Abstract
Objectives
We determined the efficacy of dietary sodium restriction (DSR) for improving vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure (SBP; 130–159 mmHg) and the associated physiological mechanisms.
Background
Vascular endothelial dysfunction develops with advancing age and elevated SBP, contributing to increased cardiovascular risk. DSR lowers BP, but its effect on vascular endothelial function and mechanisms involved are unknown.
Methods
Seventeen subjects (11M/6F; 62±7 yrs, mean±S.D.) completed a randomized, crossover study of 4 weeks of both low and normal sodium intake. Vascular endothelial function (endothelium-dependent dilation; EDD), nitric oxide (NO)/tetrahydrobiopterin (BH4) bioavailability and oxidative stress-associated mechanisms were assessed following each condition.
Results
Urinary sodium excretion was reduced by ~50% (to 70±30 mmol/day), and conduit (brachial artery flow-mediated dilation [FMDBA]) and resistance (forearm blood flow responses to acetylcholine [FBFACh]) artery EDD were 68% and 42% (peak FBFACh) higher following the low sodium diet (p<0.005). Low sodium markedly enhanced NO- mediated EDD (greater ΔFBFACh with endothelial NO synthase [eNOS] inhibition) without changing eNOS expression/activation (Ser1177 phosphorylation), restored BH4 bioactivity (less ΔFMDBA with acute BH4), abolished tonic superoxide suppression of EDD (less ΔFMDBA and ΔFBFACh with ascorbic acid infusion), and increased circulating superoxide dismutase activity (p<0.05). These effects were independent of ΔSBP. Other subject characteristics/dietary factors and endothelium-independent dilation were unchanged.
Conclusions
DSR largely reverses both macro- and microvascular endothelial dysfunction by enhancing NO and BH4 bioavailability and reducing oxidative stress. Our findings support the emerging concept that DSR induces “vascular protection” beyond that attributable to its BP-lowering effects.
Keywords: aging, nitric oxide, hypertension, diet, oxidative stress
Cardiovascular diseases [CVD] remain the leading cause of death in the United States and risk of CVD increases progressively with age (1). Vascular endothelial dysfunction, as reflected by impaired endothelium-dependent dilation [EDD] of conduit arteries and/or resistance vessels (2), is a predictor of future CVD and CV events (3,4). EDD is reduced in middle-aged and older adults in the absence of major risk factors or clinical CVD (5). Systolic blood pressure [SBP] also increases with advancing age (6), resulting in ~65% of adults 50 years of age and older with SBP ≥130 mmHg (7). Importantly, the age-associated impairment of EDD is even greater in middle-aged and older adults with elevated compared with normal SBP (2,8).
Dietary sodium restriction is a commonly recommended lifestyle modification for reducing risk of CVD (9). Based on recent analyses, the projected health and societal benefits resulting from population-wide dietary sodium restriction would be substantial (10). To date, such projections have focused on the effects of sodium restriction on BP. However, high dietary sodium intake has adverse cardiovascular effects independent of BP (11,12). Evidence from animal models and acute salt loading or cross-sectional observations in humans suggests a negative association between sodium intake and vascular endothelial function (11,13–16). However, presently it is unknown if dietary sodium restriction can reverse endothelial dysfunction in the common clinical setting of aging and elevated SBP.
The physiological mechanisms by which dietary sodium restriction may improve vascular endothelial function in humans also are unknown. Available data from rodent models suggest that high sodium diets may impair EDD by reducing nitric oxide [NO] bioavailability as a result of oxidative stress (14,17). This may be mediated via oxidation and reduced bioavailability of tetrahydrobiopterin [BH4] (18), an essential cofactor for endothelial nitric oxide synthase [eNOS]-mediated NO production, which causes uncoupling of the enzyme (14,18). Limited data in rodents suggest that high sodium also may reduce expression of eNOS (19) and suppress the activity of superoxide dismutase [SOD], an important antioxidant enzyme (20).
The aim of this study was to determine if dietary sodium restriction improves vascular endothelial dysfunction in middle-aged/older adults with moderately elevated SBP by increasing NO and BH4 bioavailability and reducing oxidative stress.
Methods
Using a double-blind, placebo-controlled, randomized, crossover design we determined both conduit artery (macrocirculatory) and resistance vessel (microcirculatory) EDD and the putative physiological mechanisms involved under well-controlled conditions of low and normal dietary sodium intake. Vascular NO and BH4 bioavailability and oxidative stress were assessed by “pharmaco-dissection” using agents that either inhibit or enhance these processes, whereas eNOS protein expression and activation (phosphorylation at Ser 1177) were measured in endothelial cells obtained from arterial sampling.
The study was conducted in the University of Colorado-Boulder Clinical and Translational Research Center [CTRC] and blood and urine assays were performed by the Colorado Clinical Translational Sciences Institute CTRC Core Lab at the University of Colorado-Denver Anschutz Medical Campus.
