expression of Gata4 and -6. This is not surprising since Gata4 is capable of protein-protein interaction with both SRF and Nkx2.5 (Belaguli et al., 2000; Lee et al., 1998; Sepulveda et al., 1998). However, consistent with our previous findings, Gata4/6 expression alone is insufficient for activation of the CNkx2.5-SHF-lux reporter (Lee et al., 2004) (Fig 1E). The transcriptional co-activator p300, which associates with GATA factors and Myocardin (Cao et al., 2005; Dai and Markham, 2001), also increases basal and BMP-stimulated activity a further 2.6–3 fold for a cumulative 50 fold increase of basal activity and 100 fold increase in BMP-stimulated activity (Lee et al., 2004).
Importantly, this high level activation of the by multiple cardiac TFs is dependent upon binding of Nkx2.5 to its consensus site: both basal and BMP-stimulated activation by the combination of Nkx2.5, SRF, Myocardin, Gata4/6 and p300 was strikingly reduced in enhancer reporters incorporating the nm Nkx2.5 binding site mutation (CNkx2.5 nm-SHF-lux) (Fig. 1F). Similarly, substitution of WT Nkx2.5 with a dominant negative Nkx2.5 protein that does not bind DNA (Kasahara et al., 2001) results in greatly reduced activation of the wild-type CNkx2.5-lux-SHF reporter (Fig. 1G). Cumulatively, these results define a requirement for Nkx2.5 autoregulation in the multi-TF activation of the CNkx2.5-SHF enhancer in vitro.
Nkx2.5 autoregulation maintains expression in cardiomyocytes during differentiation from SHF progenitors
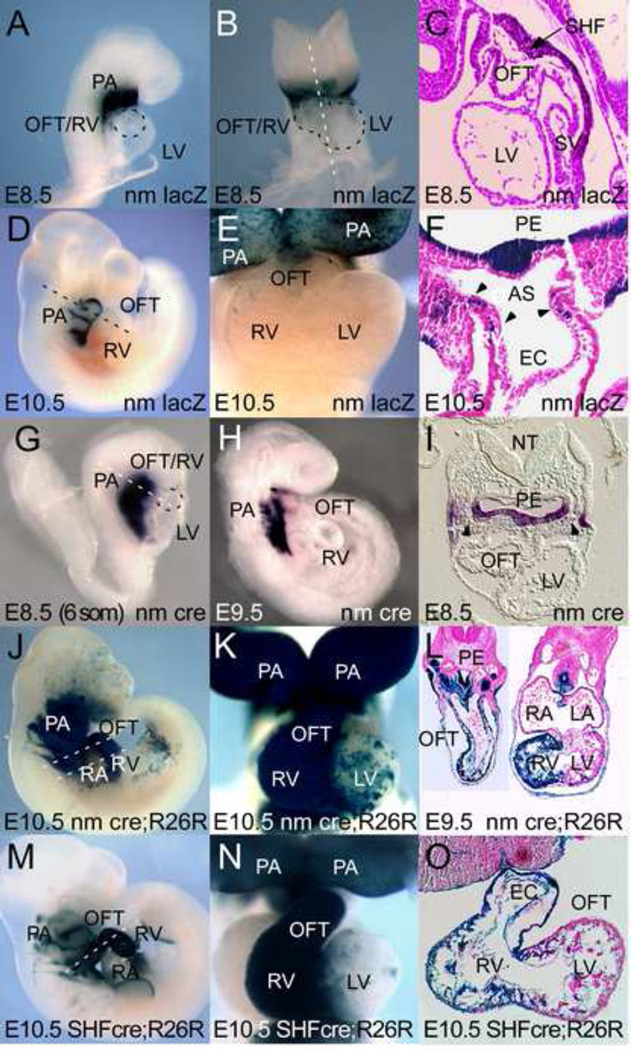
In order to assess the role of this Nkx2.5 autoregulation on expression in vivo, stable mouse lines were generated incorporating a transgenic reporter (CNkx2.5-SHF nm-lacZ) that expresses β-gal under control of the CNkx2.5-SHF enhancer containing the nm mutation (Fig. 2). We have previously described the expression of the CNkx2.5-SHF-lacZ enhancer (Lee et al., 2004). As with the unmutated enhancer, bilaterally symmetric Xgal staining is first observed in SHF splanchnic mesoderm in the pharyngeal arch region at E8.5 (6 somite stage) (Fig. 2A–C) and at this stage does not extend into the heart tube established by the primary or first heart field. Expression continues to be observed in pharyngeal endoderm and the splanchnic mesoderm of the SHF through E10.5 (Fig. 2D–F). However, and in contrast to the WT CNkx2.5-SHF-lacZ reporter, which continues to express lacZ in differentiated heart (Lee et al., 2004), the mutant CNkx2.5-SHF nm-lacZ reporter fails to maintain expression in the OFT, RV and inter ventricular septal region at E10.5, although some residual lacZ expression is observed in SHF mesodermal cells and cells of the proximal OFT/aortic sac region. Xgal staining is not observed in adult transgenic hearts (data not shown).
Figure 2. Nkx2.5 autoregulation is required for Nkx2.5-SHF enhancer expression in aortic pole myocytes but not SHF progenitors.
A–F: Shown are representative lacZ expression patterns of stable lines transgenic for the CNkx2.5-SHF nm-lacZ reporter construct (nm lacZ). A–C: LacZ expression at E8.5 in PA mesoderm, ectoderm and endoderm is similar to that seen with the wild-type Nkx2.5 SHF enhancer construct (Lee et al., 2004) with staining observed in pharyngeal arch regions containing SHF progenitors, but absent in FHF-derived heart. D–F: While still expressed in PA regions, CNkx2.5-SHF nm reporter fails to maintain lacZ expression fails in differentiating OFT and RV of E10.5 transgenic embryos except in a few cells of the SHF (F, black arrowheads). G–I: Whole mount and sectioned whole mount in situ hybridization for cre mRNA expression in CNkx2.5-SHF nm-cre stable transgenic mouse line (nm cre). Cre is expressed in pharyngeal arch endoderm, mesoderm and ectoderm overlapping with SHF mesoderm (black arrowheads) at E8.5 (6 somite stage) (I) and retained primarily in pharyngeal populations at E10.5 (H). J–L: Lineage tracing using R26R ROSA lacZ reporter strain and CNkx2.5-SHF nm-cre driver at E10.5 confirms that cre expression from the CNkx2.5-SHF nm-cre transgene marks pharyngeal arch ectoderm, endoderm, SHF progenitors (black arrowheads) and OFT and right ventricular segments of the heart similar to wild-type CNkx2.5-SHF-cre (SHF-Cre) (M–O). Abbreviations: PA: pharyngeal arch; LV: left ventricle; RA: right atrium; RV: right ventricle; OFT: out flow tract; Ao: aorta; PuA: pulmonary artery; SV: sinus venosus; SHF: second heart field mesoderm; PE: pharyngeal endoderm; AS: aortic sac; EC: endocardium. Dotted lines in A, B, and I outline developing OFT/RV (A, I) and RV and LV (B) at E8.5.
