Abstract
Introduction
This study’s objectives were to determine whether tumor response measured by CT and evaluated using Response Evaluation Criteria in Solid Tumors (RECIST) correlated with overall survival (OS) in patients with non-small cell lung cancer (NSCLC) after neoadjuvant chemotherapy and surgical resection.
Methods
We measured primary tumor size on CT before and after neoadjuvant chemotherapy in 160 NSCLC patients who underwent surgical resection. The relationship between CT-measured response (RECIST) and histopathologic response (≤10% viable tumor) and OS were assessed by Kaplan Meier survival, univariable and multivariable Cox proportional hazards regression.
Results
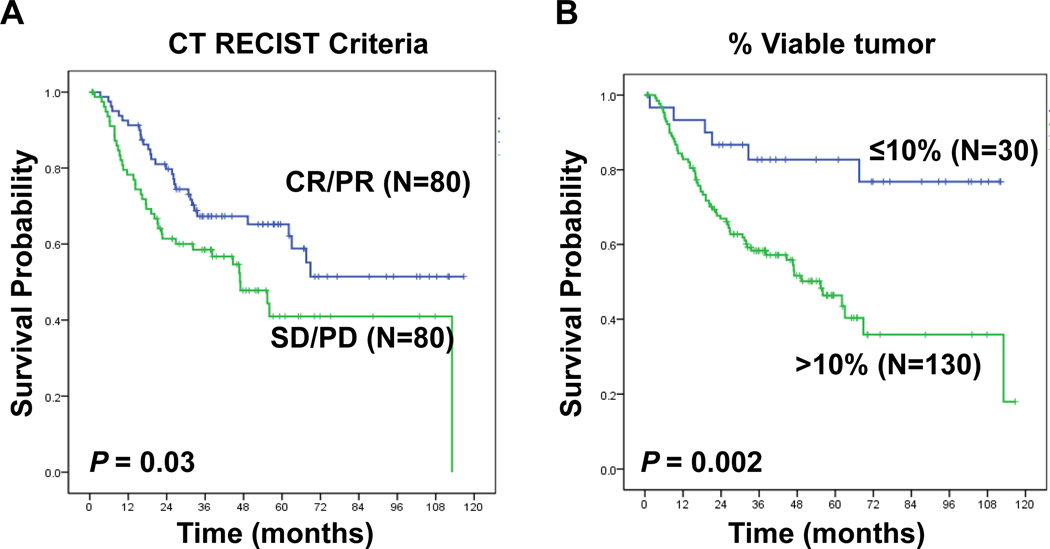
There was a statistically significant association between CT-measured response (RECIST) and OS (p=0.03). However, histopathologic response was a stronger predictor of OS (p=0.002), with a more pronounced separation of the survival curves when compared to CT-measured response. In multivariable Cox regression analysis, only pathologic stage and histopathologic response were significant predictors of OS. A 41% overall discordance rate was noted between CT RECIST response and histopathologic response. CT RECIST classified as non-responders a subset of patients with histopathologic response (8/30 pts, 27%) who demonstrated prolonged survival after neoadjuvant chemotherapy.
Conclusion
We were unable to show that CT RECIST is a reliable predictor of OS in patients with NSCLC undergoing surgical resection after neoadjuvant chemotherapy. The failure of CT RECIST to predict long-term outcome may be due to the inability of CT imaging to consistently identify patients with histopathologic response. CT RECIST may have only a limited role as an efficacy endpoint after neoadjuvant chemotherapy in patients with resectable NSCLC.
INTRODUCTION
Neoadjuvant chemotherapy has been evaluated in patients with non-metastatic non-small cell lung cancer (NSCLC) in several randomized, phase III trials1–4. Although controversial because of the small size of these trials, the impact of neoadjuvant chemotherapy on patient survival has generally been favorable. Recently, we described that histopathologic response to neoadjuvant chemotherapy was strongly associated with long-term overall survival in patients with clinical stage IB to IIIA NSCLC5; patients that exhibited ≤10% viable tumor cells after neoadjuvant chemotherapy had a significant reduction in the risk of recurrence and/or death compared to patients with >10% viable tumor cells in the surgical specimen, indicating that this could serve as an intermediary endpoint in future neoadjuvant clinical trials. The importance of histopathologic response after neoadjuvant chemotherapy was also corroborated recently by a review of two phase III neoadjuvant chemotherapy intergroup studies from France6. However, the utility of standard CT RECIST response criteria after neoadjuvant chemotherapy has not been well studied to date in patients with resectable NSCLC. We therefore investigated whether tumor response measured by CT using the Response Evaluation Criteria in Solid Tumors (RECIST)7 predicted overall survival (OS) and histopathologic response in patients with locally advanced NSCLC who received neoadjuvant chemotherapy and surgical resection.
PATIENTS AND METHODS
Patients and Treatment
We retrospectively reviewed the medical records of patients with NSCLC treated at The University of Texas M. D. Anderson Cancer Center from January 2001 to December 2008 who underwent neoadjuvant chemotherapy. During this period, 160 patients had CT imaging before and after completion of neoadjuvant therapy and underwent surgical resection with histopathologic assessment of tumor response (Table 1). From the patient medical records, we obtained detailed clinical and pathological information for all patients in the study group, including demographic data, pathological and clinical tumor-node-metastasis staging, and OS. This study was approved by The University of Texas M. D. Anderson Institutional Review Board and was performed in compliance with the Health Insurance Portability and Accountability Act.