Subjects
Enrolled subjects were 50–79 years of age and had SBP of 130–159 mmHg, i.e., high normal or stage I systolic hypertension, with diastolic BP <99 mmHg, verified on a minimum of two occasions, as described previously (21,22). These criteria were chosen to encompass the resting BP values of the majority of middle-aged and older adults at higher BP-related risk of CVD (7,23). Enrolling subjects with high normal and stage I hypertension also increased the likelihood of assessing adults who were more salt-sensitive at baseline, as salt sensitivity increases with increasing resting BP (24). All subjects were otherwise free of CVD, diabetes, kidney disease and other clinical disorders. Details of inclusion and exclusion criteria can be found in the online supplement.
Twenty middle-aged and older men and women were enrolled in the study (see Supplemental Figure 1 for CONSORT diagram). Three subjects withdrew after beginning the low-sodium diet (see below), but prior to completing the first set of vascular measurements (one was uncomfortable with study procedures; two decided they could not comply with the diet). The remaining 17 subjects (51–77 years; 11 men and 6 postmenopausal women) completed the entire 10-week protocol. All procedures were approved by the Institutional Review Board of the University of Colorado at Boulder. The nature, benefits and risks of the study were explained to the volunteers and their written informed consent was obtained prior to participation.
Experimental Design and Dietary Sodium Restriction
We used a double-blind, placebo-controlled, randomized, crossover design originally described by Cappuccio et. al. (25) and more recently by our laboratory (21). A low sodium target of 50 mmol/day (1,200 mg/day) was set, anticipating that actual mean intake would fall at ~65 mmol/day (1,500 mg/day), consistent with the Dietary Approaches to Stop Hypertension [DASH] diet (26). This was compared to the mean (“normal”) U.S. sodium intake of 150 mmol/day (3,600 mg/day) based on recent National Health and Nutrition Examination Survey [NHANES] dietary intake data (27). During the entire 10-week intervention period, subjects reduced dietary sodium intake to the target of ~50 mmol/day and were instructed to take a total of 10 tablets spread across the day with meals. For five of the weeks the tablets were placebo pills, whereas for the other five weeks the tablets were slow-release sodium chloride tablets (10 mmol [0.23 g] per tablet) (HK Pharma, UK). The slow-release salt tablets aimed to return sodium intake to the ~150 mmol/day target.
Following screening, subjects were provided with comprehensive dietary education and counseling by CTRC bionutritionists to reduce dietary sodium intake without changing caloric intake, dietary composition or potassium intake. Vascular measurements were performed after four weeks (i.e., during the fifth week) of each condition. Details of the experimental design can be found in the online supplement.
Blood Pressure, Clinical Characteristics, Assays and Diet Analysis
Brachial artery BP was assessed under quiet resting conditions in a supine position using a semi-automated device (Dynamap XL, Johnson and Johnson) in accordance with the Joint National Committee guidelines (28), as described previously (21). SOD activity was measured in plasma samples (n=12/13) using a commercially available colorimetric kit (Cell Technology) (29). 24-hr urinary sodium and potassium excretion were measured weekly as a quantitative maker of dietary intake (Figure 1). A CTRC bionutritionists analyzed a 3-day diet record recorded by subjects at baseline and at the end of each sodium condition, as described previously (21). For details of these measurements, as well as assessments of other subject characteristics, please see the online supplement.
Figure 1. 24-hour Urinary Sodium and Potassium Excretion.
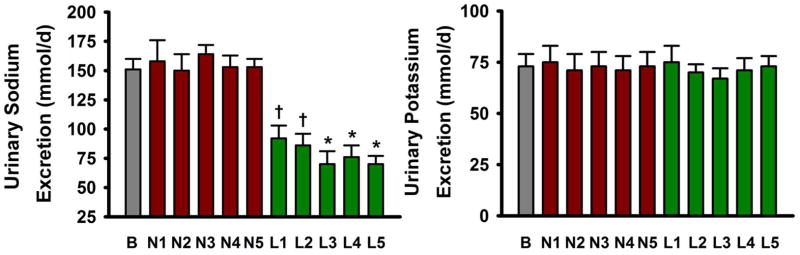
24-hour urinary excretion of sodium (left) and potassium (right) at baseline (B), weeks 1–5 of the normal sodium condition (N1–N5) and weeks 1–5 of the low sodium condition (L1–L5). Values are mean ± S.E.; * P<0.001; † P<0.005 vs. baseline or normal sodium of same week (repeated measures ANOVA with post-hoc Bonferonni corrected comparisons; n=17).
Conduit Artery Endothelium-Dependent and -Independent Dilation
Brachial artery flow-mediated dilation [FMDBA], estimated peak shear rate and endothelium-independent dilation (brachial artery dilation to 0.4 mg of sublingual nitroglycerin) were determined using duplex ultrasonography (Power Vision 6000, Toshiba) with a 7.5MHz adjustable (6–11 MHz) linear array transducer, as described previously (30,31). BH4 modulation of EDD was determined by measuring FMDBA ~3 hrs after oral administration of BH4 or placebo tablets (32) (n=14/15), and the influence of oxidative stress on FMDBA was assessed by infusing a supraphysiological dose of ascorbic acid or isovolumic saline during each of these visits (BH4 and placebo days), as described previously (31,33) (n=12–17). See the online supplement for details.