To test whether lacZ expressing cells marked by the CNkx2.5-SHF nm-lacZ reporter in E8.5 pharyngeal arch mesoderm include SHF precursors, transgenic lineage marking analysis was performed with mouse lines expressing Cre recombinase under control of the CNkx2.5-SHF nm enhancer (CNkx2.5-SHF nm-cre). In situ hybridization using a probe specific for Cre mRNA confirmed that the CNkx2.5-SHF nm-cre transgene drives Cre mRNA expression in a pattern identical to the lacZ expression seen with the CNkx2.5-SHF nm-lacZ enhancer (Fig. 2G–I). To determine the fate of cells marked with this transgene, CNkx2.5-SHF nm-cre mice were crossed with the conditional floxed lacZ reporter mouse line R26R Rosa-lacZ which permanently marks cells that have expressed the Cre transgene at any point during differentiation (Soriano, 1999). Mice derived from this cross showed Xgal positivity in cells of the splanchnic mesoderm, foregut endoderm and branchial arch ectoderm, as well as SHF-derived OFT and RV in CNkx2.5-SHF nm-cre+/−; R26R lacZ+/− double-positive embryos at E10.5 (Figure 2J–L). Cells of the left ventricle (LV) and atria remain unmarked confirming that the CNkx2.5-SHF nm-cre expression was restricted to SHF progenitors. This SHF-specific fate map is qualitatively similar both to the expression pattern of CNkx2.5-SHF-lacZ (Lee et al., 2004), and to a fate map obtained using a WT CNkx2.5-SHF-cre driver line (Fig. 2M–O). These results are consistent with a model whereby enhancer autoregulation by Nkx2.5-recruited TF complexes is dispensable for early activation in SHF progenitors, but is required for maintenance of expression in differentiated myocytes derived from these progenitors after they have entered the heart.
Some ectopic lacZ reporter activation is observed in OFT cushions using the CNkx2.5-SHF nm-cre as compared to the lineage tracing obtained with the wild-type enhancer and craniofacial ectodermal lineages are marked in both lineage traces (Fig 2J, L compared to M, O) indicating transient expression in ectoderm and a transient repressive role for Nkx2.5 autoregulation in neural crest or OFT endocardial lineages that was not appreciated with our previous WT CNkx2.5-SHF-lacZ studies. While Nkx2.5 expression has not been reported in migratory or post-migratory neural crest cells, it is transiently expressed in anterior ectoderm at the border of the neural plate and non-neural ectoderm in chicken (Schultheiss et al., 1995). As this region later gives rise to dorsal neural tube from which CNCC are derived, the ectopic expression in OFT cushions may in part represent a specific loss of appropriate Nkx2.5-mediated repression in these CNCC precursors that is specific to the chick SHF enhancer. These results are also consistent with previous lineage tracings achieved with an mNkx2.5-3’UTR-IRES-Cre allele that, in addition to marking craniofacial ectoderm populations, also marked OFT cushion cells in mosaic fashion that were likely derived from an endocardial or bi- or multi-potent Nkx2.5 (+) lineage (Stanley et al., 2002) and that at E9.5 may represent the majority of OFT cushion cells.
Conservation of Nkx2.5 SHF regulatory elements among vertebrates
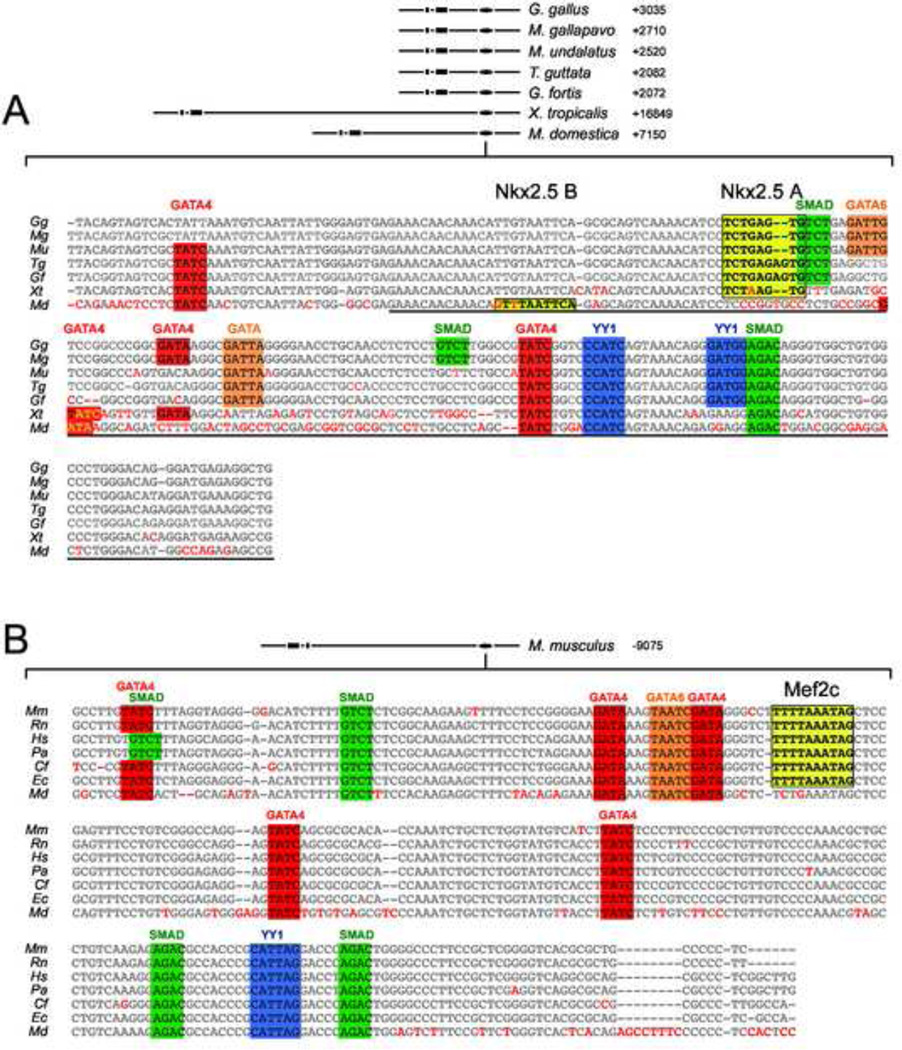
We next examined whether Nkx2.5 autoregulation of its SHF expression is an evolutionarily conserved mechanism. A comparative genomic analysis of Nkx2.5 genes of available vertebrate species revealed that a 250 bp element highly similar to a central region of the ~2.0 kb CNkx2.5-SHF enhancer module is highly conserved in the 3’ flanking genomic regions of the turkey (Meleagris gallopavo) Budgarigar or parakeet (Melopsittacus undalatus), medium ground finch (Geospiza fortis), zebra finch (Taeniopigea guttata), frog (Xenopus tropicalis) and opossum (Monodelphis domestica) Nkx2.5 genes (Fig. 3A). This region of conservation contains the BMPRE and conserved GATA, YY1 and Smad4 consensus binding sites that are essential for BMP response and SHF expression of the chicken Nkx2.5 SHF enhancer (Lee et al., 2004). Importantly, this phylogenetically conserved region also possesses the archetypal Nkx2.5 binding element in chicken, turkey, zebra finch and frog (Fig. 3A yellow boxed area). Interestingly, while the equivalent region of the opossum genome lacks this Nkx2.5 binding site, a sequence highly similar to a weaker-affinity Nkx2.5 binding site (C(a/t)TTAATTN) (Chen and Schwartz, 1995) is present in a relative position 25 bp 5’ to the expected position of the archetypal site.
