Abstract
Dengue is a mosquito-borne disease of growing global health importance. Prevention efforts focus on mosquito control, with limited success. New insights into the spatiotemporal drivers of dengue dynamics are needed to design improved disease-prevention strategies. Given the restricted range of movement of the primary mosquito vector, Aedes aegypti, local human movements may be an important driver of dengue virus (DENV) amplification and spread. Using contact-site cluster investigations in a case-control design, we demonstrate that, at an individual level, risk for human infection is defined by visits to places where contact with infected mosquitoes is likely, independent of distance from the home. Our data indicate that house-to-house human movements underlie spatial patterns of DENV incidence, causing marked heterogeneity in transmission rates. At a collective level, transmission appears to be shaped by social connections because routine movements among the same places, such as the homes of family and friends, are often similar for the infected individual and their contacts. Thus, routine, house-to-house human movements do play a key role in spread of this vector-borne pathogen at fine spatial scales. This finding has important implications for dengue prevention, challenging the appropriateness of current approaches to vector control. We argue that reexamination of existing paradigms regarding the spatiotemporal dynamics of DENV and other vector-borne pathogens, especially the importance of human movement, will lead to improvements in disease prevention.
Keywords: infectious disease, spatial epidemiology, arthropod-borne virus, emerging infections, disease ecology
Dengue is a mosquito-borne viral infection prevalent in the tropics and subtropics with an incidence and range that have increased substantially over the last three decades (1–4). The incidence of severe, life-threatening disease (dengue hemorrhagic fever or DHF) is also on the rise (5). Dengue virus (DENV) is caused by any of four closely related, but antigenically distinct and genetically diverse, virus serotypes (DENV-1, -2, -3, and -4), which are transmitted by day-biting, peridomestic Aedes mosquitoes, primarily Aedes aegypti (6). Incompletely understood immunological interactions among serotypes that can enhance disease severity (7–9) have hampered the development of effective, commercially available vaccines. A recent trial of a promising vaccine candidate demonstrated only partial (<33%) protection (10). Effective antiviral therapeutics are also not available. Thus, despite nearly a century of research, the only tools presently available to combat dengue target mosquito populations, mostly with insecticides and larval source reduction. In current practice, these tools are most often only partially effective (11). Although new vector control approaches are under development (12), innovations in dengue-control approaches are constrained by our limited understanding of virus-transmission dynamics and its drivers. In particular, the role of human movement in DENV transmission is uncertain, although it could be a key driver of fine-scale spatiotemporal disease patterns.
Few incisive, seroepidemiological studies of dengue have been conducted (13–15), in part, because of the difficulty and cost of conducting sufficiently large and long-term, cohort-based studies (16). Hence, much of what we do know about dengue is derived from passive disease-surveillance data that do not take into account that DENV infections in endemic populations are often asymptomatic or cause only mild disease (17, 18). This has led to significant uncertainty in our estimates of key factors determining the controllability of dengue, such as the basic reproductive rate (R0) (13). Nevertheless, it is well documented that dengue incidence and seroprevalence appear highly focal in both space and time (19–23). Moreover, Ae. aegypti mosquitoes are highly anthropophilic, aggregate in and around human premises, and disperse relatively short distances (<100 m) (24–27). These epidemiological and entomological observations form the basis of current vector control strategies, which include focal spraying in the geographic vicinity around the home of reported dengue cases and control of larvae developing in containers (28).
Whereas it has long been recognized that DENV dispersal is human-mediated at broad spatial scales [i.e., between communities, regionally and globally (6)], the importance of human movement at fine spatial scales has not been well studied. By contrast, human movement has long been recognized as a key underlying driver in the dynamics of directly transmitted diseases, such as measles (29, 30). Because mosquito vectors are mobile and many bite at night (as is the case for the anopheline malaria vectors), the prevalent assumption for vector-borne diseases has been that local human movements play only a nominal role in transmission (31). Dengue is one likely exception because of the day-biting habit (32–34) and limited flight range of Ae. aegypti in comparison with the distance covered by routine human movements between houses and other mosquito-harboring sites. Recent theoretical studies (31, 35–37) argue that human-mediated DENV dispersal should indeed be quite important for local virus propagation. If supported empirically, this would focus more attention on human-mediated virus dispersal, with important implications for dengue surveillance and prevention.
Here, we present data from a large-scale, longitudinal dengue cohort study in Iquitos, Peru, intended to measure the importance of house-to-house human movements on DENV transmission. We used contact tracing to identify households visited during the day by febrile participants with a diagnosed DENV infection over the previous 2 wk. Consenting contacts residing in houses visited by febrile cases were diagnosed for recent DENV infection (contact-site cluster investigations; Methods). We focused on houses because previous work by our team measured very low mosquito abundances in many nonresidential spaces in Iquitos [e.g., schools, open-air markets, recreational areas (38)] and on visited households because a house-based study design was logistically tractable and previous work in Iquitos revealed that adult Ae. aegypti cluster strongly within single houses (25). Our results show that infection risk and transmission rates are substantially elevated among households visited by DENV-infected people, pointing to a key role of human house-to-house movement in DENV transmission. This finding has important implications for dengue prevention and control, as well as our understanding of vector-borne diseases in general.