TABLE 1.
Patient Demographics and Treatment Characteristics
| Age Median (Range) | 63 (40–85) |
| Gender: n (%) | |
| Male | 92 (57%) |
| Female | 68 (43%) |
| Histology: n(%) | |
| Adenocarcinoma | 68 (43%) |
| Squamous cell carcinoma | 51 (32%) |
| Othersa | 41 (25%) |
| Tumor Size (cm):n (%) | |
| 0.0–2.0 | 40 (25%) |
| 2.1–3.0 | 40 (25%) |
| 3.1–5.0 | 47 (29%) |
| >5.0 | 33 (21%) |
| Clinical Stage: n (%) | |
| IA /B | 52 (32%) |
| IIA /B | 35 (22%) |
| IIIA /B | 64 (40%) |
| IV | 9 (6%) |
| Type of Resectionn (%) | |
| Wedge or Segmentectomy | 4 (3%) |
| Bilobectomy or Lobectomy | 143 (89%) |
| Pneumonectomy | 13 (8%) |
| Neoadjuvant Chemotherapy: n (%) | |
| T+C | 143 (89%) |
| Carboplatin | 107 (67%) |
| Cisplatin | 53 (33%) |
| Taxol | 76 (48%) |
| Taxotere | 68 (42%) |
| Gemcitabine | 13 (8%) |
| Etoposide | 3 (2%) |
| Treatm ent Cycle Median (Range) | 3 (1–11) |
Abbreviation: aOthers (32 patients with NSCLC-NOS, 4 with with adenosquamous carcinoma, 3 with neuroendocrine tumor, 1 with large cell and 1 with sarcoma); T, Taxol or Taxotere; C, Caboplatin or Cisplatin; AJCC/UICC 6th Edition.
CT and Measurements
The CTs used in this study were performed before and after neoadjuvant chemotherapy. All chest CTs were performed on a General Electric CT scanner (LiteSpeed, LightSpeed, or HiSpeed; GE Medical Systems, Milwaukee, WI). The CT scan was obtained within 2 weeks before starting chemotherapy and within 4 weeks of completion of chemotherapy. In the RECIST assessment method7, lesion size was based on the longest dimension (LD) of the primary tumor. Measurements were performed by a single board-certified thoracic radiologist (JJE) who was blinded to long-term outcome to reduce inter-observer variability and bias8. The percentage change in the size of the target lesion was calculated between the pre-chemotherapy and post-chemotherapy measurements. Patients with disappearance of the lesion were defined as achieving complete response (CR); a ≥30% decrease in the LD of the target lesion were defined as achieving partial response (PR); a ≥20% increase in LD or the appearance of new lesions were defined as having progressive disease (PD)7. All other outcomes were defined as stable disease (SD). Patients who achieved a CR or PR by RECIST were defined as radiologic responders while patient who demonstrated SD or PD were defined as radiologic non-responders.
Histopathologic Response
Histopathologic response was assessed as previously described by Pataer et al5. Hematoxylin and eosin (H&E)-stained slides were assessed of sections of the gross residual tumor resected after neoadjuvant chemotherapy (at least 1 section per cm of tumor greatest diameter). The percentage of residual tumor was quantified by comparing the estimated cross sectional area of the viable tumor foci to estimated cross sectional areas of necrosis, fibrosis and inflammation on each slide. The results for all slides were averaged together to determine the mean values of percentage of viable tumor cells for each patient. We previously demonstrated that a cut-off of 10% viable tumor cells could distinguish patients with a high versus low probability of long-term disease-free and overall survival5. As such, patients were considered to be pathologic responders if they had ≤10% viable tumor cells and pathologic non-responders if they had >10% viable tumor cells9–11.
Statistical Analysis
Correlations were evaluated using Pearson’s linear test or the Spearman rank test. Overall survival was calculated from the time of surgery to the time of death from any cause or to the time of the patient’s last follow-up visit, after which the data were censored. Survival probability as a function of time was computed by the Kaplan-Meier method. The log-rank test was used to compare OS between groups. Univariable Cox proportional hazards regression analysis was used to examine the association between various prognostic factors and OS. Variables found to be significant in univariable analysis (p < 0.25) were then evaluated by multivariable analysis using the Cox proportional hazards regression model with backward stepwise Wald elimination. In multivariable analysis, p < 0.05 was taken to be significant. Statistical analyses were performed using SPSS version 19.0 software (SPSS, Inc., Chicago, IL).
RESULTS
Patient Demographics and Treatment Characteristics
The study population included 92 men (57%) and 68 women (43%) with a median age of 64 years (range, 40–85 years). Histologic tumor types are shown in Table 1. All patients were treated with a platinum-based doublet, and the majority received a taxane and platinum (143 patients, 89%). The median number of treatment cycles was 3 (range, 1–11 cycles) and 143 patients (89%) received a lobectomy or bilobectomy (Table 1).