Figure 3. Divergent conserved enhancers mediate SHF expression of Nkx2.5 in mammalian and non-mammalian vertebrates.
Shown in A is the central BMP response element contained with the chicken Gallus gallus (Gg) Nkx2.5 SHF enhancer (CAR3) aligned with homologous 3’ flanking regions in the Nkx2.5 genes from the turkey Meleagris gallopavo (Mg), Budgarigar or parakeet Melopsittacus undalatus (Mu), medium ground finch Geospiza fortis (Gf), zebrafinch Taenopygia guttata (Tg), frog Xenopustropicalis (Xt) and opossum Monodelphis domestica (Md). Homologous flanking regions are at varying distances 3’ from the transcriptional start site in these species, as shown in the schematic at top. Conserved residues are shown in gray and divergent residues are highlighted in red. Consensus binding motifs are shown as colored boxes for cardiac GATA (red), Smad4 (green), YY1 (blue) and Nkx2.5 (yellow). Underlined region highlights the previously characterized chicken Nkx2.5 BMPRE. B. SHF enhancer conserved in mammalian species. Shown are aligned Nkx2.5-5’ enhancer sequences from mouse Mus musculus (Mm), rat Rattus norvegicus (Rn), human Homo sapiens (Hs), chimp Pan troglodytes (Pt), orangutan Pongo pygmaetus abelii (Pa), dog Canis familiaris (Cf), horse Equus caballus (Ec), and opossum Monodelphis domestica (Md). TF site highlighting is as in A, except that a conserved Mef2 CArG binding consensus rather than an NKE is in yellow. Note that both enhancer motifs are conserved in opossum, but lack either a strong Nkx2.5 binding motif (3’ enhancer) or Mef2 binding CArG consensus (5’ enhancer).
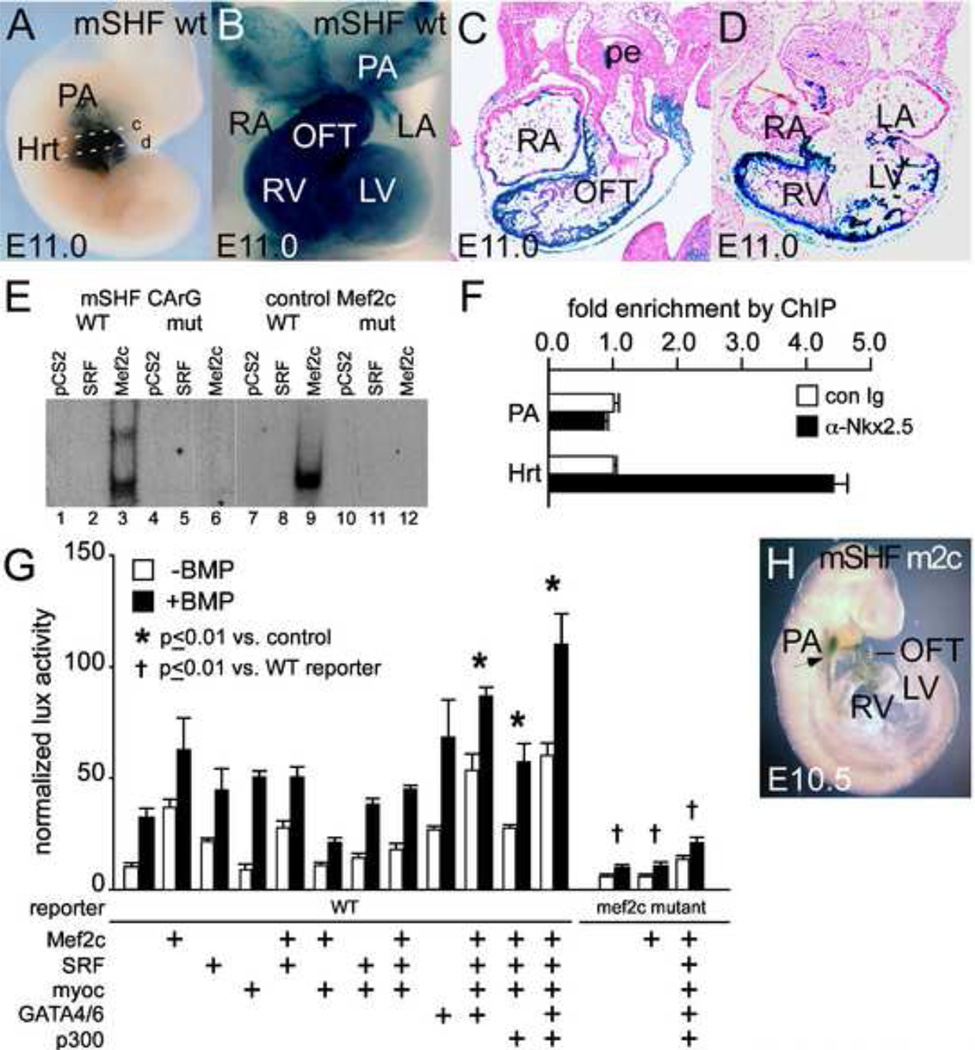
By contrast, an enhancer directly homologous to the CNkx2.5-SHF enhancer was not apparent in Nkx2.5 genomic flanking regions of mice or other placental mammals. Several enhancer elements of the mouse Nkx2.5 (mNkx2.5) gene are known to regulate transgene expression in the E8.5–10.5 mouse heart, some of which are responsive to BMP or the BMP pathway-specific Smad proteins (Brown, 2003; Liberatore et al., 2002; Lien et al., 1999; Reecy et al., 1999; Searcy et al., 1998). One of these mNkx2.5 enhancers is well conserved specifically in placental mammals and opossum (Fig. 3B) but strikingly absent in the non-mammalian species analyzed above. This approximately 500 bp enhancer is located 9 kb 5’ to the two major coding exons of mNkx2.5. This distal 5’ enhancer of mNkx2.5 regulates OFT and RV expression in transgenic mouse reporter constructs utilizing the heterologous hsp68 lacZ promoter, requires interaction with cardiogenic GATA transcription factors via consensus binding elements for expression, and responds to Smad-mediated BMP signaling in cell culture assays (Lien et al., 1999; Takeuchi et al., 2005). Multispecies alignment of this enhancer in mammals revealed conserved TF binding consensus sites for GATA, YY1 and Smad4 transcription factors also found in the conserved central region of the CNkx2.5-SHF enhancer (Fig. 3B). This pattern of conserved TF binding sites raised the possibility that this mammalian enhancer, while divergent in sequence from the chick enhancer, might regulate expression of Nkx2.5 in an analogous manner. As shown in Figure 4A–D, a chimeric reporter gene (here after referred to as mNkx2.5-SHF-lacZ) consisting of this distal 5’ 500 bp enhancer of mNkx2.5 and the chicken Nkx2.5 basal promoter indeed drives lacZ expression in SHF, OFT and RV, in a pattern highly similar to that seen with CNkx2.5-SHF-lacZ (Lee et al., 2004). This mNkx2.5-SHF enhancer mediates this function in its native 5’ position relative to the basal promoter (10/10 transient transgenic embryos), or when placed 3’ to promoter and coding sequences (5/5 transient transgenic embryos) (Fig. 4A–D and data not shown).