Results
We report results from 54 contact-site cluster investigations over two transmission seasons (SI Appendix, Table S1). In a case-control design (Methods), we compared clusters initiated by DENV-infected participants (DENV+; 23 clusters) with others initiated by febrile participants negative for DENV (DENV−; 25 clusters); 6 clusters were indeterminate (SI Appendix, Tables S1 and S2). A total of 906 people ≥5 y of age residing in 191 visited houses provided blood samples for DENV diagnosis (16.8 ± 8.2 SD people per cluster; 5.0 ± 2.4 SD per house; Fig. 1 A and B and SI Appendix, Tables S1 and S2). Just before the start of this study in early 2008, a new dengue serotype, DENV-4, invaded Iquitos and became predominant for the two subsequent transmission seasons of our study (>99% of virus isolates; Fig. 1C and SI Appendix, Table S3). Overall, 171 cluster participants were diagnosed positive for DENV infection. We estimated that 82.4% of infections were asymptomatic in DENV+ clusters, for a ratio of 1:4.56 apparent:inapparent infections (SI Appendix, Results and Table S4).
Fig. 1.
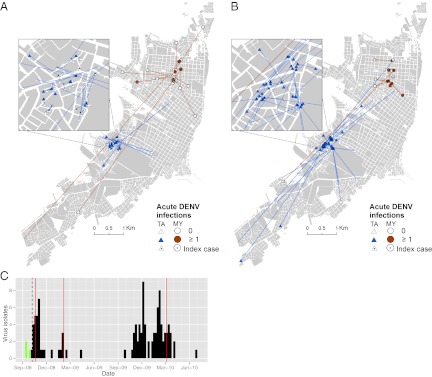
Summary of cluster investigations. Geographic distribution of households participating in DENV− (A) and DENV+ (B) cluster investigations (n = 48). Lines connect contact sites with the homes of index cases. Symbols denote the neighborhood of the index case’s home: circle, MY neighborhood; triangle, TA neighborhood. Filled symbols indicate any house with ≥one acute DENV infection (in all cluster investigations). (C) DENV isolates by week from febrile cases enrolled in the two study neighborhoods (green, DENV-3; black, DENV-4). The dashed vertical line indicates the beginning of the study period. Red vertical lines indicate when city-wide insecticide fumigation campaigns were instigated by local health authorities.
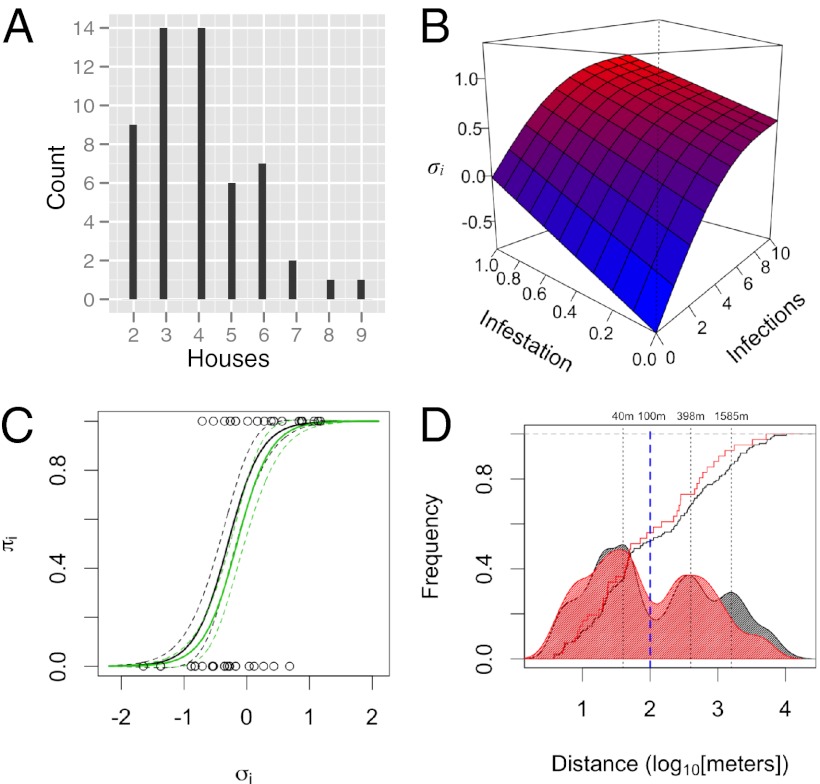
Of the 54 cluster investigation index cases (SI Appendix, Table S1), two-thirds (68%) were students, of which the majority (70%; SI Appendix, Fig. S3) were under 22 y of age and who, on average, reported visiting 8.55 places over the prior 2 wk between 0500 and 2200 hours (best fit by a negative binomial distribution, θ = 11.94). The most common type of place visited was residential, often houses of friends or family (35.3%; SI Appendix, Fig. S4). Index cases visited 3 other houses on average (range, 1–8; Figs. 1 A and B and 2A), with this number decreasing with age [β = −0.014; P < 0.05; Poisson generalized linear model]. People visited houses several times during the 15-d study period (median, 4 visits) for about 1–2 h each visit (median, 90 min; SI Appendix, Fig. S5). The houses they visited were distributed throughout urban Iquitos, with the majority very close to the index case’s home (52% within 100 m; Figs. 1 A and B and 2D and SI Appendix, Figs. S4 and S6). Residents of MY visited more houses outside their neighborhood than residents of TA. Nonresidential locations tended to be further away from the index home than residential locations, reflecting the centralized locations of commercial and public places in Iquitos (SI Appendix, Fig. S6). Generally, the movements of index cases from the two neighborhoods overlapped in the downtown commercial area and at a large outdoor market in the southeast corner of the city.