Resistance Vessel Endothelium-Dependent and -Independent Dilation
Forearm blood flow [FBF] was measured in the experimental (catheterized) and the control forearm with strain-gauge venous occlusion plethysmography (AI6 Arterial Inflow System, Hokanson), as previously described (n=12/13) (34). All FBF values are presented as percent increase in flow (ml · 100 ml forearm tissue−1 · min−1) in response to intrabrachial artery drug infusion (acetylcholine [ACh], sodium nitroprusside, ACh + NG monomethyl-L-arginine [L-NMMA] or ACh + ascorbic acid) compared with saline control. Forearm vascular conductance [FVC] was calculated from intra-arterial BP and FBF (FVC=FBF/mean arterial pressure) to account for any differences in arterial BP between sodium conditions. See the online supplement for details.
Endothelial Cell Protein Assessments
The procedures for collection and analysis of arterial endothelial have been described in detail previously (30,31). Vascular endothelial cells were obtained from the brachial artery and analyzed for expression of total eNOS and eNOS phosphorylated at Ser 1177 using immuno-fluorescence imaging (n=12/13). For details, please see the online supplement.
Statistics
Changes in EDD in response to dietary sodium restriction (low vs. normal sodium condition) were analyzed using a series of linear mixed-effects models (Supplemental Table 1; see supplement for details and power calculations). Differences in other variables were assessed using paired t-tests (between sodium conditions) or repeated measures ANOVA with post-hoc Bonferonni corrected comparisons (sodium conditions vs. baseline). Bivariate relations were determined using the Pearson correlation coefficient. All data are reported as means ± S.D.
Results
Effectiveness of Dietary Sodium Restriction and Clinical Characteristics
Subjects were non-Hispanic Caucasian (88%) or Asian (12%) with a mean age of 62±7 years (Table 1). They successfully reduced sodium intake without altering potassium intake based on both urinary excretion (sodium: 151±37 mmol/d, baseline; 153±27 mmol/d, normal sodium condition; 70±30 mmol/d, low sodium condition [week 5 values]) (Figure 1) and dietary analysis (Table 2). Other than sodium intake, diet composition was unchanged (Table 2). As anticipated, SBP was reduced during the low sodium condition, but other clinical characteristics, including diastolic BP, were unaffected (Table 1). Circulating humoral factors also were unchanged (Table 3).
Table 1.
Clinical Characteristics
| Clinical Characteristics | Baseline | LS | NS |
|---|---|---|---|
| Body Mass (kg) | 80.5±17.5 | 79.3±17.1 | 79.8±17.4 |
| BMI (kg·m2) | 27.1±4.1 | 26.4±4.2 | 26.6±4.3 |
| Systolic Blood Pressure (mmHg) | 138±7 | 128±10* | 140±15 |
| Diastolic Blood Pressure (mmHg) | 83±7 | 79±6 | 82±6 |
| Total Cholesterol (mg·dL−1) | 196±27 | 187±27 | 194±22 |
| LDL Cholesterol (mg·dL−1) | 123±23 | 118±21 | 127±23 |
| HDL-Cholesterol (mg·dL−1) | 52±16 | 49±16 | 50±15 |
| Triglycerides (mg·dL−1) | 104±61 | 98±39 | 88±42 |
| Fasting Glucose (mg·dL−1) | 80±16 | 81±15 | 78±15 |
| Plasma Sodium (mmol) | 139.4±2.7 | 138.1±2.8 | 139.0±1.8 |
| Creatinine Clearance (mL·min−1· 1.73m2) | 96±16 | 92±26 | 99±29 |
| MDRD eGFR (mL·min−1· 1.73m2) | 81±13 | 82±15 | 86±19 |
| PA (ActiGraph) (kcals·wk−1) | N/A | 3242±1285 | 3016±1320 |
| PA (questionnaire) (MET hrs·wk−1) | N/A | 27±29 | 28±28 |
Data are mean ± S.D.
P<0.01 vs. baseline or NS.
BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate (Modification of Diet in Renal Disease (MDRD) Study Equation); PA, physical activity; MET, metabolic equivalent. ActiGraph data are on a sub-sample of n=10 subjects.
Table 2.
Diet Composition
| Diet Composition | Baseline | LS | NS |
|---|---|---|---|
| Total kcals per day | 2191±562 | 2045±509 | 2019±466 |
| Carbohydrates (% of total kcals) | 47±8 | 52±9 | 47±12 |
| Protein (% of total kcals) | 16±3 | 15±3 | 17±3 |
| Fat (% of total kcals) | 34±6 | 33±9 | 33±9 |
| Calcium (mmol·day−1) | 23±8 | 19±6 | 19±5 |
| Magnesium (mmol·day−1) | 11±5 | 12±5 | 11±3 |
| Potassium (mmol·day−1) | 63±18 | 64±17 | 64±18 |
| Sodium (mmol·day−1)† | 136±48 | 56±15* | 57±12* |
| Fruits and Vegetables (cups·day−1) | 3.7±1.9 | 3.6±1.6 | 3.7±1.8 |
Data are mean ± S.D;
P<0.001 vs. baseline.
Note, sodium intake is based on 3-day diet records and excludes sodium added back in pill form.