Figure 4. Mef2c interaction is required for SHF-specific expression of mNkx2.5-SHF enhancer in OFT and RV.
Shown in A–D are representative transient whole mount and section expression data for mNkx2.5-SHF-lacZ reporter transgenes combining the conserved 5’ enhancer from mouse Nkx2.5 and the chicken Nkx2.5 proximal promoter. Expression at E11.0 is observed in branchial arch ectoderm and mesoderm, and in the SHF derivatives of OFT and RV in the wild-type transgene mNkx2.5-SHF-lacZ (mSHF WT). Planes of section in C and D are shown in A by white dashed lines c and d. E. EMSA demonstrates preferential binding of Mef2c to the CArG consensus in the conserved 5’ enhancer of mouse Nkx2.5 over SRF (compare lane 3 to lane 4 and to binding of Mef2c to control Mef2c site in control lanes 8 and 9). Binding is lost upon mutation of the central AT residues in the 5’ mouse enhancer site (lane 4–6; 10–11). F. ChIP experiments show that Mef2c is associated with the 5’ mNkx2.5 SHF enhancer region and mNkx2.5 promoter proximal region in E10.5 heart tissue (Hrt) vs. pharyngeal arch tissue. G. While overexpression of Mef2c alone has a modest impact on mNkx2.5-SHF lux activity, much greater enhancement of basal and BMP-activated expression is observed with combined expression of Mef2c with SRF, Myocardin, p300 and cardiogenic GATAs. Increased activation is lost with Mef2c CArG box mutation (right-most columns). H. Mutation of Mef2c binding CArG box consensus results in loss of mNkx2.5-SHF enhancer activity. Representative Xgal staining pattern of E10.5 mouse embryo transgenic for mNkx2.5-SHF m2c-lacZ transgenic reporter bearing a point mutation of the consensus Mef2c binding site (mSHF m2c). LacZ expression is lost in OFT and RV while expression in PA SHF mesoderm is relatively maintained (arrow). Embryonic stages are shown in lower left. Abbreviations are as in previous figures.
Mef2c mediates sustained expression in SHF-derived conotruncal and right ventricular myocytes of the mouse Nkx2.5 SHF enhancer
While the mNkx2.5-SHF enhancer shares several consensus elements in common with the CNkx2.5-SHF enhancer, it lacks an apparent consensus-binding element for Nkx2.5. We noted that the mNkx2.5-SHF enhancer instead contains a conserved CArG box binding consensus potentially binding the MADS domain cardiac transcription factor Mef2c (Martin et al., 1993) and that this site is correspondingly absent in the chicken and non-mammalian SHF enhancers (Fig. 3B, compare to Fig. 3A). Gel shift or EMSA analysis shows that this site binds to Mef2c but not to SRF, another MADS domain TF that recognizes distinct A/T rich CArG sites Fig. 4E). Moreover, as shown in Figure 4F ChIP assay of chromatin derived from E10.5 mouse hearts using anti-Mef2c antibody demonstrates enrichment for amplicons within the mNkx2.5-SHF enhancer surrounding the mNkx2.5-SHF Mef2c binding site, consistent with direct enhancer binding in vivo. With respect to the mouse mNkx2.5-SHF enhancer, Mef2c expression appears to play a role similar or parallel to that of Nkx2.5 expression for the chicken CNkx2.5-SHF enhancer in that overexpression of Mef2c alone resulted in minimal activation of the reporter whereas it greatly enhanced reporter activation in response to the GATA4/6, Smad, SRF, Myocardin and p300 transcription factor cocktail (Fig. 4G). The requirement for Mef2c binding for enhancer activation was demonstrated by greatly diminished activation of a construct (mNkx2.5-SHF m2c-lux) bearing a mutation of the CArG consensus though BMP response is not affected by this mutation (Fig 4G, right hand columns). Similarly, in vivo expression of an mNkx2.5-SHF m2c-lacZ transgenic reporter was greatly reduced in PA, RV and OFT of transient transgenic embryos (9/9 transgenic embryos) (Fig. 4H).