Fig. 2.
Individual movement and infection risk. (A) Distribution of the number of houses included in each cluster. Our analysis was restricted to individuals who reported visiting ≥one house beyond the home (SI Appendix). (B) Relationship between σi, cluster-infestation rates, and the total number of acute infections in a cluster [binomial generalized additive model, adjusted R2 = 76.7%; SI Appendix]. (C) Predicted risk for infection as a function of σi (black line, no prior exposure to DENV-4 in a cluster; green line, ≥one person with prior DENV-4 exposure). Open circles indicate DENV diagnosis for index cases (1 = DENV+). (D) Cumulative and frequency distribution of distance to visited houses (black, all houses; red, houses with ≥one acute DENV-4 infection). Although nearby houses were most common, most individuals (72.2%) visited places relatively far away from their home, with nearly half of all visited houses located >100 m away.
Because we were limited in our ability to estimate contact with mosquitoes in terms of the actual time spent at houses in the activity space, we developed an index, σi, to approximate virus exposure for an individual person, i (the index case; SI Appendix, Methods). Calculation of σi is based on the number of concurrent acute DENV infections that occurred throughout all places recently visited by index cases (i.e., all households participating in a cluster investigation):
 |
Here, pj is the proportion of all acute infections in cluster i that occurred in house j, J is the total number of households in the cluster investigation, n is the total number of acute infections in the cluster, and c is a correction factor. The second item on the right side of Eq. 1 is a penalty that increases with the ratio of houses to acute infections. Although σi correlates strongly with the total number of infections in a cluster and with the number of infested households (Fig. 2B and SI Appendix, Results), it is a better measure of contact than either alone because the probability that a visited household held an infective mosquito should increase with the number of concurrent infections in the same household. Time is finite, so visits to uninfested houses reduce contact at infested houses. Observing no acute infections in a cluster does not mean that the participating locations were not infested with infected vectors but that the probability was low. The correction term, c, allows for continuous values of contact even in the absence of any observed acute infections (SI Appendix, Methods).
DENV-infection risk was strongly correlated with σi (Figs. 2B and see Fig. 4 A and B), more so than with percentage of infestation or the total number of infections in a cluster, validating it as a measure of contact (SI Appendix, Results). Our best logistic regression model included σi (P < 0.001) and a measure of herd immunity among household residents [number of immune people; odds ratio (OR): 0.58; 95% confidence interval (CI): 0.292–0.953; Fig. 2C and SI Appendix, Tables S5 and S6]. Other effects, such as the neighborhood of the index case’s home and the average distance between visited houses (Fig. 2D), had little effect on risk estimates. Alternative models including only a term for the infection rate in the home of the index case or time in visited houses were poorer fits to the data (SI Appendix, Table S6). Total time spent at a location was not a good predictor of infection; rather, the frequency of visitation appeared to be more important (P < 0.05; SI Appendix, Results) (35).
Fig. 4.
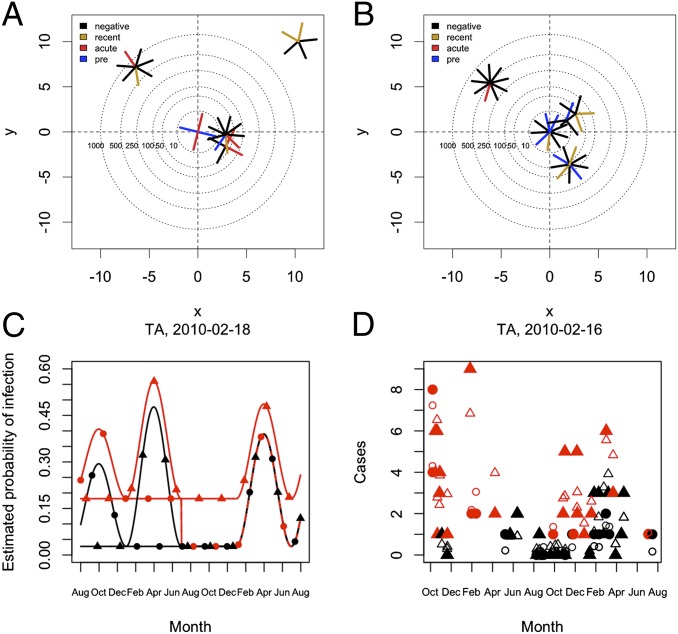
DENV-infection risk among contacts. Diagrams A and B show two clusters plotted in relative space (index house is at the center). Each segment represents one participant, color indicates serological status (recent, elevated acute IgM; acute, virus isolation or IgM seroconversion; pre, previous exposure). Distances on the x and y axes are the cube-root of actual distances; concentric circles around the index house demarcate different radii. (A) DENV+ cluster (σ = 1.1). (B) DENV− cluster (σ = −0.71). Both clusters were initiated in the same neighborhood, in the same week of the second season of transmission. (C) Estimates of infection risk over the period of the study based on a model assuming seasonally forced transmission (SI Appendix). Red indicates infection probability within DENV+ activity spaces, and black indicates infection probability within DENV− activity spaces. (D) Actual (solid symbols) and predicted (open symbols) number of cases based on the model. In C and D: circle, MY neighborhood; triangle, TA neighborhood.