Table 3.
Circulating Humoral Factors
| Circulating Humoral Factors | LS | NS | P-value |
|---|---|---|---|
| Log C-Reactive Protein (mg·L−1) | 0.27±0.53 | 0.15±0.51 | 0.53 |
| Log Interleukin-6 (pg·mL−1) | 0.13±0.25 | 0.08±0.32 | 0.59 |
| Log Tumor Necrosis Factor α (pg·mL−1) | 0.05±0.10 | 0.03±0.17 | 0.66 |
| Angiotensin II (pg·mL−1) | 4.7±2.7 | 3.9±2.3 | 0.42 |
| Plasma Renin Activity (ng·mL−1·hr−1) | 1.48±1.2 | 0.84±0.54 | 0.36 |
| Aldosterone (ng·dL−1) | 5.7±2.6 | 4.1±1.6 | 0.32 |
| Endothelin-1 (pg·mL−1) | 6.3±1.4 | 5.9±1.3 | 0.58 |
| Norepinephrine (pg·mL−1) | 400±170 | 392±140 | 0.88 |
| Cystatin C (mg·L−1) | 0.76±0.15 | 0.77±0.13 | 0.93 |
| Insulin (μU·mL−1) | 11.4±4.7 | 9.3±3.9 | 0.19 |
Data are mean ± S.D. LDL, low-density lipoprotein.
Dietary Sodium Restriction Improves Conduit Artery (Macrocirculatory) Endothelial Function
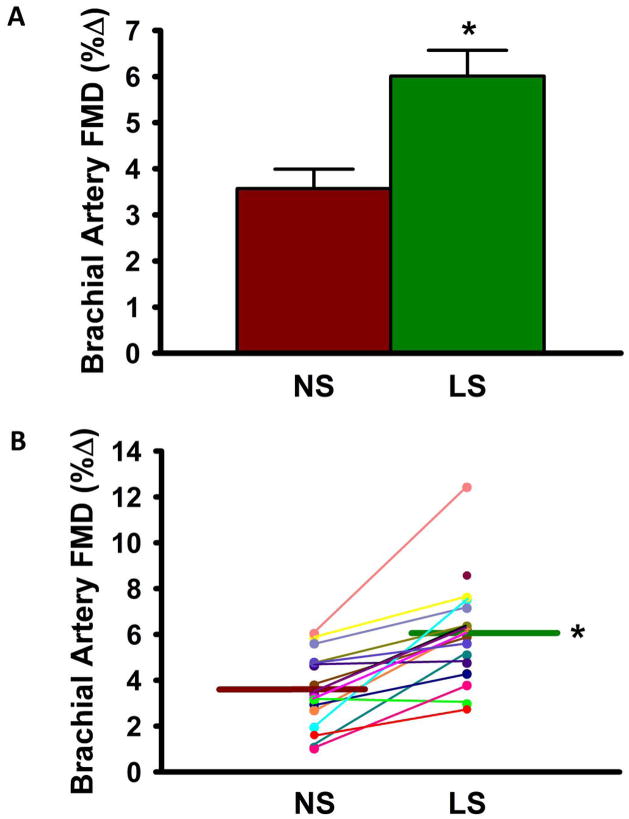
FMDBA was 68% greater during the low sodium vs. normal sodium condition (6.01±2.31 vs. 3.57±1.69 %Δ, p<0.001) (Figure 2A). The response was consistent, with all but two subjects demonstrating higher FMDBA in the low vs. normal sodium state (Figure 2B). When the two sodium conditions were pooled, there was a significant inverse relation between sodium excretion and FMDBA (r= −0.48, p<0.01). The difference in FMDBA between sodium conditions remained highly significant (p<0.001) after correcting for SBP and medication use, and ΔSBP with sodium restriction did not correlate with FMDBA (r= −0.05, p=0.85). No trends for gender differences were observed, although the study was not powered to detect such differences. Baseline diameter and shear rate did not differ across sodium conditions (Table 4) or with ascorbic acid ± BH4 administration, and the difference in FMDBA between conditions remained highly significant (p<0.001) when covarying for these factors. Endothelium-independent dilation to sublingual nitroglycerin was unaffected by dietary sodium restriction (Table 4). The associated mixed-effects regression models are shown in Supplemental Table 1.
Figure 2. Brachial Artery Flow-Mediated Dilation.
Mean group (A) and individual subject (B) brachial artery FMD %Δ during normal sodium [NS] vs. low sodium [LS] condition. Values are mean ± S.E.; *P<0.001 vs. NS (linear mixed-effects models (see supplement for details); n=16/17). Note, all seventeen subjects are shown in (B), but due to similarities some subjects overlap and may not be individually distinguishable.
Table 4.
