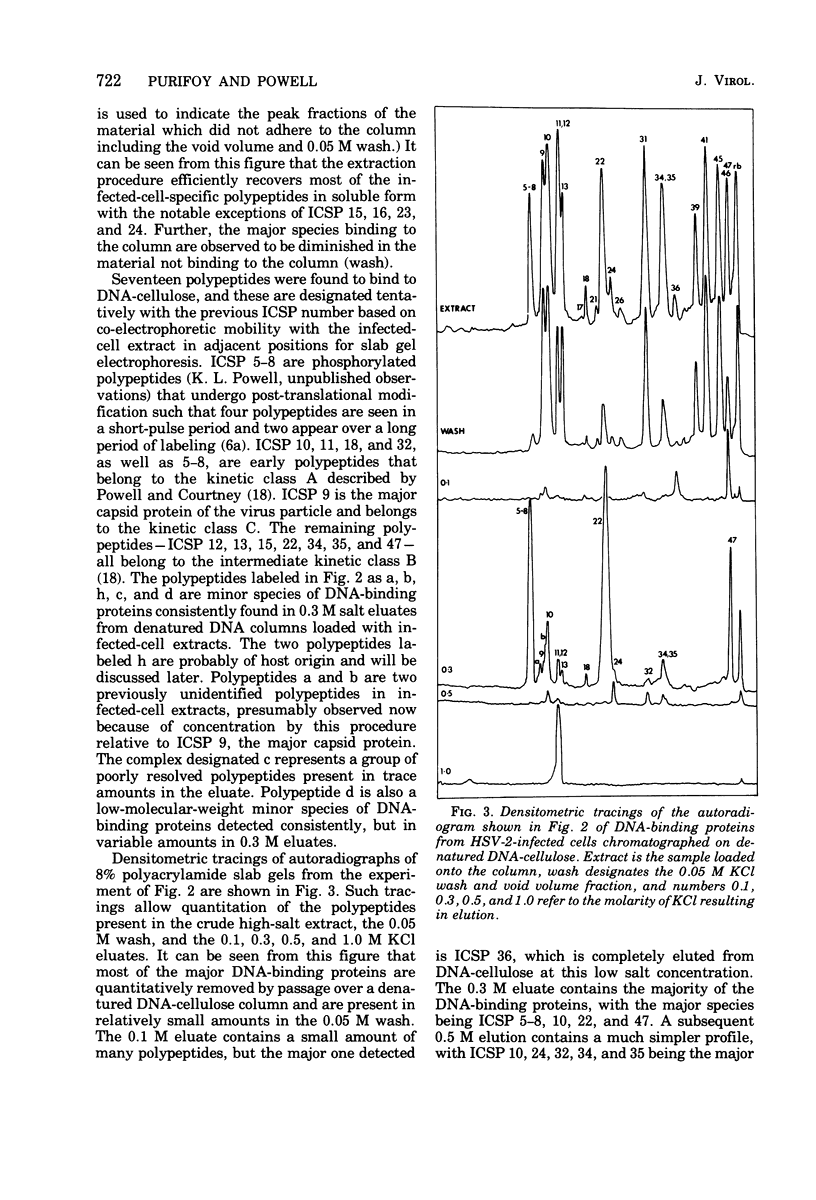
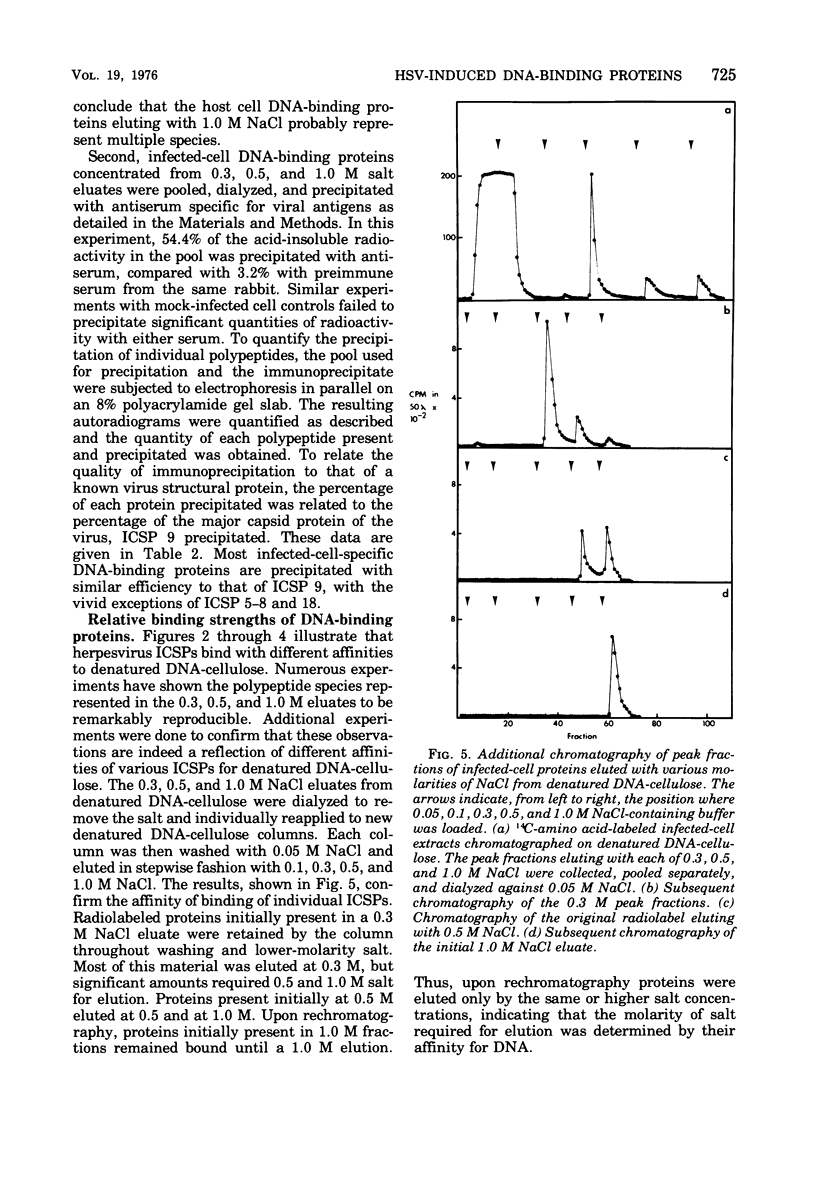
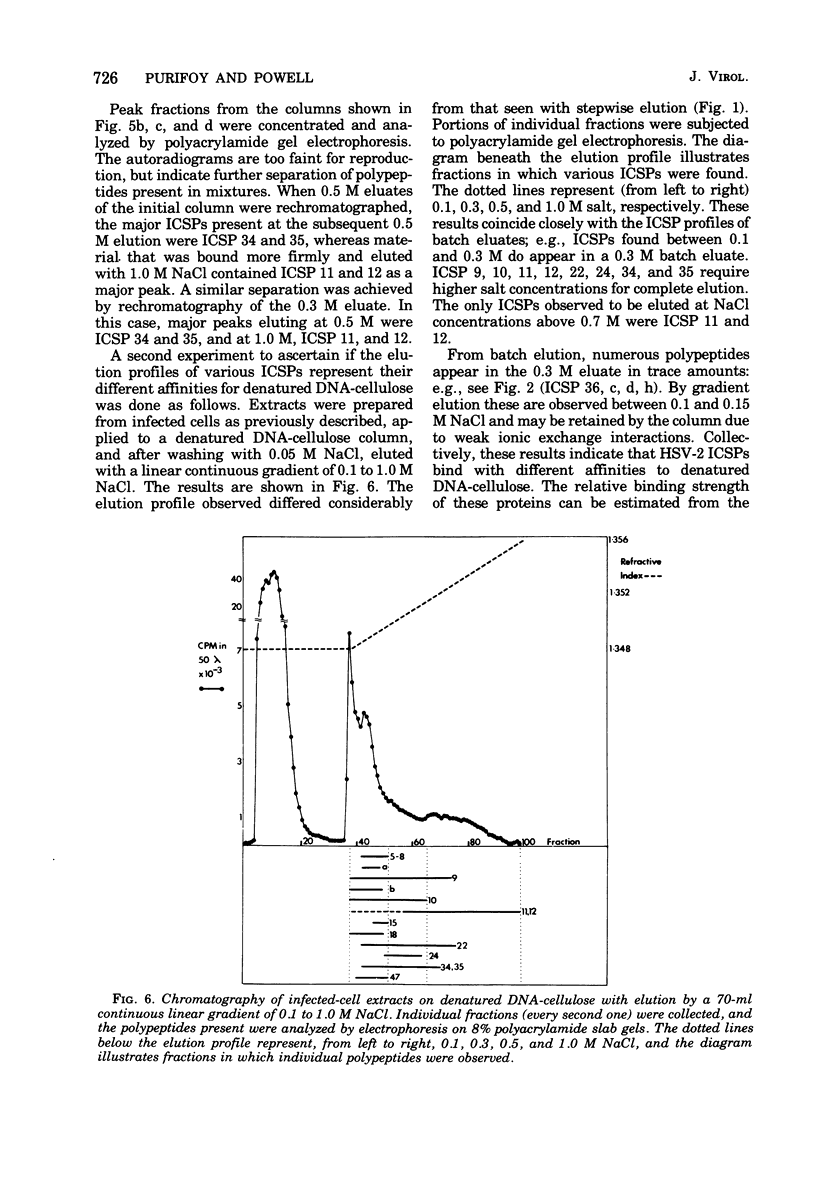
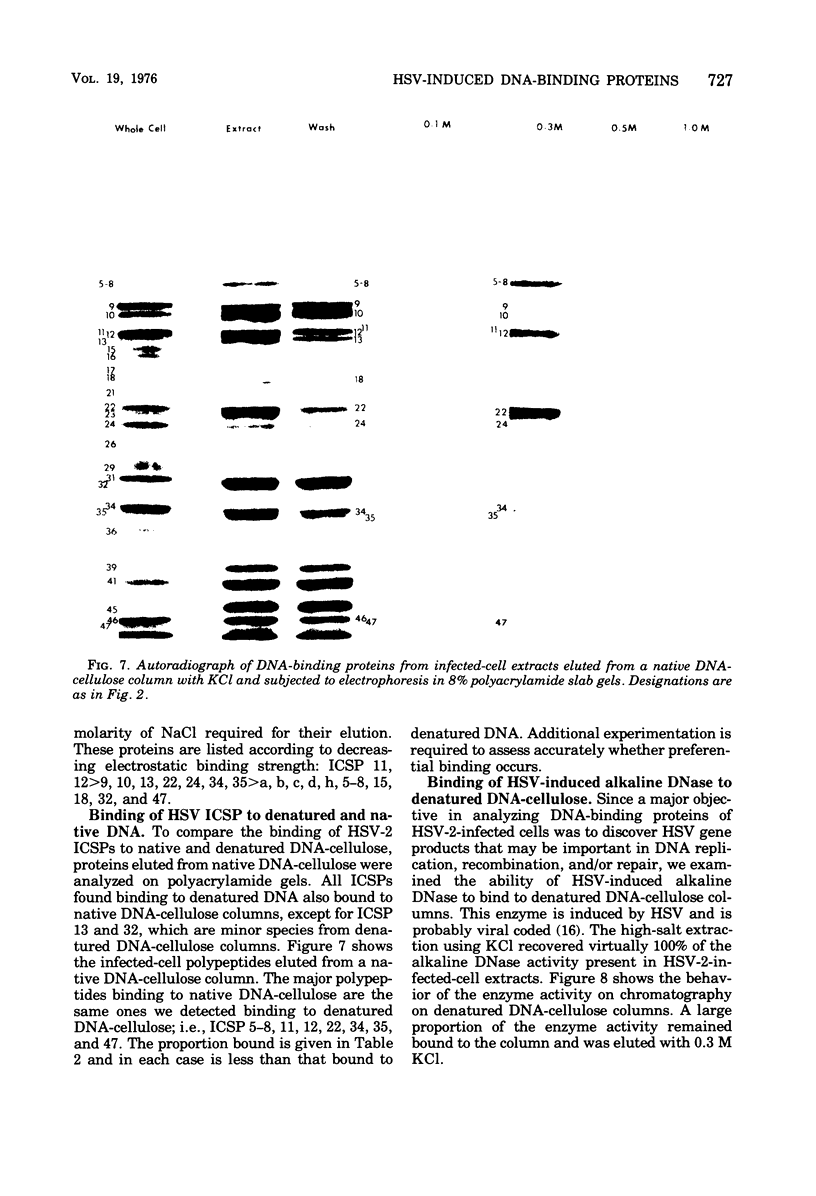
Abstract
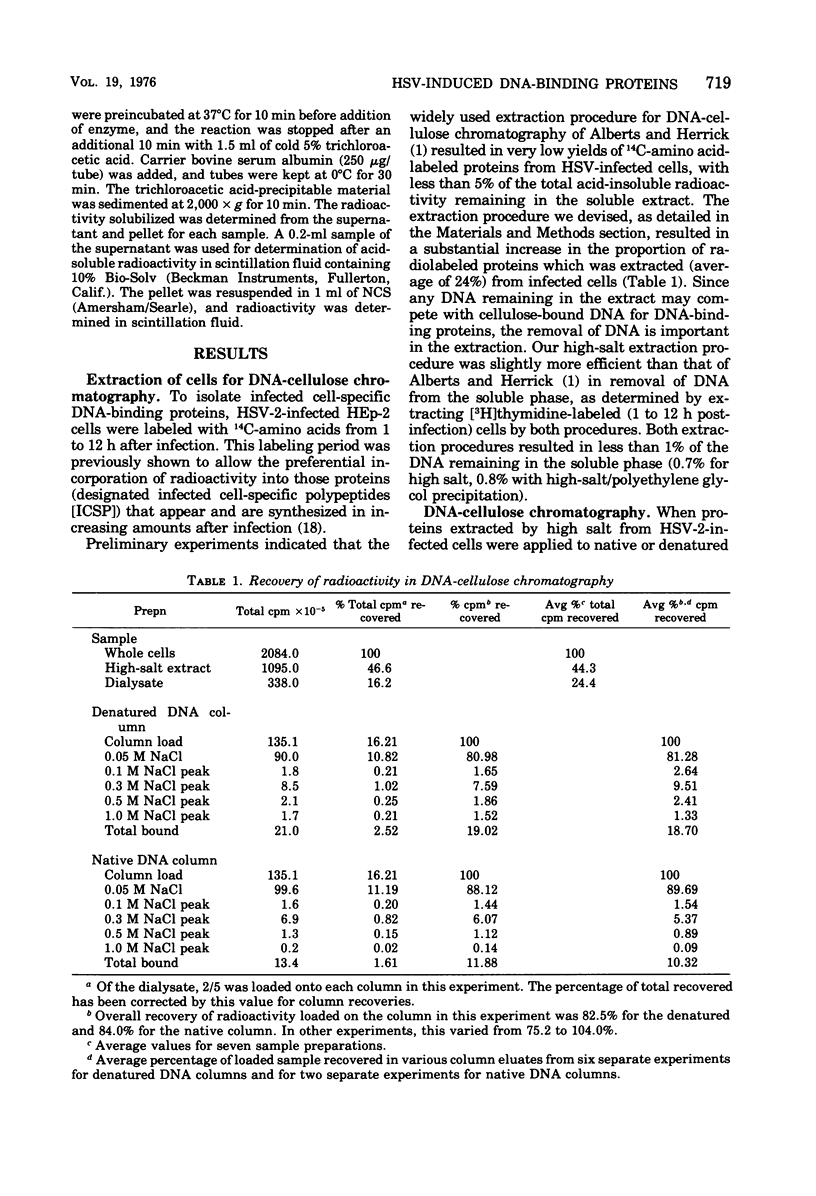
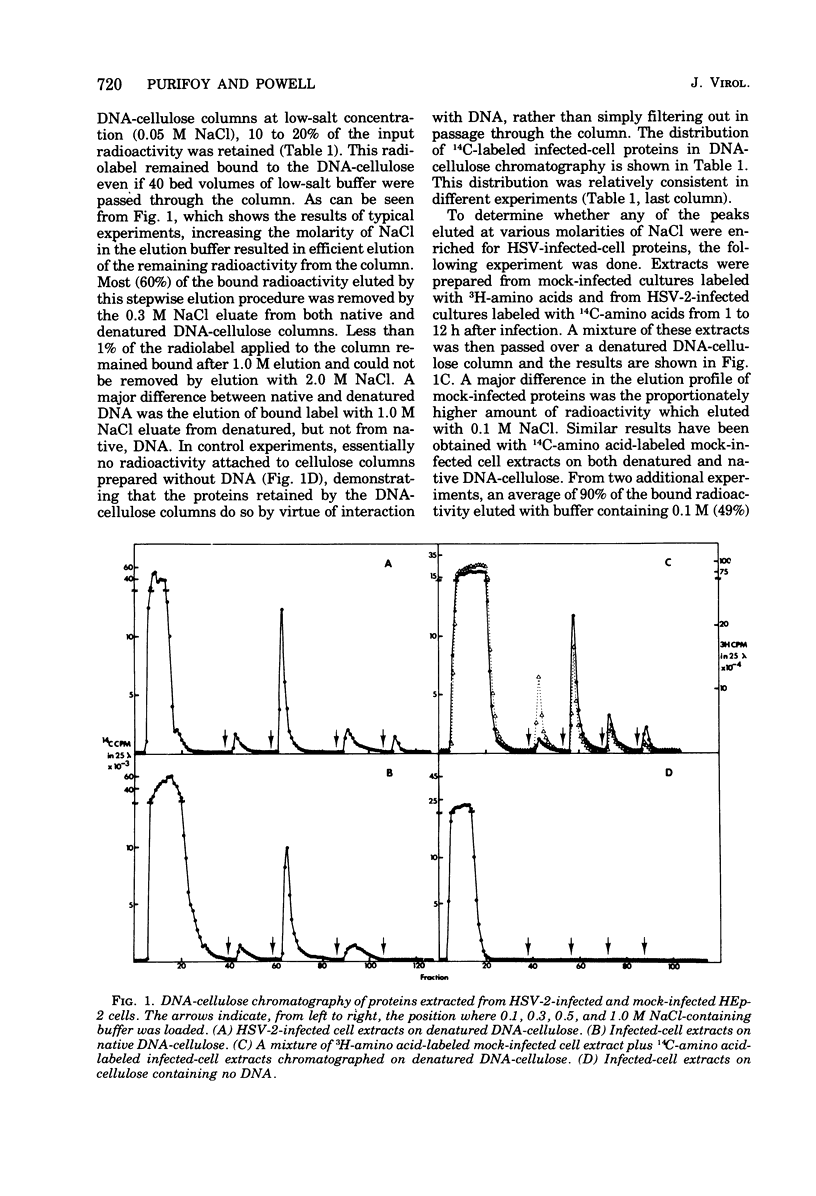
Affinity chromatography on single-stranded and double-stranded DNA-cellulose indicates that 12 proteins previously identified from herpes simplex virus type 2-infected cells, ranging in molecular weight from 28 X 10(3) to 186 X 10(3), bind to DNA-cellulose. The DNA-binding proteins found in infected cells differed in relative binding strengths for denatured DNA-cellulose. The virus specificity of these DNA-binding proteins was further studied by