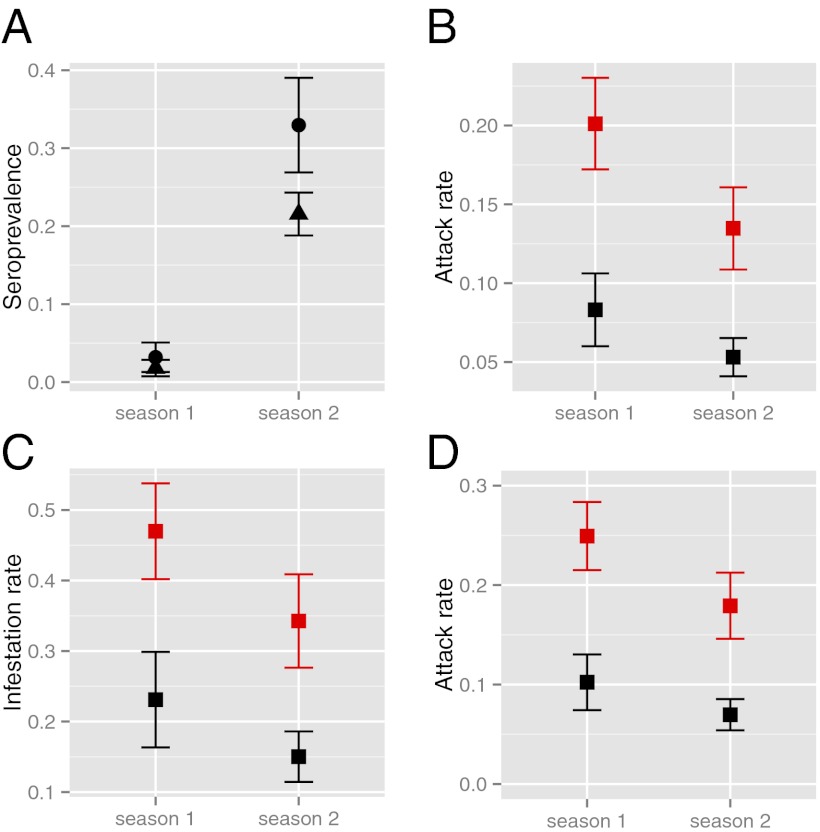
DENV incidence within clusters and among households was markedly heterogeneous and defined by how houses were connected through human movement. Despite declining transmission over the two seasons associated with increasing herd immunity (Fig. 3A) and citywide vector control efforts (Fig. 1C), there was significantly more transmission in DENV+ than in DENV− clusters, as estimated by binomial regression (Fig. 3B). Infestation rates (Methods,Data Analysis,Endpoints) were also markedly higher for DENV+ clusters (Figs. 3C and 4 A and B). Household attack rates showed the same pattern (Fig. 3D), with no difference between the homes of index cases and visited houses. Risk for infestation (≥one acute infection) was primarily determined by association with a DENV+ index case (OR = 5.53; 95% CI: 2.4–13.7; SI Appendix, Table S12), with a weak (and not significant) effect of distance from the index case’s home, which we attribute to lower infection rates in distant locations (>2 km; OR = 0.67; 95% CI: 0.41–1.07; SI Appendix, Table S12). The distributions of distances to all visited houses and those with ≥1 acute infection were remarkably similar except at long distances (>2 km; Fig. 2D). Over half of all visited houses (56%) with DENV infections were within 100 m of the home (Fig. 4 A and B).
Fig. 3.
DENV transmission in contact clusters. (A) Average prevalence of DENV-4–neutralizing antibodies within clusters at their initiation, across both transmission seasons (solid circle, MY neighborhood; triangle, TA neighborhood). (B) Cluster attack rates. (C) Cluster infestation rates. (D) Household attack rates (Methods, Data Analysis, Endpoints). In B through D, black represents DENV− clusters, and red represents DENV+ clusters. In all cases, the point indicates the predicted rate based on the best model selected by AICc (SI Appendix, Tables S7–S11). Error bars are ±1 SEM.
Using a transmission model (SI Appendix, Methods, Transmission Model), we found that overlap (i.e., residing in the activity space of a DENV-infected individual) significantly increased infection risk. The importance of overlap was estimated by decomposing individual infection risk into (i) a “within-cluster” risk derived from association with the activity space of a DENV-infected individual and (ii) a background infection risk associated with living in Iquitos (i.e., rates measured in DENV− control clusters). The within-cluster component was highly significant [maximum-likelihood estimation: 0.158; 95% CI: 0.105–0.220; Fig. 4 C and D and SI Appendix, Fig. S10 and Table S14] and independent of distance between the home of a contact and the home of an index case (SI Appendix, Fig. S11). Coupled with the high rate of concurrently infected people across houses in DENV+ clusters (SI Appendix, Fig. S8), this indicates that people’s movements do not overlap randomly. Visited houses were often the home of relatives and friends, but houses also commonly serve as stores or workshops in Iquitos. We interviewed a subset of participants and found that it was common for contacts to visit the same places as other contacts: in two-thirds of clusters, two or more interviewed contacts reported visiting the same place routinely.