Hemodynamic Factors
| Hemodynamic Factors | LS | NS | P-Value |
|---|---|---|---|
| Brachial artery FMD (mm Δ) | 0.23±0.07 | 0.15±0.07 | <0.001 |
| Baseline brachial artery diameter (mm) | 3.89±0.56 | 4.07±0.44 | 0.31 |
| Peak shear rate (sec−1) | 391±104 | 378±118 | 0.50 |
| Brachial artery dilation to NTG (% Δ) | 24.0±4.5 | 23.4±2.3 | 0.54 |
| Brachial artery dilation to NTG (mm Δ) | 0.91±0.14 | 0.91±0.15 | 0.82 |
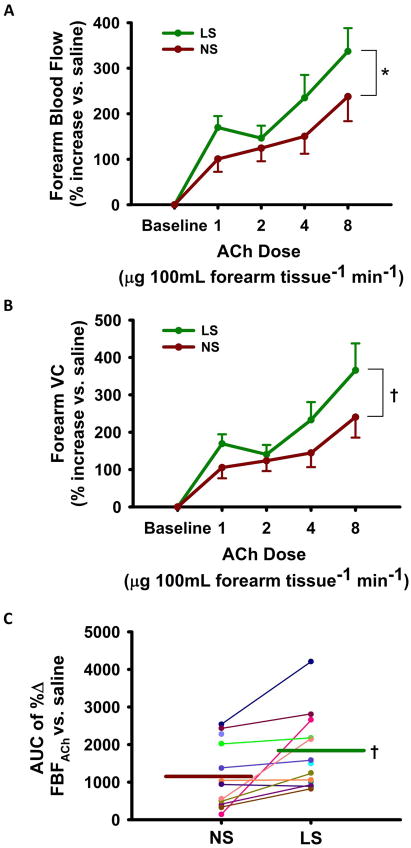
| AUC of %Δ FBFACh vs. saline | 1768±1002 | 1213±890 | <0.05 |
| AUC of %Δ FVCACh vs. saline | 1811±1006 | 1220±873 | <0.05 |
| AUC of %Δ FBFSNP vs. saline | 1035±377 | 995±635 | 0.93 |
| AUC of %Δ FVCSNP vs. saline | 1040±465 | 1000±655 | 0.93 |
Data are mean ± S.D. FMD, flow-mediated dilation; NTG, nitroglycerin; AUC, area under the curve; FBFACh, forearm blood flow to acetylcholine; FVC, forearm vascular conductance; SNP, sodium nitroprusside.
Dietary Sodium Restriction Improves Resistance Vessel (Microcirculatory) Endothelial Function
The low sodium condition improved EDD to ACh whether expressed as FBF (+42% at peak dose) or as BP-corrected forearm vascular conductance (+52% at peak dose) (both p<0.05) (Figure 3A–B). EDD also was greater in the low vs. normal sodium state when expressed as area under the dose-response curve (AUC, Table 4). Again, the effect of sodium restriction was consistent among the subjects, with all but two individuals demonstrating higher values for the AUC of FBFACh compared with the normal sodium state (Figure 3C). The FBF and forearm vascular conductance response to the endothelium-independent dilator sodium nitroprusside was unchanged across sodium conditions (Table 4). FBFACh in the control arm did not change with sodium condition or drug infusions (not shown).
Figure 3. Forearm Blood Flow to Acetylcholine.
Forearm blood flow [FBF] (A) and vascular conductance [VC] (B) response to acetylcholine [ACh] during normal sodium [NS] vs. low sodium [LS]. Individual subject FBFAch (area under the curve, AUC) (C). Values are mean ± S.E.; * P<0.005; † p<0.05 LS vs. NS (linear mixed-effects model; n=12/13).
Dietary Sodium Restriction Improves EDD by Increasing NO Bioavailability
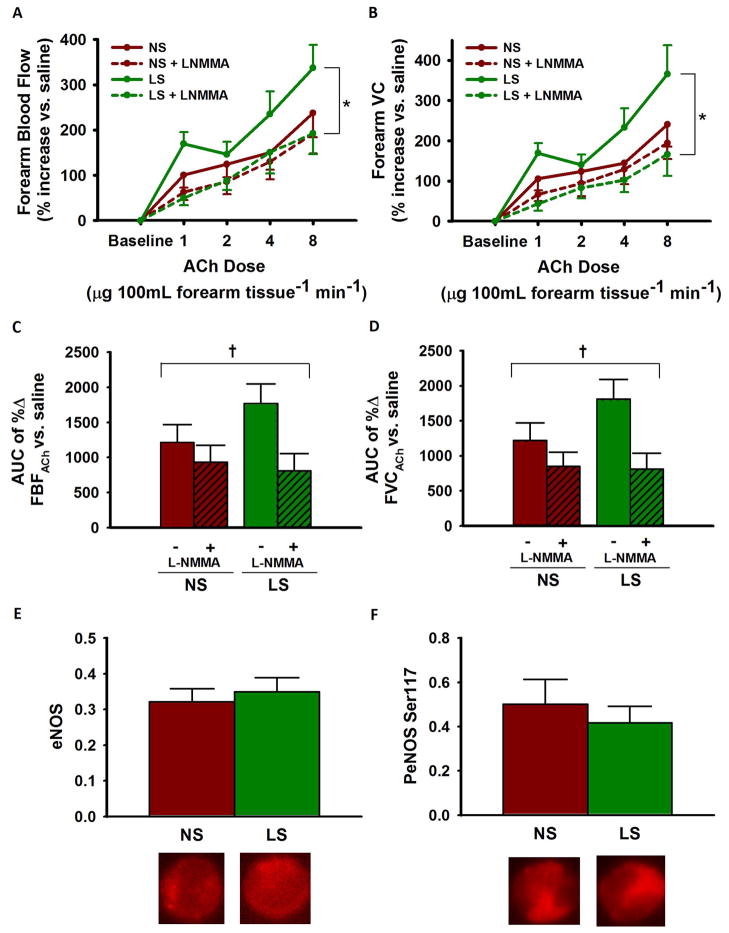
Co-infusion of the eNOS inhibitor L-NMMA markedly reduced FBFACh and BP-corrected forearm vascular conductance during the low sodium state, but not during the normal sodium condition (Figure 4A–D). As a result, FBFACh no longer differed between the low and normal sodium conditions during eNOS inhibition, indicating increased NO bioavailability during the low sodium state. In vascular endothelial cells sampled from the brachial artery, eNOS protein expression and activation, the latter indicated by phosphorylation at Ser 1177, did not differ between sodium conditions (Figure 4E–F).