Mef2c mediation of Nkx2.5 autoregulation
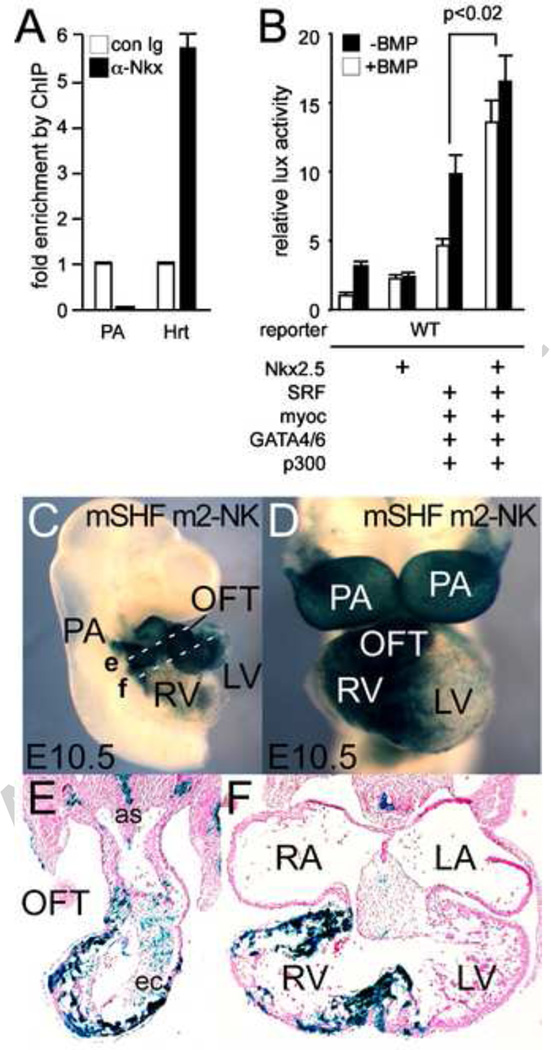
As the activation of the mNkx2.5-SHF reporter in cell assays by Mef2c and combinatorial cardiac TFs was weaker than that observed with the chick enhancer, we speculated that other factors were yet required for more robust activation. Since Nkx2.5 and Mef2c are capable of protein-protein interaction (Vincentz et al., 2008), we considered the possibility that Mef2c binding actually mediates Nkx2.5 recruitment and indirect autoregulation. Despite the lack of an apparent NKE in its sequence, ChIP assay of chromatin derived from E10.5 mouse hearts confirms that Nkx2.5 binds in vivo to the mNkx2.5-SHF enhancer region in E9.5 heart tissue (Fig 5A). Cell assay reveals that while Nkx2.5 by itself has no effect on mNkx2.5-SHF activation, addition of Nkx2.5 with Mef2c, GATA4/6, SRF, Myocardin, and p300 in cell-based assays results in an additional 2× activation of the mNkx2.5-SHF-lux reporter (p<0.02) (Fig 5B). In order to test the hypothesis that Nkx2.5 recruitment is the major function of Mef2c binding to the mNkx2.5-SHF enhancer, we replaced the Mef2c binding CArG consensus site in this enhancer with the Nkx2.5 binding consensus from the CNkx2.5-SHF chick enhancer and tested the corresponding lacZ reporter construct in transient transgenic mouse assays. As shown in Figure 5 (C–F), the resulting lacZ reporter (mNkx2.5-SHF m2-NK-lacZ), like the wild-type reporter, is expressed in SHF-derived PA and RV at E10.5 (4/6 transgenic embryos; 2 with no expression), though PA mesodermal SHF-expression is not as apparent in these transient embryos. By demonstrating that direct recruitment of Nkx2.5 to this enhancer can in effect by pass the requirement for Mef2c binding, this experiment both supports the hypothesis that the principle role of Mef2c binding to the mNkx2.5 SHF enhancer is Nkx2.5 recruitment, and confirms that Nkx2.5 autoregulation of SHF expression is conserved among vertebrates.
Figure 5. Mef2c CArG and NKE consensus sites are functionally interchangeable on the mNkx2.5 SHF enhancer.
A: ChIP results demonstrating Nkx2.5 binding to the native 5’ mNkx2.5-SHF enhancer region in vivo in E10.5 heart vs. PA tissue. B: mNkx2.5-lux reporter gene is not significantly activated by Nkx2.5 co-expression alone, but addition of Nkx2.5 along with Mef2c and other cardiac TFs (multi-TF: SRF, Myocardin. Gata4 and 6, p300) results in significantly increased activation. C–F: Whole mount (C, D) and section (E, F) analysis of E10.5 embryo transgenic for the mNkx2.5-SHF (m2→NKE) lacZ reporter where the CArG consensus binding site has been altered to the Nkx2.5 binding NKE site from the cNkx2.5-SHF enhancer. LacZ expression is highly similar to both CNkx2.5-SHF and mNkx2.5-SHF lacZ reporters with expression largely in pharyngeal arch, OFT and RV myocardium. Planes of section in E and F are shown in C by dashed white lines e and f, respectively. Abbreviations are as in previous figures.
Discussion
Conservation of Nkx2.5 autoregulation and its relationship to Mef2c
Here we identify autoregulation as an important aspect of Nkx2.5 maintenance in the SHF. In addition we found that this regulation is conserved in vertebrate species and is operative at a critical juncture in OFT development. Interestingly, such a role for Nkx2.5 in its own regulation may be evolutionarily conserved since enhancer analysis of the archetypal tinman gene in Drosophila revealed that maintenance of Tinman in cardiogenic mesoderm requires binding of Tinman itself (Xu et al., 1998). Similarly cross-regulation of Nkx2.5 and Mef2 may represent a deeply conserved module for early heart development. For example, in Drosophila, sustained expression of tinman in cardiac mesoderm requires binding of Tinman itself along with the Smad homolog Mad to the 3’ tin-D enhancer element that is conserved in multiple Drosophila strains (Xu et al., 1998). The tin-D region also contains several well-conserved but as yet uncharacterized CArG-like consensus sites that might serve as binding sites for DMef2. DMef2 homozygous mutants initiate visceral and cardiac mesoderm formation normally but later show, abnormal cardiac and somatic myocyte development indicating that DMef2 is dispensable for early cardiac specification but plays a more important role in differentiation of myocytes from cardiac progenitors. (Bour et al., 1995). In addition, web-published databases* report direct association of DMef2 to the 3’ flanking regions of tinman as detected by ChIP assay of wild-type embryos at embryonic stages 10–13, and altered tinman mRNA expression is detected in Dmef2 null mutant embryos at these same stages. However, the issue of direct regulation of tinman by Drosophila DMef2 remains to be defined.