Discussion
Human house-to-house movements played a key role in defining individual infection risk, local patterns of incidence, rapid urban-wide virus spread, and heterogeneity in rates of DENV transmission in Iquitos. Contact with other households likely to be infested with infected mosquitoes at the time of infection was the best indicator of risk, consistent with our model for DENV in Iquitos (31), which was based on prior data indicating that houses are the primary point of human exposure to mosquitoes. In other parts of the world, relevant movements may differ depending on how and where exposure takes place [e.g., in schools (39)]. DENV transmission was markedly higher among participants of DENV+ clusters, a pattern that was largely independent of distance from the home of the index case. The distribution of cases was a reflection of the distribution of distances to visited houses. Transmission appeared to be driven by collective movement patterns that were nonrandom and influenced by social connections. Thus, human-mobility patterns on both individual and collective levels had a powerful impact on how DENV moved through the human population.
Our results, in combination with conclusions from other studies describing highly focal patterns of DENV transmission around the home (<50 m) (18, 20) indicate that transmission occurs among multiple, fine-scale foci connected by the movements of infected and susceptible people. At these short distances from the home, the relative contribution of mosquitoes versus humans to pathogen dispersal is still uncertain, but clearly both are important.
The improved understanding of the level of human-mediated virus dispersal that we detected has implications for improving dengue surveillance and control. For instance, on average (accounting for participation rates), for every apparent DENV-4 infection, we would expect at least five contacts to also be acutely infected, ∼four of whom would be inapparent infections. These would be spread across four houses, the home of the index, another neighboring house, and two farther away (>100 m): well beyond the range of focal insecticide applications. Moreover, concurrent control clusters in the same neighborhoods indicate that transmission was highly heterogeneous at this scale. As noted for other targeted intervention strategies (40), use of contact tracing to better characterize DENV transmission and to inform focused vector control (21, 41) will depend on the methods used and the cost of identifying transmission hot spots. In most dengue endemic areas with existing tools and resources, we expect this to be logistically impractical. Our data highlight, however, that conventional control misses important foci of transmission, resulting in an ineffective allocation of resources to locations where the risk is low. A priori knowledge about household commuting behavior could be applied to define zones for targeted control. Although still logistically challenging, interventions targeted at the neighborhoods in our study could be effective, given that most movements were contained within their boundaries. In addition to interventions targeting vectors, our findings, by better informing the design, parameterization, and validation of transmission models, will assist in estimation of immunization thresholds and delivery strategies once an effective dengue vaccine becomes commercial available (13). Human movement in and out of treatment areas also needs to be accounted for during the evaluation of novel vector control strategies, such as Wolbachia-infected, refractory Ae. aegypti (12).
Although we expect that the details of human movement and its influence on virus transmission will vary as a function of factors like socioeconomics, culture, geography, and population density, there appear to be common underlying processes that are applicable across different contexts. We expect that the importance of human movement that we observed in Iquitos will hold in other populations and for other pathogens transmitted by Ae. aegypti (i.e., chikungunya and yellow fever viruses) and other day-biting mosquitoes (i.e., Aedes albopictus). This will be particularly true where the scale of human movements exceeds the scale of vector movements (31), as is the case in large urban centers where dengue is endemic. The possibility that individual movements between houses are socially determined reinforces and adds a geographic component to the notion of communities in social networks (42, 43) and provides a different way for thinking about how a vector-borne pathogen may spread through a population.
Our results were statistically robust, but our study had limitations. We assessed recent movements through a self-reporting mechanism, which is prone to recall error and potential bias. We are confident, however, that the households that individuals reported visiting were indeed visited because they were part of a routine. Activity spaces were, if anything, under-sampled. We also focused on individuals who reported visiting at least one other household because we could only assess exposure in houses with participants who agreed to provide blood samples. Exposure could occur wherever infectious mosquitoes are present (44–46). In Iquitos, people commonly visit other residences, but in other geographic areas, people may be more or less mobile, making the home or other locations comparatively more important for transmission. Our DENV-4 plaque reduction neutralization test (PRNT) had high specificity, but, as for other investigators, sensitivity was low (47). Consequently, we have confidence in our positive results, but false negative results may underestimate seroprevalence. In addition, contact-cluster studies are logistically complicated, resource-demanding, and ultimately dependent on the natural dynamics of transmission, which limited our sample size. We were able to detect variation in infection rates, however, because the population was fully susceptible to DENV-4. Finally, we did not determine whether all infected people in clusters were infected with the same virus. Preliminary analyses based on Envelope gene sequence data did not reveal significant variation among DENV-4 isolates. We are pursuing more extensive sequencing to more fully examine relationships among virus isolates. Despite these constraints, our data provide previously unavailable, fine-scale details on virus transmission by explicitly accounting for human movements and infection.