Figure 4. Contribution of eNOS to EDD.
Dose-response for forearm blood flow [FBF] (A) and forearm vascular conductance [FVC] (B) to acetylcholine [ACh] + NG monomethyl-L-arginine [L-NMMA] during normal sodium [NS] and low sodium [LS]; area under the curve [AUC] values (C–D). Total endothelial nitric oxide synthase [eNOS] protein (E) and eNOS phosphorylated at Ser 1177 [PeNOS] (F) in subjects’ endothelial cells relative to human umbilical vein endothelial cell [HUVEC] control; representative images shown below. Values are mean ± S.E.; * P<0.001; † P<0.05 (drug x sodium condition interaction; linear mixed-effects model; n=12/13).
Dietary Sodium Restriction Improves EDD by Reducing Oxidative Stress and Increasing BH4 Bioavailability
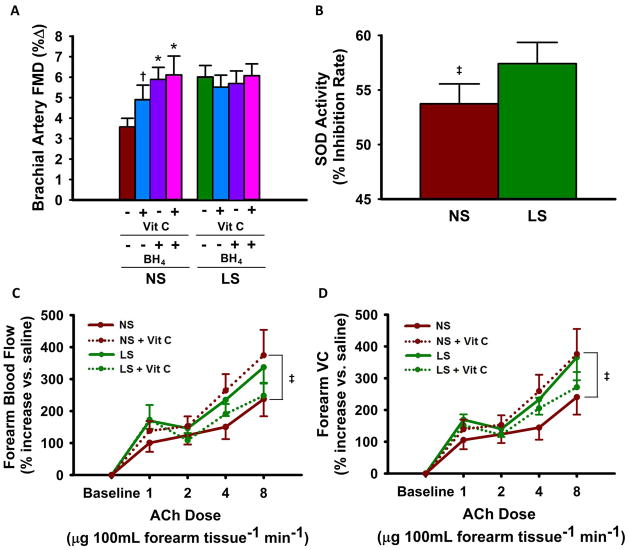
Infusion of ascorbic acid increased FMDBA during normal sodium (+37%, p<0.05), but not during the low sodium condition (Figure 5A), indicating reduced oxidative stress-related suppression of EDD during the low sodium condition. Similarly, oral BH4 administration improved FMDBA only in the normal sodium state (+65%, p<0.005) (Figure 5A). No further improvement in FMDBA was observed when ascorbic acid and BH4 were co-administered (Figure 5A). FMDBA no longer differed between the low and normal sodium conditions following administration of ascorbic acid and/or BH4. These responses were independent of the sodium condition-related influence on SBP. Endothelium-independent dilation to nitroglycerin did not change with ascorbic acid + BH4 during either sodium condition (data not shown). Circulating SOD activity was modestly greater (p<0.05) during the low vs. normal sodium condition (Figure 5B).
Figure 5. Contributions of Oxidative Stress and BH4 Bioavailability to EDD.
Brachial artery FMD %Δ during normal sodium [NS] vs. low sodium [LS] ± co-administration of ascorbic acid [Vit C] and/or tetrahydrobiopterin [BH4] (A). Circulating superoxide dismutase [SOD] activity (B). Forearm blood flow (C) and vascular conductance (D) response to acetylcholine [ACh] ± Vit C. Values are mean ± S.E.; * p<0.001; † p<0.005; ‡ p<0.05 (drug x sodium condition interaction or main effect of sodium condition; linear mixed-effects model; n=12–17).
Co-infusion of ascorbic acid also improved FBFACh (peak dose, +57%) under the normal sodium compared with the low sodium condition (Figure 5C) condition. FBFACh no longer differed between the low and normal sodium conditions during co-infusion of ascorbic acid. Results were similar when EDD was expressed as forearm vascular conductance (Figure 5D).