Further evidence supporting parallel roles of Mef2c and Nkx2.5 in regulating SHF expression of Nkx2.5 in the mouse comes from the intriguing observation that homozygous mutations in both genes lead to similar looping defects (Lin et al., 1997; Lints et al., 1993). In addition, Mef2c is a known downstream target of Nkx2.5, (Tanaka et al., 1999) and we have preliminary evidence of a quantitative loss of Nkx2.5 transcripts in Mef2c−/− null hearts (Clark and Lee, unpublished). These results potentially add another dimension to the cooperative nature of Nkx2.5 and Mef2c transcriptional regulation in the SHF in addition to their ability to co-regulate target genes through protein-protein interaction (Vincentz et al., 2008). While past work has found evidence for SHF-specific regulation of Mef2c (Dodou et al., 2004), a direct role for Mef2c function in SHF formation parallel to that of Nkx2.5 has yet to be determined either through examination of the behavior of SHF lineages in Mef2c−/− constituitive knockouts, or through analysis of embryonic cardiac phenotypes resulting from SHF-specific deletion of Mef2c..
Multifactor complex recruitment underlies SHF enhancer function
Our findings and those of other researchers present a model of Nkx2.5 autoregulation in the SHF in which Nkx2.5 recruits similar multi-TF complexes to enhancers that are functionally conserved but divergent in sequence. This is consistent with an existing paradigm for cardiac field specification suggesting that phenotypic differentiation requires spatiotemporally overlapping of multiple TFs of the homeobox, zinc finger and helix-loop helix domain familes. This paradigm of TF cooperativity has been reinforced by the finding of reciprocal regulation between early cardiac TFs during development (Davis et al., 2000; Lien et al., 1999; Molkentin et al., 2000; Searcy et al., 1998; Ueyama et al., 2003). Given the ability of many cardiac TFs to interact with and recruit one another through protein-protein interactions, regulatory regions like the mouse and chicken SHF enhancers could attract functionally similar or identical TF complexes specifying similar cardiac fields while evincing limited sequence homology.
These findings also resonate with high-throughput analyses of cardiac enhancers. A recent survey of cardiac-specific enhancers associated with p300 recruitment in E11.5 day hearts of mice found relatively poor sequence conservation of functionally confirmed heart enhancers as compared to fore brain-specifying enhancers (Blow et al., 2010). Interestingly, this study also found that the majority of predicted mouse heart enhancers were conserved at the level of sequence only among other placental mammals, indicating significant divergence of such enhancers in avians and amphibians much as we found with the Nkx2.5 SHF enhancers. In this respect it is interesting to note that the opossum Nkx2.5 gene possesses both the mammailan 5’ and non-mammalian 3’ Nkx2.5 SHF enhancers (Fig 3A, B) but that the 5’ enhancer lacks a strong binding consensus for Mef2c, and the 3’ enhancer contains a low-affinity Nkx2.5 binding consensus (Chen and Schwartz, 1995). This may suggest that SHF regulation of the opossum gene depends upon the collaboration of two weak enhancers rather than one strong one. As a marsupial, opossum (Monodelphia domestica) is evolutionarily more distant from the other mammals identified in our analysis and, along with monotremes, may represent an independent evolutionary lineage from those of avians and placental mammals.
Nkx2.5 Regulation and OFT Malformation: Integration of Signals Controlling Progenitor Proliferation and Differentiation
Nkx2.5 null mutant embryos have severe OFT and right heart hypoplasia and previous studies have that this is caused in part by disrupting the normal balance between proliferation and differentiation of SHF cells. (Prall et al., 2007). Indeed, Nkx2.5 regulation is known to be intimately tied to several important signaling pathways including BMP, the fibroblast growth factors, especially FGF8 and 4 (Alsan and Schultheiss, 2002; Barron et al., 2000; Ilagan et al., 2006; Schultheiss et al., 1997; Tirosh-Finkel et al., 2010; Waldo et al., 2001) and sonic hedgehog (shh) (Dyer et al., 2010; Zhang et al., 2001). Each of these signaling pathways has been shown to regulate Nkx2.5 in either the first or second heart field or, in the context of OFT morphogenesis. In our current study we demonstrate that Nkx2.5 SHF regulatory regions contain consensus binding sites for Smad4, GATA and YY1, all of which have been implicated in the direct activation of Nkx2.5 by BMP (Fig 3.) (Lee et al., 2004). While it is possible that FGF and Shh act at some level through secondary regulation of BMP signaling, our ongoing work on species-comparative enhancer analysis will explore the possibility that these and other morphogenic signals directly regulate Nkx2.5 SHF expression through transcriptional pathways collaborating with or competing against YY1/Smad-mediated BMP activation.
Downstream of Nkx2.5 in the SHF, we have recently found that Nkx2.5 directly regulates the early cardiac transcriptional regulator Jarid2 in SHF cells (Barth et al., 2010). As Jarid2 is a known repressor of both cyclin D1 and cardiomyocyte proliferation in embryonic hearts (Lee et al., 2000; Toyoda et al., 2003) and is a key component of the PRC2 repressive complex that regulates the activation of embryonic gene programs during the differentiation of embryonic stem cells (Landeira et al., 2010; Li et al., 2010; Pasini et al., 2010; Peng et al., 2009; Shen et al., 2009). Jarid2's dependence on normal Nkx2.5 expression for proper regulation is yet another example of how the induction and maintenance of normal Nkx2.5 expression controls OFT growth and morphogenesis. Through these coordinated studies we hope to obtain a more global understanding of the Nkx2.5-centered regulatory transcriptome controlling normal OFT development.
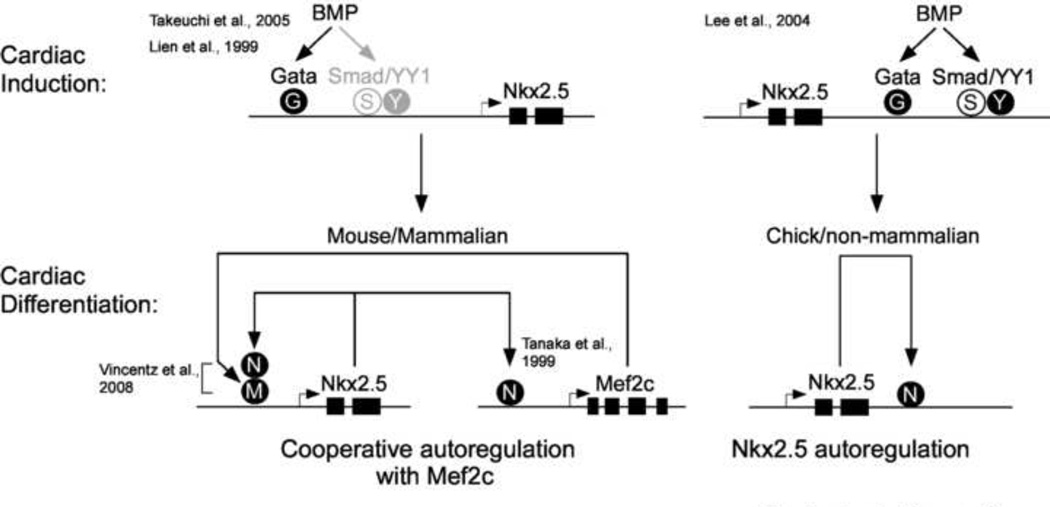
Figure 6. Summary model of direct and indirect autoregulation of Nkx2.5 SHF enhancers.