Results from this study indicate that human movement is a key component in determining DENV-infection risk, shaping incidence patterns, and driving virus spread. At the individual level, the risk for DENV infection is defined by virus contact across recently visited places that are conducive to mosquito exposure. Fine-scale human movements, therefore, underlie patterns of infection and result in pronounced temporal and spatial heterogeneity in dengue incidence. At a collective or population level, people who visit the same places, where infection and transmission take place, shape patterns of DENV transmission. In Iquitos, this translates into movement between a person’s residence and the households of family and friends. Given the integral role of human movement in dengue epidemiology, disease outbreaks cannot be fully understood and the development, evaluation, and application of novel interventions will not be optimized unless human movement is incorporated into the analyses. In addition, we emphasize that the role of humans in the spread of other vector-borne pathogens is likely to be more important than historically thought because individual human movements will lead to variation in exposure. Continued, simultaneous quantification of human movement and infection across vector-borne disease systems will lead to an increasingly refined understanding of their dynamics and help guide prevention and control efforts.
Methods
Approval of Experiments Involving Human Subjects.
The study protocol was approved by the University of California at Davis (Protocol 2007-15244) and Naval Medical Research Unit Six (Protocol NMRCD.2007.0007) Institutional Review Boards (IRBs), with the latter including Peruvian representation, in compliance with all US Federal and Peruvian regulations governing the protection of human subjects. IRB authorization agreements were established between the University of California, Davis, and the University of Illinois at Urbana–Champaign, Emory University, and San Diego State University; and between the Naval Medical Research Unit Six and Tulane University. The protocol was reviewed and approved by the Loreto Regional Health Department (LRHD), which oversees health research in Iquitos. In all instances, written consent was provided by study participants.
Study Area.
Our study was conducted in Iquitos in 2008, an urban area of ∼370,000 residents accessible only by river or air travel located in the Amazon Basin of northeastern Peru (73.2°W, 3.7°S; 120 m above sea level) in the Department of Loreto [Instituto Nacional de Estadística e Informática]. Iquitos has been the site of dengue research since the early 1990s with continuous community-based, cohort research since 1999 (48, 49).
Long-Term Prospective, Longitudinal Cohort.
Based on differences in historical records of DENV transmission and relative geographic independence (local availability of services such as schools and health clinics), two previously described neighborhoods, MY and TA (49), were selected for febrile surveillance and longitudinal monitoring of anti–dengue virus-neutralizing antibodies. Enrollment in the study was offered to people living in all households on contiguous blocks with an estimated population of ∼3,000 in each neighborhood between November 2007 and May 2008 until we recruited 2,444 longitudinal participants ≥5-y old, divided evenly between the two neighborhoods. Longitudinal participants provided blood samples at ∼6-mo intervals to test for serological evidence of DENV infection (SI Appendix, Fig. S1).
Febrile Surveillance.
In April 2008, active community-based surveillance was initiated in all consenting households based on a strategy described previously (48), where study personnel visited each household a minimum of three times per week to ask whether anyone living there had a febrile illness. When a febrile person (≥3 y old) was identified, written informed consent was obtained from adult participants or the parents of participants <18 y of age. Assent was obtained from participating children (3–18). Blood was drawn at the time of case identification (acute sample), followed by a convalescent blood sample 14–21 d later. We offered two options for study participation: (i) acute and convalescent blood samples with clinical evaluation by a project physician (option 1); and (ii) option 1 procedures with an interview inquiring about the participant’s movement patterns over the previous 15 d (option 2) (SI Appendix, Fig. S1). In the latter situation, we asked for permission to visit houses identified in the interview. These individuals, thus, served as the “index cases” of contact-site cluster investigations.
Retrospective Movement Interviews.
We developed interviews to describe the activity space of febrile individuals, defined as all locations visited between 0500 and 2200 hours in the prior 15 d when infection most likely occurred. These were designed to be culturally sensitive and were administered by trained, Peruvian research technicians. The interview instrument was validated with global-positioning system tracking in a separate study. Interviews lasted ∼20 min. Households identified in interviews were located and geocoded if they were located within the Iquitos urban area. Sites outside of Iquitos (4% of all places identified in interviews) were excluded for logistical reasons and because risk for DENV is low in rural areas outside of the city.
Contact-Site Cluster Investigations.
Our design called for balancing clusters between those initiated by individuals diagnosed with dengue (DENV+) and those negative for dengue (DENV−) and between the two neighborhoods. Logistically, we were limited to two to three cluster investigations per week. Thus, depending on our needs and the availability of options, we selected which cluster to initiate each week (SI Appendix, Methods). All residents >5 y of age of houses identified in interviews (contacts) were then invited to participate by providing two blood samples 14–21 d apart for diagnosis of acute DENV infection. Participating households were visited every other day for the 2- to 3-wk study period to inquire about fever and other dengue symptoms. Individuals developing a fever during a cluster investigation were invited to participate in the study as febrile cases (option 1, above).
Sample Processing.