Response to Neoadjuvant Chemotherapy by Radiologic and Pathologic Criteria
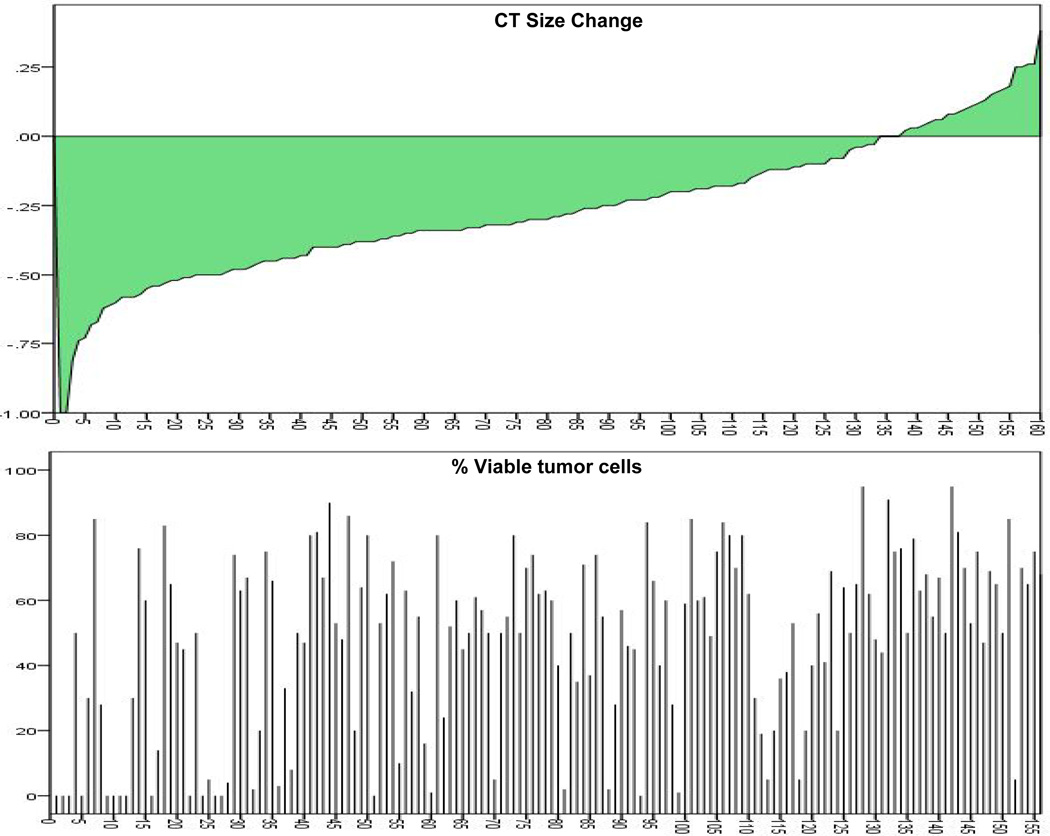
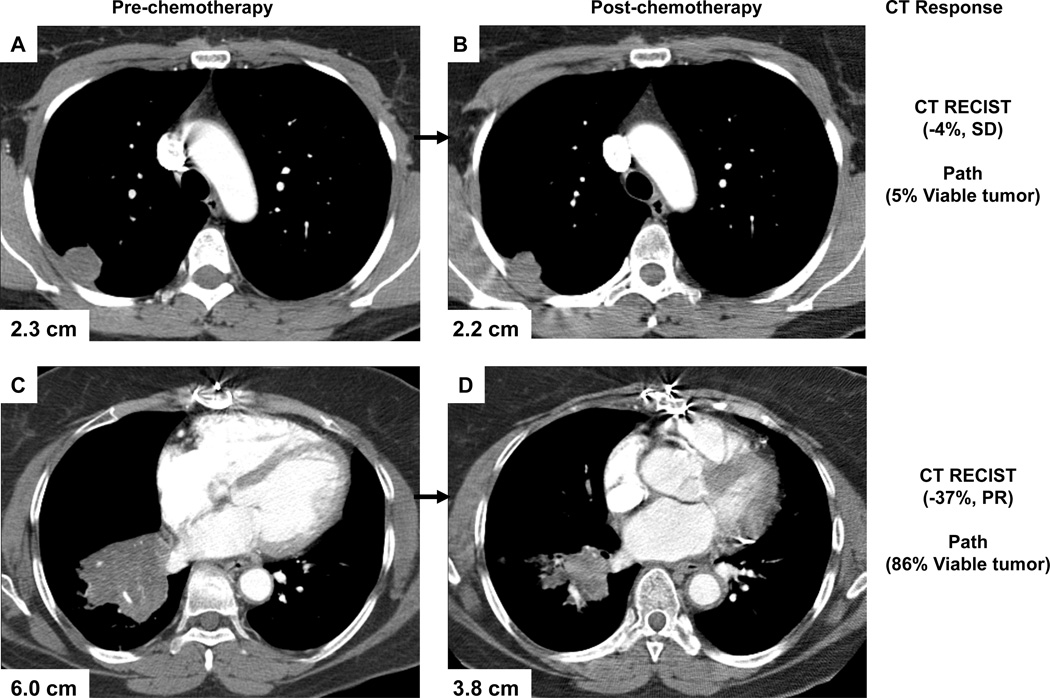
CT RECIST demonstrated two (1%) patients with a complete response and 78 (49%) patients with a partial response. Stable disease occurred in 75 (47%) patients and disease progression was rare and seen in only 5 (3%) patients after neoadjuvant chemotherapy. Histopathologic response (≤ 10% viable tumor) was seen in 30/160 patients (19%) and occurred more frequently in patients with CR/PR by CT criteria, compared to patients with SD/PD (27% versus 10%, P<0.005) (Table 2). There was, however, a 41% discordance rate between histopathologic response and CT RECIST response (8/80 patients had a histopathologic response despite being classified as SD/PD by CT criteria, and 58/80 patients did not achieve pathologic response despite being classified as CR/PR by CT criteria) (Figure 1). The sensitivity of CT RECIST to identify histopathologic responders was 73% and the specificity was 55%. Representative examples of the dissociation between response by CT and pathologic criteria are shown in Figure 2.