comparison with DNA-binding proteins isolated from mock-infected cells, and by immunoprecipitation of infected-cell DNA-binding proteins with antisera specific for viral antigens. The promise this technique holds for the purification and study of polypeptides involved in virus DNA replication, recombination, or repair is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Brody T. A DNA-binding form of the main structural protein of lambda heads. Virology. 1973 Aug;54(2):441–451. doi: 10.1016/0042-6822(73)90155-4. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Jacob R. J., Honess R. W., Hayward G. S., Locker H., Roizman B. Anatomy of herpes simplex virus DNA. III. Characterization of defective DNA molecules and biological properties of virus populations containing them. J Virol. 1975 Jul;16(1):153–167. doi: 10.1128/jvi.16.1.153-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Arens M. Q., Bhaduri S., Shanmugam G., Green M. Tumour antigen specificity of a DNA-binding protein from cells infected with adenovirus 2. Nature. 1975 Apr 10;254(5500):533–536. doi: 10.1038/254533a0. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Huang W. M., Buchanan J. M. Synergistic interactions of T4 early proteins concerned with their binding to DNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2226–2230. doi: 10.1073/pnas.71.6.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M. A new DNA-exonuclease in cells infected with herpes virus: partial purification and properties of the enzyme. J Gen Virol. 1968 Dec;3(3):337–347. doi: 10.1099/0022-1317-3-3-337. [DOI] [PubMed] [Google Scholar]
- Murray B. K., Biswal N., Bookout J. B., Lanford R. E., Courtney R. J., Melnick J. L. Cyclic appearance of defective interfering particles of herpes simplex virus and the concomitant accumulation of early polypeptide VP175. Intervirology. 1975;5(3-4):173–184. doi: 10.1159/000149894. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J., Courtney R. J. The synthesis of herpes simplex virus proteins in the absence of virus DNA synthesis. Biochem Biophys Res Commun. 1975 Sep 2;66(1):262–271. doi: 10.1016/s0006-291x(75)80323-8. [DOI] [PubMed] [Google Scholar]