Discussion
The present findings are the first to show that restricting dietary sodium intake to a level consistent with the DASH diet improves both conduit artery (macrovascular) and resistance vessel (microvascular) endothelial function in middle-aged/older adults with moderately elevated SBP, a large clinical demographic with significant CVD risk burden. The results also provide the first insight in humans regarding the physiological mechanisms mediating improvements in vascular endothelial function with dietary sodium restriction, which include increased NO and BH4 bioavailability and reduced oxidative stress. None of the vascular effects observed were related to changes in BP, other dietary factors, clinical characteristics or humoral influences. Overall, our findings support the emerging concept that maintaining low dietary sodium intake exerts “vasculo-protective” effects beyond those attributable to BP-lowering.
Dietary Sodium Restriction and Vascular Endothelial Function
The present results demonstrate that dietary sodium restriction improves EDD as assessed by both FMDBA, a measure of peripheral conduit artery dilation to a mechanical (shear) stress, and FBFACh, an assessment of resistance vessel EDD in response to a chemical stimulus. These findings are in agreement with previous reports of impaired EDD in animals (13,18) and younger humans (11,15) following a high sodium load, as well as improved FMDBA after short-term sodium restriction in overweight/obese normotensive adults (35). The improvements in both micro- and macrocirculatory EDD with dietary sodium restriction in the present investigation were observed in almost all subjects and did not differ between the men and women studied. We also found that conduit artery EDD (FMDBA) was inversely related to urinary sodium excretion, an objective measure of dietary sodium intake.
The reduction in SBP with low sodium (12 mmHg) was similar to our previous studies on dietary sodium restriction (21,22) and consistent with other crossover studies employing well controlled BP assessments (25,36). The present magnitude of reduction was observed despite the inclusion of subjects on antihypertensive medications. Importantly, the improvements in EDD with sodium restriction remained significant after statistically correcting for SBP, and the changes in EDD were not related to the changes in SBP, whereas diastolic BP was unaffected. Thus, dietary sodium restriction improved vascular endothelial function beyond its influence on BP alone.
Physiological Mechanisms
High salt intake in younger humans and rodents impairs EDD by reducing NO bioavailability (13–15), and the chronic impairment in EDD in salt-sensitive compared with salt-resistant adults with hypertension is associated with reductions in bioavailable NO (37). Consistent with these observations, in the present study we show that lowering dietary sodium improves resistance vessel EDD by increasing NO bioavailability. The latter was not obviously associated with changes in eNOS or its activation as eNOS protein expression and phosphorylation of eNOS at ser 1177 did not differ between sodium conditions in vascular endothelial cells obtained from brachial artery sampling.
In rodents fed a high salt diet, impairments in EDD are linked to reduced bioavailability of BH4, an essential co-factor for NO production by eNOS (18), as well as oxidative stress (14,17), as indicated in part by improved EDD following acute administration of an antioxidant (17). In the present study, we found that a single oral dose of BH4 previously shown to improve EDD in settings of endothelial dysfunction (32) improved EDD (FMDBA) during normal, but not low sodium intake. Moreover, infusion of supraphysiological doses of ascorbic acid (vitamin C), a well-established method for acutely reducing oxidative stress-related suppression of EDD (8,33), improved both conduit artery (FMDBA) and resistance vessel (FBFACh) EDD during normal, but not lower sodium intake. Indeed, administration of BH4, ascorbic acid or both abolished the differences in EDD between the normal and low sodium conditions. We also found a modest but significant increase in circulating SOD activity in the low sodium condition, in agreement with the inhibitory effects of high salt feeding on SOD reported previously in rats (20).
It is important to note that BH4 bioavailability modulates NO-mediated EDD in the absence of changes in eNOS protein expression or activation (38). Without adequate BH4, eNOS is “uncoupled”, producing more superoxide anion and less NO (39). It is possible that dietary sodium restriction “recoupled” eNOS, increasing NO production without further activation of the enzyme. The apparent increase in BH4 bioavailability with sodium restriction most likely was mediated by reduced oxidative stress, as reactive oxygen species, particularly peroxynitrite formed by the reaction of superoxide and NO, readily oxidize BH4 to its inactive form, BH2 (39). Alternatively, sodium restriction may have increased NO bioavailability by inhibiting superoxide production by oxidant enzymes such as NADPH oxidase or by mitochondria, resulting in less superoxide to react with/reduce the bioavailability of NO.
Clinical Significance
The concept that high sodium intake has adverse cardiovascular effects independent of BP has been advanced previously (9,12). High dietary sodium impairs EDD even in rodents that are salt-resistant and, thus, do not exhibit increases in BP in response to a high salt diet (13,18,20). Acute impairment of EDD in normotensive adults after sodium loading also is BP-independent (11), and adults with elevated SBP who report lower sodium intake have enhanced EDD independent of BP (16). The present results extend these findings to sodium restriction and lend support to the overall hypothesis that sodium intake not only elevates BP, but exerts other adverse influences (12). The effects of sodium restriction on endothelial function reported here also complement previous findings that reducing sodium intake can rapidly de-stiffen large elastic arteries (21), another independent vascular risk factor for CVD (40). The improvements in these two common forms of arterial dysfunction, both predictors of future CV events (3,4,40), suggest that sodium restriction has strong potential for reducing CVD risk via broad vasculo-protective effects.