TABLE 2.
Distribution of CT Response and Pathologic Response to Neoadjuvant Chemotherapy in 160 NSCLC Patients
| Path Response | ||
|---|---|---|
| Category | ||
| CT Response Category |
≤10% Viable tumor cells | >10% Viable tumor cells |
| No. of Patients (%) | No. of Patients (%) | |
| Responder (CR/PR) |
22 (27%) | 58 (73%) |
| Non-responder (SD/PD) |
8 (10%) | 72 (90%) |
P=0.005
Figure 1.
Distribution of the percentage change in CT measured size of the primary tumor between pre-chemotherapy and post-chemotherapy measurements in 160 NSCLC patients who received neoadjuvant chemotherapy.
Figure 2.
CTs of lung tumors, showing examples of dissociation between radiological assessment of tumors and pathologic response. (A, B) No CT response to treatment by RECIST, despite 5% of viable tumor cells remaining after neoadjuvant therapy. (C, D) PR to treatment by CT criteria, but 86% of viable tumor cells remained in the resected specimen. The percentages shown are the change in the size of the target lesion between pre-chemotherapy and post-chemotherapy measurements.
Relationship Between CT and Histopathologic Response and OS
We analyzed the relationship between response assessed with CT radiologic criteria (RECIST), histopathologic criteria and OS in NSCLC patients who received neoadjuvant chemotherapy. The Kaplan-Meier survival curves in Fig 3A show that patients with CR or PR by radiologic criteria have improved OS compared to patients with SD or PD (p = 0.03). Patients with a histopathologic response have a statistically significant improvement in OS compared to patients that did not achieve a histopathologic response (p = 0.002) (Fig 3B). The separation of the curves in Fig 3B is more pronounced when compared to Fig 3A, suggesting that histopathologic response may more accurately identify patients with a higher chance of long-term survival compared to RECIST.
Figure 3.
Kaplan-Meier estimates of OS for CT (RECIST) and histopathologic response criteria. (A) CT-RECIST grouping into responders and non-responders demonstrates a difference in OS (p=0.03) (B) With histopathologic response, OS was significantly different between responders (≤10% viable tumor) and non-responders (>10% viable tumor, p=0.002), with a more pronounced separation of the curves when compared to CT-RECIST.
On univariable analysis, CT response, histopathologic response, and pathologic stage were significantly associated with OS (Table 3). These variables were then included on the multivariable analysis. Wald stepwise elimination excluded CT response from the multivariable model, indicating a stronger association of OS with histopathologic response compared with CT response. Multivariable Cox proportional hazards regression analysis revealed an association of both pathologic stage (p<0.001) and histopathologic response (p=0.05) with OS (Table 3). We repeated the multivariable analysis using the Cox proportional hazards regression model with backward stepwise Wald elimination, applying more stringent criteria for CT response (i.e., at least 50% or 70% reduction in tumor size). In both cases, CT response was not significantly associated with overall survival (p=0.23 for CT response at the 50% threshold, and p=0.98 for CT response at the 70% threshold). As observed for the 30% threshold, in both cases (50% and 70%), backward stepwise Wald elimination excluded CT response from the multivariable model, while maintaining percentage of viable tumor cells and pathological stage. We conclude, from these findings, that even when using more stringent thresholds to define CT response, pathologic response still outperforms CT response in predicting overall survival.
TABLE 3.