- Powell K. L., Watson D. H. Some structural antigens of herpes simplex virus type 1. J Gen Virol. 1975 Nov;29(2):167–178. doi: 10.1099/0022-1317-29-2-167. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Benyesh-Melnick M. DNA polymerase induction by DNA-negative temperature-sensitive mutants of herpes simplex virus type 2. Virology. 1975 Dec;68(2):374–386. doi: 10.1016/0042-6822(75)90280-9. [DOI] [PubMed] [Google Scholar]
- Rubio V., Tsai W. P., Rand K., Long C. A comparison of DNA binding proteins from normal and virus-transformed mouse cells. Int J Cancer. 1973 Nov 15;12(3):545–550. doi: 10.1002/ijc.2910120302. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A. Temperature-sensitive mutants of herpesviruses. Curr Top Microbiol Immunol. 1975;70:51–100. doi: 10.1007/978-3-642-66101-3_3. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Brown S. M., Ritchie D. A., Timbury M. C., Macnab J. C., Marsden H. S., Hay J. Genetic and biochemical studies with herpesvirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):717–730. doi: 10.1101/sqb.1974.039.01.085. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Shedden W. I., Elliot A., Tetsuka T., Wildy P., Bourgaux-Ramoisy D., Gold E. Virus specific antigens in mammalian cells infected with herpes simplex virus. Immunology. 1966 Oct;11(4):399–408. [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]