Strengths and Limitations
Diet was well-controlled in the present study with only sodium intake differing between our two conditions, as documented by “gold standard” 24-hour urine collections combined with subject diet records. This feature, along with the placebo controlled crossover design employed allowed us to isolate dietary sodium as the modulating factor between conditions. Importantly, our results are generalizable to community settings in that sodium restriction was achieved by subjects on their own, guided by dietary counseling, rather than delivered as prepared meals in a research setting. The assessments of macro- and microcirculatory endothelial function, both of which are involved in age- and BP-related CV pathology, as well as the unique insight into mechanisms of action acquired by the use of novel translational research techniques, are additional strengths of the study.
Due to the complexity, burden and, in some cases, invasiveness of the study design and procedures, we were able to study only a limited number of subjects. However, as recently emphasized by Donald et al., a much smaller n is required when assessing EDD using a crossover compared with a parallel group design (41), as shown in previous investigations (31,42). As such, we were well powered to assess change in EDD (see supplement), and importantly, our approach allowed investigation of the mechanisms underlying the beneficial vascular effects of sodium intake at a depth and level of detail that would not be possible in large cohorts.
Conclusions
The results of the present study support the hypothesis that dietary sodium restriction to a level consistent with the DASH diet improves both conduit artery (macrovascular) and resistance vessel (microvascular) endothelial function in middle-aged/older men and women with elevated SBP by increasing NO and BH4 bioavailability and reducing oxidative stress. These findings provide further support for the idea that dietary sodium restriction has the potential to improve CV health and reduce the risk of CVD beyond its BP-lowering effects.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health awards NIH AG013038, AG006537, AG03114, AG033994, AG03167 and TR000154
The authors thank Keri Nelson, Eric Chung, Elena Pellicer, Kate Howell and the staff of the University of Colorado-Boulder CTRC, particularly the bionutritionists Kathleen Farrell and Rebecca Staley, for their technical assistance.
Abbreviations list
- BH4
tetrahydrobiopterin
- CTRC
Clinical and Translational Research Center
- CVD
cardiovascular diseases
- EDD
endothelium-dependent dilation
- FBFACh
forearm blood flow to acetylcholine
- FMDBA
brachial artery flow-mediated dilation
- eNOS
endothelial nitric oxide synthase
- SOD
superoxide dismutase
- SBP
systolic blood pressure
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–75. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 4.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 7.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population: Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Frohlich ED, Hall JE, et al. The Importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the American Heart Association. Circulation. 2011;123:1138–1143. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–9. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr. 2011;93:500–5. doi: 10.3945/ajcn.110.006155. [DOI] [PubMed] [Google Scholar]
- 12.Frohlich ED. The salt conundrum: A hypothesis. Hypertension. 2007;50:161–6. doi: 10.1161/HYPERTENSIONAHA.107.088328. [DOI] [PubMed] [Google Scholar]
- 13.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol. 1993;264:H1810–6. doi: 10.1152/ajpheart.1993.264.6.H1810. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol. 2004;286:H575–83. doi: 10.1152/ajpheart.00331.2003. [DOI] [PubMed] [Google Scholar]
- 15.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 16.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;3:347–356. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1550–6. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 18.Nurkiewicz TR, Wu G, Li P, Boegehold MA. Decreased arteriolar tetrahydrobiopterin is linked to superoxide generation from nitric oxide synthase in mice fed high salt. Microcirculation. 2010;17:147–57. doi: 10.1111/j.1549-8719.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155–63. doi: 10.1016/s0895-7061(00)01234-6. [DOI] [PubMed] [Google Scholar]
- 20.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39:41–50. doi: 10.1159/000048992. [DOI] [PubMed] [Google Scholar]
- 21.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 22.Seals DR, Tanaka H, Clevenger CM, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: Role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–13. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 24.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 25.Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double-blind randomised trial of modest salt restriction in older people. Lancet. 1997;350:850–4. doi: 10.1016/S0140-6736(97)02264-2. [DOI] [PubMed] [Google Scholar]
- 26.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Agriculture ARS. What We Eat in America. NHANES; 2007–2008. [Accessed 6/26/12]. Total nutrient intakes: Percent reporting and mean amounts of selected vitamins and minerals from food and dietary supplements, by gender and age. Available: www.ars.usda.gov/ba/bhnrc/fsrg. [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 29.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: Relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–70. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 30.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-kappa B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–65. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–24. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105:1359–63. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009;89:485–90. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 36.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet. 1989;2:1244–7. doi: 10.1016/s0140-6736(89)91852-7. [DOI] [PubMed] [Google Scholar]
- 37.Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–8. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi K, Hamadate N, Matsuzaki T, et al. Increasing dihydrobiopterin causes dysfunction of endothelial nitric oxide synthase in rats in vivo. Am J Physiol Heart Circ Physiol. 2011;301:H721–9. doi: 10.1152/ajpheart.01089.2010. [DOI] [PubMed] [Google Scholar]
- 39.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: The Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donald AE, Halcox JP, Charakida M, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–64. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 42.Walsh JH, Bilsborough W, Maiorana A, et al. Exercise training improves conduit vessel function in patients with coronary artery disease. J Appl Physiol. 2003;95:20–5. doi: 10.1152/japplphysiol.00012.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.