Univariable and Multivariable Cox Proportional Hazard Analyses for Overall Survival in 160 NSCLC Patients Who Received Neoadjuvant Chemotherapy.
| Characteristics | No. of Patients |
HR | 95%CI |
P- Value |
|---|---|---|---|---|
| Univariable Cox Regression Model | ||||
| CT Response | ||||
| Responder (CR/PR) | 80 | 1.00 | ||
| Non-responder (SD /PD ) | 80 | 1.68 | 1.04–2.7 | 0.03 |
| Viable tumor | ||||
| ≥10% | 30 | 1.00 | ||
| >10% | 130 | 3.56 | 1.52–8.32 | 0.003 |
| Pathological Stages | <0.001 | |||
| 0/IA/IB | 67 | 1.00 | ||
| IIA/IIB | 43 | 2.08 | 1.07–4.07 | 0.03 |
| IIIA /IIIB | 41 | 4.40 | 2.41–8.03 | <0.001 |
| IV | 9 | 5.46 | 2.13–13.98 | <0.001 |
| Histology | ||||
| Adenocarcinoma (Reference) | 68 | 1.00 | ||
| Squamous Cell Carcinoma | 51 | 0.67 | 0.38–1.19 | 0.18 |
| Others | 41 | 0.92 | 0.52–1.61 | 0.76 |
| Age (Continuous) | 160 | 1.01 | 0.98–1.04 | 0.42 |
| Gender | ||||
| Female (Reference) | 68 | 1.00 | ||
| Male | 92 | 0.88 | 0.55–1.41 | 0.59 |
| Multivariable Cox Regression Model | ||||
| Viable tumor | ||||
| ≥10% | 30 | 1.00 | ||
| >10% | 130 | 2.39 | 0.99–5.78 | 0.05 |
| Pathological Stages | <0.001 | |||
| 0/IA /IB | 67 | 1.00 | ||
| IIA /IIB | 43 | 1.70 | 0.86–3.36 | 0.13 |
| IIIA /IIIB | 41 | 3.54 | 1.91–6.58 | <0.001 |
| IV | 9 | 4.71 | 1.83–12.11 | <0.001 |
Abbreviations: CT, Computed Tomography; CI, Confidence Interval; HR, Hazard Ratio. AJCC/UICC 6th edition
Complementary Prognostic Value of Radiological and Histopathologic Criteria
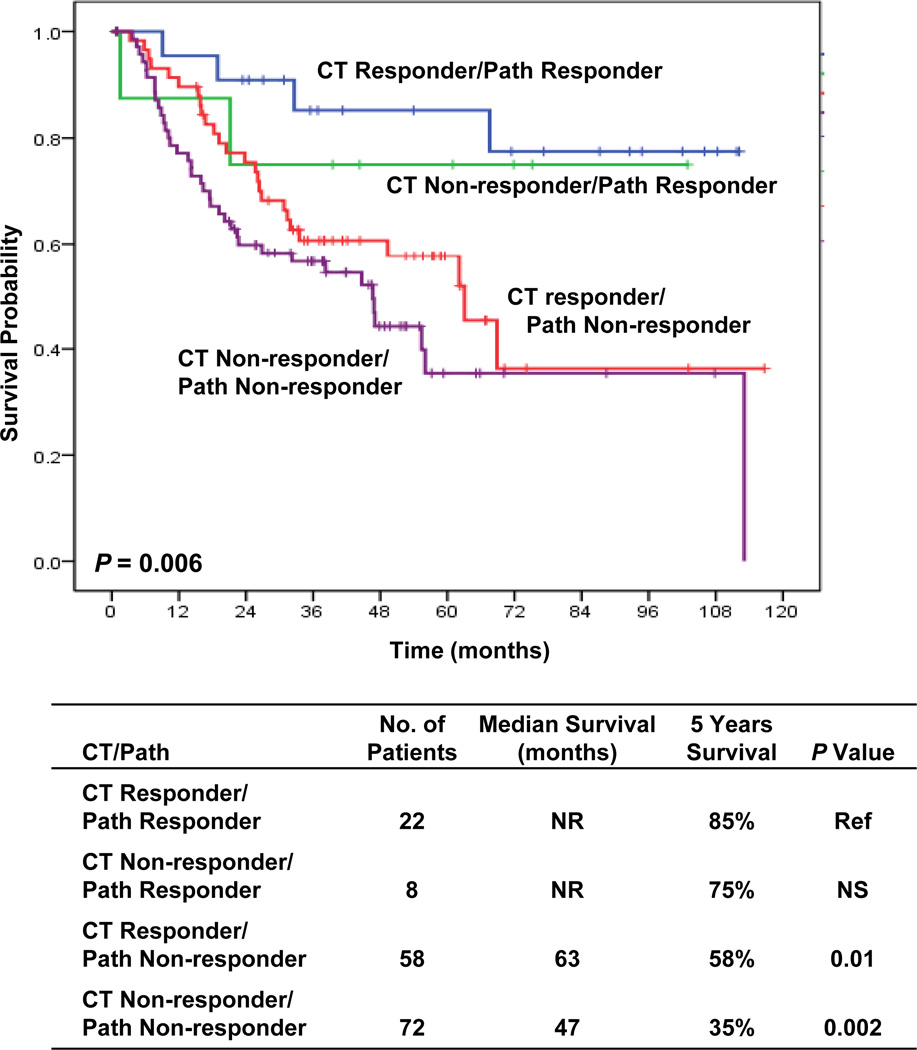
To determine whether the failure of CT RECIST response criteria to predict OS was due to lack of correlation with histopathologic response we combined radiologic CT RECIST and histopathologic criteria into four subgroups: (1) patients who were CT responders and histopathologic responders, (2) patients who were CT responders but histopathologic non-responders, (3) patients who were CT non-responders but histopathologic responders, (4) patients who were CT non- responders and histopathologic non-responders. As shown in Fig 4, Kaplan-Meier survival analysis indicated that the four subgroups had significantly different OS (p = 0.006). Patients who were CT responders and histopathologic responders had prolonged OS but CT non-responders with histopathologic responders also had prolonged survival even greater than CT responders and histopathologic non-responders. These results suggest that histopathologic response may be the most important predictor of long-term survival and that CT response may not be predictive in all patients because CT response does not identify all patients who have a pathologic response. Furthermore, in patients that were pathologic non-responders, there was no significant difference in survival between CT responders and CT non-responder (p=0.14, data not shown) suggesting that CT response does not compensate for a lack of histopathologic response after neoadjuvant chemotherapy as regards to improvement in OS.