Annotated model shows models of early activation of both mouse/mammalian 5’ Nkx2.5 and Chick/non-mammalian 3’ SHF enhancers by BMP via Gata, Smad and YY1 mediated mechanisms during early cardiac induction in SHF progenitors and later maintenance of expression in cardiac myocytes of the aortic pole. Mouse/mammalian enhancer expression is maintained during cardiac differentiation in part through recruitment of Nkx2.5 by protein-protein interaction with Mef2c in emerging heart cells (left). In chick and other avian or non-mammalian species SHF expression is maintained by direct recruitment of Nkx2.5 to consensus enhancer binding sites. Model incorporates both findings in this work and referenced findings from previous studies.
Highlights.
-
-
We compared regulation of SHF enhancers of chick and mouse Nkx2.5 genes
-
-
We found evidence for systematic divergence of mammalian vs. avian SHF enhancers
-
-
Autoregulation by Nkx2.5 appears to be conserved from flies to man
-
-
Nkx2.5 autoregulationis necessary during differentiation of SHF progenitors
-
-
Nkx2.5 autoregulation requires cooperation with multiple cardiac TFs
Acknowledgements
We thank Lena Du and Arlene Sharpe of the Brigham and Women’s Core Transgenic Facility, Tam Thompson of the Children’s Hospital, Boston, Core Transgenic Facility and Alexander Awgulewitsch and Donna Jacobs of the MUSC Core Transgenic Facility for assistance with transient and stable transgenic mouse generation. We thank Stuart Orkin, Tucker Collins, Seigo Izumo, Eric Olson, and Malcolm Whitman, for expression constructs. We thank Clayton Carlson and Aseem Ansari for anti-Nkx2.5 antiserum used in ChIP experiments. We thank Jessica Trombetta, Marie Lockhart and Danielle Geeting for technical assistance. We thank Jeremy Barth, W. Scott Argraves, Ann Foley and Tom Borgfor critical reading of our manuscript. KHL and ABL received support from NHLBI R01 (HL58862); KHL received support from NHLBI K08 (K08HL003371), the South Carolina COBRE for Developmentally-based Cardiovascular Disease (NCRR P20RR016434), the American Heart Association (Mid-Atlantic Affiliate, BGIA 12060120) and facilities support from the Darby Children’s Research Institute at MUSC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development (Cambridge, England) 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]
- Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Barron M, Gao M, Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev Dyn. 2000;218:383–393. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Barth JL, Clark CD, Fresco VM, Knoll EP, Lee B, Argraves WS, Lee KH. Jarid2 is among a set of genes differentially regulated by Nkx2.5 during outflow tract morphogenesis. Dev Dyn. 2010;239:2024–2033. doi: 10.1002/dvdy.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol. Cell Biol. 2000;20:7550–7558. doi: 10.1128/mcb.20.20.7550-7558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DW. Genetic origins of pediatric heart disease. Pediatr Cardiol. 2010;31:422–429. doi: 10.1007/s00246-009-9607-y. [DOI] [PubMed] [Google Scholar]
- Blow MJ, McCulley DJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, Afzal V, Bristow J, Ren B, Black BL, Rubin EM, Visel A, Pennacchio LA. ChIP-Seq identification of weakly conserved heart enhancers. Nat Genet. 2010;42:806–810. doi: 10.1038/ng.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour BA, O'Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, Abmayr SM, Nguyen HT. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- Brown CO, Chi X, Shirai M, Feng X-H, Schwartz RJ. The cardiac determining factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. Journal of Biological Chemistry. 2003;279:10659–10669. doi: 10.1074/jbc.M301648200. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor popultaion that proliferates prior to differentiation and contributes a majority of cells to the heart. Developmental Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Wang Z, Zhang CL, Oh J, Xing W, Li S, Richardson JA, Wang DZ, Olson EN. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol. Cell Biol. 2005;25:364–376. doi: 10.1128/MCB.25.1.364-376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Croissant J, Majesky M, Topouzis S, McQuinn T, Frankovsky MJ, Schwartz RJ. Activation of the cardiac alpha-actin promoter depends upon serum response factor, Tinman homologue, Nkx-2.5, and intact serum response elements. Devel. Genet. 1996;19:119–130. doi: 10.1002/(SICI)1520-6408(1996)19:2<119::AID-DVG3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Chen CY, Schwartz RJ. Identification of novel DNA binding targets and regulatory domains of a murine tinman homeodomain factor, nkx-2.5. J. Biol. Chem. 1995;270:15628–15633. doi: 10.1074/jbc.270.26.15628. [DOI] [PubMed] [Google Scholar]
- Chen CY, Schwartz RJ. Recruitment of the tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol. Cell Biol. 1996;16:6372–6384. doi: 10.1128/mcb.16.11.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Whiteley M, Felsenfeld G. A 5' element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- Dai YS, Markham BE. p300 Functions as a coactivator of transcription factor GATA-4. Journal of Biological Chemistry. 2001;276:37178–37185. doi: 10.1074/jbc.M103731200. [DOI] [PubMed] [Google Scholar]
- Davis DL, Wessels A, Burch JB. An Nkx-dependent enhancer regulates cGATA-6 gene expression during early stages of heart development. Dev. Biol. 2000;217:310–322. doi: 10.1006/dbio.1999.9561. [DOI] [PubMed] [Google Scholar]
- Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- Dyer LA, Makadia FA, Scott A, Pegram K, Hutson MR, Kirby ML. BMP signaling modulates hedgehog-induced secondary heart field proliferation. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmuntz E, Geiger E, Benson DW. NKX2.5 mutations in patients with tetralogy of fallot. Circulation. 2001;104:2565–2568. doi: 10.1161/hc4601.098427. [DOI] [PubMed] [Google Scholar]
- Granados-Riveron JT, Pope M, Bu'lock FA, Thornborough C, Eason J, Setchfield K, Ketley A, Kirk EP, Fatkin D, Feneley MP, Harvey RP, Brook JD. Combined Mutation Screening of NKX2-5, GATA4, and TBX5 in Congenital Heart Disease: Multiple Heterozygosity and Novel Mutations. Congenit Heart Dis. 2011 doi: 10.1111/j.1747-0803.2011.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development (Cambridge, England) 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- Kasahara H, Lee B, Schott JJ, Benson DW, Seidman JG, Seidman CE, Izumo S. Loss of function and inhibitory effects of human CSX/NKX2.5 homeoprotein mutations associated with congenital heart disease. J Clin Invest. 2000;106:299–308. doi: 10.1172/JCI9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H, Usheva A, Ueyama T, Aoki H, Horikoshi N, Izumo S. Characterization of homo- and heterodimerization of cardiac Csx/Nkx2.5 homeoprotein. J. Biol. Chem. 2001;276:4570–4580. doi: 10.1074/jbc.M004995200. [DOI] [PubMed] [Google Scholar]