Longitudinal blood samples were analyzed by four-dilution PRNTs with a 70% cutoff for all four DENV serotypes (PRNT70; SI Appendix, Methods). We estimated 99% specificity and 85% sensitivity for detecting DENV-4 seroconversions by PRNT assay. Acute samples from febrile cases and cluster participants were tested by RT-PCR and inoculated onto C6/36 cells for virus isolation as described previously (50). Acute and convalescent samples from cluster participants were assayed for virus by RT-PCE and virus isolation. All samples were also assayed for DENV-specific IgM by antibody-capture ELISA (50). Individuals opting for option 2 had their acute blood tested by RT-PCR (50) for DENV within 48 h of the initial blood draw to initiate cluster investigations.
Data Analysis.
Based on serological results, contacts were classified as an acute DENV-4 infection, a recent (within last 5 mo) DENV infection, previously exposed to DENV-4, or negative (SI Appendix, Methods). These data were used to tabulate attack rates at the household and cluster (all houses within activity space of a febrile case) levels.
Endpoints.
The endpoints were as follows: σi, index of potential virus exposure; “cluster attack rate,” proportion of previously susceptible contacts in a cluster with an acute DENV infection; “cluster infestation rate,” proportion of households with ≥one acute DENV infection; and “household attack rate,” proportion of previously susceptible household residents with an acute DENV infection.
Statistics.
Data were fit to multiple candidate models using generalized binomial linear and additive models including fixed and random effects, as deemed appropriate. Residuals were evaluated in reference to all independent variables. Models were compared and ranked using Akaike’s information criterion with correction for small sample size (AICc) and log-likelihood ratio tests. All analyses were conducted in R Version 2.13. See SI Appendix, Methods for details.
Transmission Model.
We developed a seasonally forced transmission model to estimate the additional risk for infection attributable to association with the activity space of a DENV infected person. Briefly, we partitioned infection risk into two components: (i) a “within-cluster” risk derived from residing in the activity space of a DENV-infected person; and (ii) a background risk associated with living in Iquitos (estimated from negative clusters). This model was then fit to the data from contact-cluster investigations using likelihood and Bayesian approaches (see SI Appendix, Transmission Model for details).
Supplementary Material
Acknowledgments
We thank the residents of Iquitos for welcoming us into their homes and permitting these studies to be conducted. We appreciate the support of the Loreto Regional Health Department, our Peruvian research team in Lima and Iquitos, and many others who helped to make this study happen (see SI Appendix for a full list). This work is supported by National Institutes of Health Grant R01 AI069341-01 (to T.W.S.), US Department of Defense Global Emerging Infections Systems Research Program Work Unit No. 847705.82000.25GB.B0016 (http://www.afrims.org/geis.html), and Military Infectious Disease Research Program Work Unit No. 6000 RAD1.S.B0302.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213349110/-/DCSupplemental.
References
- 1.Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 2.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Fauci AS. Dengue and hemorrhagic fever: A potential threat to public health in the United States. JAMA. 2008;299(2):214–216. doi: 10.1001/jama.2007.31-a. [DOI] [PubMed] [Google Scholar]
- 4.Brady OJ, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuno G. Emergence of the severe syndrome and mortality associated with dengue and dengue-like illness: Historical records (1890 to 1950) and their compatibility with current hypotheses on the shift of disease manifestation. Clin Microbiol Rev. 2009;22(2):186–201. doi: 10.1128/CMR.00052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17(2):321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 7.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: Implications for vaccine development. Cell Host Microbe. 2008;4(3):229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman AL. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 9.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29(42):7221–7228. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Sabchareon A, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet. 2012;380(9853):1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 11.Scott TW, Morrison AC. Vector dynamics and transmission of dengue virus: Implications for dengue surveillance and prevention strategies. Curr Top Microbiol Immunol. 2010;338:115–128. doi: 10.1007/978-3-642-02215-9_9. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann AA, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- 13.Johansson MA, Hombach J, Cummings DA. Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine. 2011;29(35):5860–5868. doi: 10.1016/j.vaccine.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beatty M, et al. WHO-VMI Dengue Vaccine Modeling Group Assessing the potential of a candidate dengue vaccine with mathematical modeling. PLoS Negl Trop Dis. 2012;6(3):e1450. doi: 10.1371/journal.pntd.0001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endy TP, Yoon I-K, Mammen MP. In: Dengue Virus, Current Topics in Microbiology and Immunology. Rothman AL, editor. Berlin, Heidelberg: Springer-Verlag; 2010. pp. 1–13. [DOI] [PubMed] [Google Scholar]
- 16.Scott TW, Morrison AC. Vector-Borne Diseases: Understanding the Environmental, Human Health, and Ecological Connections. Washington: National Academies Press; 2008. pp. 132–149. [PubMed] [Google Scholar]
- 17.Endy TP, et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis. 2011;5(3):e975. doi: 10.1371/journal.pntd.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon IK, et al. Underrecognized mildly symptomatic viremic dengue virus infections in rural Thai schools and villages. J Infect Dis. 2012;206(3):389–398. doi: 10.1093/infdis/jis357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison AC, Getis A, Santiago M, Rigau-Perez JG, Reiter P. Exploratory space-time analysis of reported dengue cases during an outbreak in Florida, Puerto Rico, 1991-1992. Am J Trop Med Hyg. 1998;58(3):287–298. doi: 10.4269/ajtmh.1998.58.287. [DOI] [PubMed] [Google Scholar]