Figure 4.
Kaplan-Meier estimates of OS for response assessment based on both CT RECIST and histopathologic criteria: Survival correlates with histopathologic response even when combined with CT RECIST criteria (5 yr survival – histopathologic response 85% and 75% for CT responders and non-responders, respecyively, vs non histopathologic response 53% and 38%, for CT responders and non-responders, respectively, p<0.001)
DISCUSSION
The current standard of care in North America and Europe following surgical resection of lymph node-positive NSCLC patients is cisplatin-based adjuvant chemotherapy. These recommendations are based on data from three randomized controlled studies12–14 and a meta-analysis from 5 trials with 4584 patients15. Additionally, neoadjuvant chemotherapy is also being used in patients with non-metastatic NSCLC1–4 and a recent meta-analysis from 13 randomized neoadjuvant trials (3206 patients), demonstrated a HR for death of 0.84 (95% CI 0.77–0.92, p=0.0001) in favor of neoadjuvant treatment, translating into an absolute improvement of overall survival at 5 years of 6%16. These studies also demonstrated that neoadjuvant treatment has activity in NSCLC and (1) elicits objective responses (assessed by imaging studies) in at least 40% of the patients; (2) has no significant increase in peri-operative mortality17; and (3) does not appear to negatively impact disease resectability.
A potential advantage of developing neoadjuvant treatment strategies for resectable NSCLC is the opportunity to evaluate response as an intermediary endpoint of efficacy. If a close correlation between response to treatment and overall survival is demonstrated then it would be possible to design more efficient clinical trials incorporating novel neoadjuvant therapies that would evaluate response as a surrogate marker for improved long-term outcomes. This strategy would allow for an early readout of efficacy, and could streamline drug development. It would also allow investigation of intensification of adjuvant treatment in patients that did not respond adequately to neoadjuvant therapy, in an attempt to improve long term outcomes.
In this study, we demonstrate that in 160 patients with resectable NSCLC who received neoadjuvant chemotherapy, there was an association between CT-measured tumor response (RECIST) and OS (p=0.03). However, histopathologic response was a stronger predictor of OS (p=0.002), with a more pronounced separation of the survival curves when compared to CT-measured response (Fig 3). The lower performance of CT-measured tumor response (RECIST) in predicting OS after neoadjuvant chemotherapy may be due in part to the inability of standard measurements of CT tumor size changes to predict histopathologic response. As demonstrated in Figure 4, 58/80 patients with a CT response failed to have a histopathologic response while 8/30 patients with a histopathologic response failed to demonstrate a response on CT RECIST response. Sensitivity was 73% but specificity was only 55%. This inability of RECIST CT-measured tumor size changes to predict histopathologic response may be due to various factors including the fact that NSCLC tumors are pathologically heterogeneous in composition and include cancer cells, stromal tissue and associated inflammatory cells18,19. Because of this, CT RECIST response assessment may provide only a macroscopic evaluation of the primary tumor and it is possible that the CT RECIST measured tumor size changes are confounded by inflammatory or fibrotic changes. This latter possibility has been reported previously in patients with advanced stage NSCLC18.
These observations have significant implications for ongoing clinical trials that utilize CT imaging response criteria (RECIST) as intermediary endpoints of treatment response in both metastatic and non-metastatic NSCLC20 as well as other tumor types19. Several studies have suggested that there may be more accurate CT response criteria than RECIST 21,22 such as volumetric response measurements with automatic deformable image registration (ADIR). Similarly in other tumor types, Choi and colleagues demonstrated that GIST tumors treated with imatinib were more accurately assessed with small CT changes in tumor size or density rather than standard RECIST criteria, while Chun and colleagues found that colorectal liver metastases were more accurately assessed with morphologic CT criteria than RECIST23. Other authors have suggested that monitoring response with apoptosis molecular imaging or contrast-enhanced MRI may be more accurate24–26.