- Kelly RG. The second heart field. Curr Top Dev Biol. 2012;100:33–65. doi: 10.1016/B978-0-12-387786-4.00002-6. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Developmental Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Landeira D, Sauer S, Poot R, Dvorkina M, Mazzarella L, Jorgensen HF, Pereira CF, Leleu M, Piccolo FM, Spivakov M, Brookes E, Pombo A, Fisher C, Skarnes WC, Snoek T, Bezstarosti K, Demmers J, Klose RJ, Casanova M, Tavares L, Brockdorff N, Merkenschlager M, Fisher AG. Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nat Cell Biol. 2010;12:618–624. doi: 10.1038/ncb2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Evans S, Ruan TY, Lassar AB. SMAD-mediated modulation of YY1 activity regulates the BMP response and cardiac-specific expression of a GATA4/5/6-dependent chick Nkx2.5 enhancer. Development. 2004;131:4709–4723. doi: 10.1242/dev.01344. [DOI] [PubMed] [Google Scholar]
- Lee Y, Shioi T, Kasahara H, Jobe SM, Wiese RJ, Markham BE, Izumo S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol. Cell Biol. 1998;18:3120–3129. doi: 10.1128/mcb.18.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Song AJ, Baker R, Micales B, Conway SJ, Lyons GE. Jumonji, a nuclear protein that is necessary for normal heart development. Circ. Res. 2000;86:932–938. doi: 10.1161/01.res.86.9.932. [DOI] [PubMed] [Google Scholar]
- Li G, Margueron R, Ku M, Chambon P, Bernstein BE, Reinberg D. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010;24:368–380. doi: 10.1101/gad.1886410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore CM, Searcy-Schrick RD, Vincent EB, Yutzey KE. Nkx-2.5 gene induction in mice is mediated by a Smad consensus regulatory region. Dev. Biol. 2002;244:243–256. doi: 10.1006/dbio.2002.0604. [DOI] [PubMed] [Google Scholar]
- Lien CL, Wu C, Mercer B, Webb R, Richardson JA, Olson EN. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development (Cambridge, England) 1999;126:75–84. doi: 10.1242/dev.126.1.75. [DOI] [PubMed] [Google Scholar]
- Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science (New York, NY) 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development (Cambridge, England) 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Martin JF, Schwarz JJ, Olson EN. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci U S A. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinney DB, Geiger E, Blinder J, Benson DW, Goldmuntz E. NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol. 2003;42:1650–1655. doi: 10.1016/j.jacc.2003.05.004. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicholas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Developmental Cell. 2004;6:685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Antos C, Mercer B, Taigen T, Miano JM, Olson EN. Direct activation of a GATA6 cardiac enhancer by Nkx2.5: evidence for a reinforcing regulatory network of Nkx2.5 and GATA transcription factors in the developing heart. Dev. Biol. 2000;217:301–309. doi: 10.1006/dbio.1999.9544. [DOI] [PubMed] [Google Scholar]
- Pasini D, Cloos PA, Walfridsson J, Olsson L, Bukowski JP, Johansen JV, Bak M, Tommerup N, Rappsilber J, Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, Wysocka J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reecy JM, Li X, Yamada M, DeMayo FJ, Newman CS, Harvey RP, Schwartz RJ. Identification of upstream regulatory regions in the heart-expressed homeobox gene Nkx2-5. Development. 1999;126:839–849. doi: 10.1242/dev.126.4.839. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JBE, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Devel. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development (Cambridge, England) 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- Searcy RD, Vincent EB, Liberatore CM, Yutzey KE. A GATA-dependent nkx-2.5 regulatory element activates early cardiac gene expression in transgenic mice. Development (Cambridge, England) 1998;125:4461–4470. doi: 10.1242/dev.125.22.4461. [DOI] [PubMed] [Google Scholar]
- Sepulveda JL, Belaguli N, Nigam V, Chen CY, Nemer M, Schwartz RJ. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: role for regulating early cardiac gene expression. Mol. Cell Biol. 1998;18:3405–3415. doi: 10.1128/mcb.18.6.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, Yuan GC, Lee Y, Orkin SH. Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3'UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int J Dev Biol. 2002;46:431–439. [PubMed] [Google Scholar]
- Takeuchi JK, Mileikovskaia M, Koshiba-Takeuchi K, Heidt AB, Mori AD, Arruda EP, Gertsenstein M, Georges R, Davidson L, Mo R, Hui CC, Henkelman RM, Nemer M, Black BL, Nagy A, Bruneau BG. Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development (Cambridge, England) 2005;132:2463–2474. doi: 10.1242/dev.01827. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development (Cambridge, England) 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Zeisel A, Brodt-Ivenshitz M, Shamai A, Yao Z, Seger R, Domany E, Tzahor E. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development. 2010;137:2989–3000. doi: 10.1242/dev.051649. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Shirato H, Nakajima K, Kojima M, Takahashi M, Kubota M, Suzuki-Migishima R, Motegi Y, Yokoyama M, Takeuchi T. jumonji downregulates cardiac cell proliferation by repressing cyclin D1 expression. Dev Cell. 2003;5:85–97. doi: 10.1016/s1534-5807(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Kasahara H, Ishiwata T, Nie Q, Izumo S. Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol. Cell Biol. 2003;23:9222–9232. doi: 10.1128/MCB.23.24.9222-9232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz JW, Barnes RM, Firulli BA, Conway SJ, Firulli AB. Cooperative interaction of Nkx2.5 and Mef2c transcription factors during heart development. Dev Dyn. 2008;237:3809–3819. doi: 10.1002/dvdy.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development (Cambridge, England) 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Xu X, Yin Z, Hudson JB, Ferguson EL, Frasch M. Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev. 1998;12:2354–2370. doi: 10.1101/gad.12.15.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y-T, Breitbart RE, Smoot LB, Lee Y, Mahdavi V, Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992;6:1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Kelly RG, Meilhac SM, Buckingham ME, Brown NA. Right ventricular myocardium derives from the anterior heart field. Circ. Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, Vassileva G, McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]