- 20.Mammen MP, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5(11):e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis. 2010;4(12):e920. doi: 10.1371/journal.pntd.0000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salje H, et al. Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci USA. 2012;109(24):9535–9538. doi: 10.1073/pnas.1120621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liebman KA, et al. Spatial dimensions of dengue virus transmission across interepidemic and epidemic periods in Iquitos, Peru (1999-2003) PLoS Negl Trop Dis. 2012;6(2):e1472. doi: 10.1371/journal.pntd.0001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington LC, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72(2):209–220. [PubMed] [Google Scholar]
- 25.Getis A, Morrison AC, Gray K, Scott TW. Characteristics of the spatial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am J Trop Med Hyg. 2003;69(5):494–505. [PubMed] [Google Scholar]
- 26.Maciel-de-Freitas R, Souza-Santos R, Codeço CT, Lourenço-de-Oliveira R. Influence of the spatial distribution of human hosts and large size containers on the dispersal of the mosquito Aedes aegypti within the first gonotrophic cycle. Med Vet Entomol. 2010;24(1):74–82. doi: 10.1111/j.1365-2915.2009.00851.x. [DOI] [PubMed] [Google Scholar]
- 27.Reiter P. Oviposition, dispersal, and survival in Aedes aegypti: Implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 2007;7(2):261–273. doi: 10.1089/vbz.2006.0630. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Special Programme for Research and Training in Tropical Diseases . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. [Google Scholar]
- 29.Bharti N, et al. Explaining seasonal fluctuations of measles in Niger using nighttime lights imagery. Science. 2011;334(6061):1424–1427. doi: 10.1126/science.1210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley S. Large-scale spatial-transmission models of infectious disease. Science. 2007;316(5829):1298–1301. doi: 10.1126/science.1134695. [DOI] [PubMed] [Google Scholar]
- 31.Stoddard ST, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3(7):e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong J, Astete H, Morrison AC, Scott TW. Sampling considerations for designing Aedes aegypti (Diptera:Culicidae) oviposition studies in Iquitos, Peru: Substrate preference, diurnal periodicity, and gonotrophic cycle length. J Med Entomol. 2011;48(1):45–52. doi: 10.1603/me10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuno M, Tonn RJ. A study of biting habits of Aedes aegypti in Bangkok, Thailand. Bull World Health Organ. 1970;43(2):319–325. [PMC free article] [PubMed] [Google Scholar]
- 34.Chadee DD. Landing periodicity of the mosquito Aedes aegypti in Trinidad in relation to the timing of insecticidal space-spraying. Med Vet Entomol. 1988;2(2):189–192. doi: 10.1111/j.1365-2915.1988.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 35.Adams B, Kapan DD. Man bites mosquito: Understanding the contribution of human movement to vector-borne disease dynamics. PLoS ONE. 2009;4(8):e6763. doi: 10.1371/journal.pone.0006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barmak DH, Dorso CO, Otero M, Solari HG. 2011. Dengue epidemics and human mobility. arXiv:1102.3869.
- 37.Cosner C, et al. The effects of human movement on the persistence of vector-borne diseases. J Theor Biol. 2009;258(4):550–560. doi: 10.1016/j.jtbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison AC, et al. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100(Suppl 1):S73–S86. doi: 10.1179/136485906X105534. [DOI] [PubMed] [Google Scholar]
- 39.Méndez F, et al. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am J Trop Med Hyg. 2006;74(4):678–683. [PubMed] [Google Scholar]
- 40.Woolhouse ME, et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94(1):338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bousema T, et al. Hitting hotspots: Spatial targeting of malaria for control and elimination. PLoS Med. 2012;9(1):e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salathé M, et al. A high-resolution human contact network for infectious disease transmission. Proc Natl Acad Sci USA. 2010;107(51):22020–22025. doi: 10.1073/pnas.1009094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palla G, Derényi I, Farkas I, Vicsek T. Uncovering the overlapping community structure of complex networks in nature and society. Nature. 2005;435(7043):814–818. doi: 10.1038/nature03607. [DOI] [PubMed] [Google Scholar]
- 44.Adams B, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci USA. 2006;103(38):14234–14239. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honório NA, et al. Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2009;3(11):e545. doi: 10.1371/journal.pntd.0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.García-Rejón JE, et al. Mosquito infestation and dengue virus infection in Aedes aegypti females in schools in Merida, Mexico. Am J Trop Med Hyg. 2011;84(3):489–496. doi: 10.4269/ajtmh.2011.10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas SJ, et al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am J Trop Med Hyg. 2009;81(5):825–833. doi: 10.4269/ajtmh.2009.08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocha C, et al. Comparison of two active surveillance programs for the detection of clinical dengue cases in Iquitos, Peru. Am J Trop Med Hyg. 2009;80(4):656–660. [PubMed] [Google Scholar]
- 49.Morrison AC, et al. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: Interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4(5):e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forshey BM, et al. NMRCD Febrile Surveillance Working Group Arboviral etiologies of acute febrile illnesses in Western South America, 2000-2007. PLoS Negl Trop Dis. 2010;4(8):e787. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.