It has also been suggested that response assessed by [18F] fluorodeoxyglucose (FDG) positron emission tomography (PET) after chemotherapy may be more accurate than CT measured responses (RECIST) in patients with NSCLC27,28,29–31. Not all authors are in agreement with this finding, however, as demonstrated by Tanvetyanon et al who evaluated two consecutive phase II neoadjuvant chemotherapy trials and found that CT response (RECIST) was more accurate than PET32. This is not unreasonable since FDG-PET imaging may be affected by the cellular composition of the primary tumor as well as the therapeutic-induced inflammatory response33. In this regard, the exact mechanism of FDG uptake and distribution among cells within the primary tumor is unknown and although FDG uptake in lung cancer is thought to be primarily due to the tumor cells, there is a variable contribution from the inflammatory response due to competitive uptake in macrophages and lymphocytes33. Animal studies have shown that up to 30% of the FDG-uptake in a tumor may be caused by the macrophage/monocyte system and that some tumors retain high FDG uptake at the end of therapy even with complete histopathological response at the time of resection34,35. It has recently been reported that the prediction of histopathologic response in patients with locally advanced NSCLC who received neoadjuvant chemotherapy followed by curative surgery is more accurate when defined by a combined radiologic-metabolic response using CT and FDG-PET compared to radiologic and metabolic response alone36,37. However, even so the accuracy for the prediction of histopathologic response was only 73 to 82% in radiologic-metabolic responders (compared with 70% in radiologic responders and 52 to 75% in metabolic responders)37.
In conclusion, our study suggests that changes in CT measured tumor size by standard RECIST response criteria are unreliable in predicting OS or histopathologic response after neoadjuvant therapy in resectable NSCLC. Because of the overall poor reliability of CT in predicting therapeutic response and OS, CT RECIST may have only a limited role as an endpoint for efficacy in clinical trials with novel therapeutics in metastatic and non-metastatic NSCLC. In the future, novel CT, PET or molecular imaging response criteria may need to be developed beyond standard CT RECIST changes in tumor size to accurately serve as surrogate endpoints for treatment efficacy.
ACKNOWLEDGMENTS
We thank Wanda L. Reese for her assistance in preparing the manuscript.
Grant Support: This work is supported in part by the Department of Defense W81XWH-07-1-0306, National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA 016672 - Lung Program, Specialized Program of Research Excellence (SPORE) Grant CA070907, and by support from the Homer Flower Gene Therapy Fund, the Charles Rogers Gene Therapy Fund, the Margaret Wiess Elkins Endowed Research Fund, the Flora and Stuart Mason Lung Cancer Research Fund, and the Phalan Thoracic Gene Therapy Fund, Rexanna’s Foundation, Mayberry Memorial Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: William William has received research grants from Sanofi-Aventis, Astellas, and Eli-Lilly, and American Society of Clinical Oncology. Reginald Munden has received grants from the Department of Defense, has provided expert testimony to the U. S. Government and has received honoraria from the British Oncology Group. Vassiliki Papadimitrakopoulou has received research grants from Astellas, Astra-Zeneca, Bristol Myers Squibb, Novartis, Merck, Celgene, Clovis. Stephen Swisher has received honoraria from Glaxo Smith Kline. Kathryn Gold has received a grant from the American Society of Clinical Oncology and honoraria from the M. D. Anderson Physicians Network. The other authors do not have any other conflicts of interest to declare.
MD Anderson Lung Cancer Collaborative Research Group: Lauren Byers, Joseph Chang, George Blumenschein, James D. Cox, Wayne Hofstetter, Bingliang Fang, Frank Fossella, Don Gibbons, Bonnie Glisson, Waun Ki Hong, Faye Johnson, Daniel Karp, Merrill S Kies, Jonathan Kurie, Zhongxing Liao, Steven Lin, Charles Lu, Ritsuko Komaki, Cesar Moran, Michael O’Reilly, Vali Papadimitrakopoulou, Katherine M. W. Pisters, David Rice, Pierre Saintigny, George Simon, Anne Tsao, Garrett L. Walsh, James Welsh.
REFERENCES
- 1.Roth JA, Atkinson EN, Fossella F, et al. Long term follow-up of patients enrolled in a randomized trial comparing peri-operative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Gomez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized clinical trial. Lung Cancer. 1999;26:7–14. doi: 10.1016/s0169-5002(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 3.Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, IIIa non-small-cell cancer. J Clin Oncol. 2002;20:247–253. doi: 10.1200/JCO.2002.20.1.247. [DOI] [PubMed] [Google Scholar]
- 4.Pisters KM, Vallieres E, Crowley J, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group trial S9900, an intergroup, randomized, phase III trial. JCO. 2011;28:1843–1849. doi: 10.1200/JCO.2009.26.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pataer A, Kalhor n, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825–832. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouillet G, Monnet E, Milleron B, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7(5):841–849. doi: 10.1097/JTO.0b013e31824c7d92. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Erasmus JJ, Gladish GW, Broemeling L, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: Implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–2582. doi: 10.1200/JCO.2003.01.144. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Jarin X, Stoopler MB, Raftopoulos H, et al. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol. 2003;16:1102–1108. doi: 10.1097/01.MP.0000096041.13859.AB. [DOI] [PubMed] [Google Scholar]
- 10.Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol. 1997;123:469–477. doi: 10.1007/BF01192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junker K, Langner K, Klinke F, et al. Grading of tumour regression in non-small-cell lung cancer: Morphology and prognosis. Chest. 2001;120:1584–1591. doi: 10.1378/chest.120.5.1584. [DOI] [PubMed] [Google Scholar]
- 12.The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 13.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 14.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine Trialist Association [ANITA]: a randomized controlled trial. Lancet Oncol. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 15.Fruh M, Rolland E, Paignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3573–3581. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 16.Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. J Thorac Oncol. 2010;5:510–516. doi: 10.1097/JTO.0b013e3181cd3345. [DOI] [PubMed] [Google Scholar]
- 17.Siegenthaler MP, Pisters KM, Merriman KW, et al. Preoperative chemotherapy for lung cancer does not increase surgical morbidity. Ann Thorac Surg. 2001;71(4):1105–1111. doi: 10.1016/s0003-4975(01)02406-7. [DOI] [PubMed] [Google Scholar]
- 18.Birchard KR, Hoang JK, Herndon JE, et al. Early changes in tumor size in patients treated for advanced stage nonsmall cell lung cancer do not correlate with survival. Cancer. 2009;115:581–586. doi: 10.1002/cncr.24060. [DOI] [PubMed] [Google Scholar]
- 19.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 20.Milano A, Perri F, Ciarmiello A, Caponigro F. Targeted-therapy and imaging response: a new paradigm for clinical evaluation? Rev Recent Clin Trials. 2011;6(3):259–265. doi: 10.2174/157488711796575540. [DOI] [PubMed] [Google Scholar]
- 21.Kozak MM, Murphy JD, Schipper ML. Tumor volume as a potential imaging-based risk-stratification factor in trimodality therapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(5):920–926. doi: 10.1097/jto.0b013e31821517db. e4t al. [DOI] [PubMed] [Google Scholar]
- 22.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198(4):737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302(21):2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graves EE, Maity A, Le QT. The tumor microenvironment in non-small-cell lung cancer. Semin Radiat Oncol. 2010;20(3):156–163. doi: 10.1016/j.semradonc.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Langen AJ, van den Boogaart V, Lubberink M, et al. Monitoring response to antiangiogenic therapy in non-small cell lung cancer using imaging markers derived from PET and dynamic contrast-enhanced MRI. J Nucl Med. 2011;52(1):48–55. doi: 10.2967/jnumed.110.078261. [DOI] [PubMed] [Google Scholar]
- 26.de Saint-Hubert HM, Bauwens M, Verbruggen A, Mottaghy FM. Apoptosis imaging to monitor cancer therapy: the road to fast treatment evaluation? Curr Pharm Biotechnol. 2012;13(4):571–583. doi: 10.2174/138920112799436320. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JS, Choi NC, Fischman AJ, et al. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: Correlation with histopathology. Lung Cancer. 2002;35:179–187. doi: 10.1016/s0169-5002(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 28.de Geus-Oei LF, van der Heijden HF, Visser EP, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–1598. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 29.Akhurst T, Downey RJ, Ginsberg MS, et al. An initial experience with FDG-PET in the imaging of residual disease after induction chemotherapy for lung cancer. Ann Thorac Surg. 2002;73:529–536. doi: 10.1016/s0003-4975(01)03257-x. [DOI] [PubMed] [Google Scholar]
- 30.Schmücking M, Baum RP, Bonnet R, et al. Correlation of histologic results with PET findings for tumor regression and survival in locally advanced non-small cell lung cancer after neoadjuvant treatment. Pathologe. 2005;26:178–190. doi: 10.1007/s00292-005-0758-1. [DOI] [PubMed] [Google Scholar]
- 31.Pöttgen C, Levegrün S, Theegarten D, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res. 2006;12:97–106. doi: 10.1158/1078-0432.CCR-05-0510. [DOI] [PubMed] [Google Scholar]
- 32.Tanvetyanon T, Eikman EA, Sommers E, et al. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol. 2008;26:4610–4616. doi: 10.1200/JCO.2008.16.9383. [DOI] [PubMed] [Google Scholar]
- 33.Christensen JD, Colby TV, Patz EF., Jr. Correlation of [18F]-2-fluoro-deoxy-D-glucose positron emission tomography standard uptake values with the cellular composition of stage I nonsmall cell lung cancer. Cancer. 2010 Jun 8; doi: 10.1002/cncr.25302. [DOI] [PubMed] [Google Scholar]
- 34.Maschauer S, Prante O, Hoffmann M, et al. Characterization of 18F-FDG uptake in human endothelial cells in vitro. J Nucl Med. 2004;45:455–460. [PubMed] [Google Scholar]
- 35.Deichen JT, Prante O, Gack M, et al. Uptake of [18F]fluorodeoxyglucose in human monocyte-macrophages in vitro. Eur J Nucl Med Mol Imaging. 2003 doi: 10.1007/s00259-002-1018-8. [DOI] [PubMed] [Google Scholar]
- 36.Poettgen C, Theegarten D, Eberhardt W, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 2007;73:316–323. doi: 10.1159/000134474. [DOI] [PubMed] [Google Scholar]
- 37.Lee HY, Lee HJ, Kim YT, et al. Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol. 2010;5:497–503. doi: 10.1097/JTO.0b013e3181d2efe7. [DOI] [PubMed] [Google Scholar]