Abstract
One strategy to restore vision in retinitis pigmentosa and age-related macular degeneration is cell replacement. Typically, patients lose vision when the outer retinal photoreceptor layer is lost, and so the therapeutic goal would be to restore vision at this stage of disease. It is not currently known if a degenerate retina lacking the outer nuclear layer of photoreceptor cells would allow the survival, maturation, and reconnection of replacement photoreceptors, as prior studies used hosts with a preexisting outer nuclear layer at the time of treatment. Here, using a murine model of severe human retinitis pigmentosa at a stage when no host rod cells remain, we show that transplanted rod precursors can reform an anatomically distinct and appropriately polarized outer nuclear layer. A trilaminar organization was returned to rd1 hosts that had only two retinal layers before treatment. The newly introduced precursors were able to resume their developmental program in the degenerate host niche to become mature rods with light-sensitive outer segments, reconnecting with host neurons downstream. Visual function, assayed in the same animals before and after transplantation, was restored in animals with zero rod function at baseline. These observations suggest that a cell therapy approach may reconstitute a light-sensitive cell layer de novo and hence repair a structurally damaged visual circuit. Rather than placing discrete photoreceptors among preexisting host outer retinal cells, total photoreceptor layer reconstruction may provide a clinically relevant model to investigate cell-based strategies for retinal repair.
Keywords: stem cell, blindness, retinal surgery, visual cortex, neural regeneration
Degeneration of the outer retina resulting in sight loss may occur in retinitis pigmentosa (RP) and other retinal degenerations in which there is a progressive loss of photoreceptor cells from the outer nuclear layer (ONL). A potential treatment for patients who are blind from outer retinal degeneration would be photoreceptor transplantation using a cell therapy approach. The observation that previously blind patients could read after stimulation by subretinal electrodes (1) indicates that the downstream circuitry of the human inner retina retains a capacity to be reactivated to restore vision long after all photoreceptors are lost.
Rod precursor cells up to the point of neural retina leucine zipper (Nrl) transcription factor expression have been generated by another group through 3D murine embryonic stem (ES) cell culture (2). Separately, it was shown that cells at this ontogenetic stage could survive and function in a retina in early degeneration when the ONL is relatively well-formed (3). For these findings to be translated into clinical applications, one requirement is that cell therapy must be shown to restore vision at the stage of disease characterized by the loss of photoreceptors. For the return of visual function in end-stage degeneration, newly introduced precursors at the Nrl-expressing stage would have to resume their developmental program to form a polarized ONL after transplantation, with the formation of light-sensitive outer segments (OS) containing enzymes critical for light sensitivity. Furthermore, the cells would need to reconnect synaptically with residual downstream retinal neurons for light-evoked signals to be relayed to central targets.
Photoreceptor replacement in the absence of the host ONL remains relatively uninvestigated, and it is not known if precursor cell transplantation could be a successful approach for the end-stage degenerate host with a zero anatomical and functional baseline. Rather than adding more photoreceptor cells into a preexisting host ONL, we aimed instead to investigate whether a functional ONL could be reconstructed de novo by precursor cell transplantation.
Results
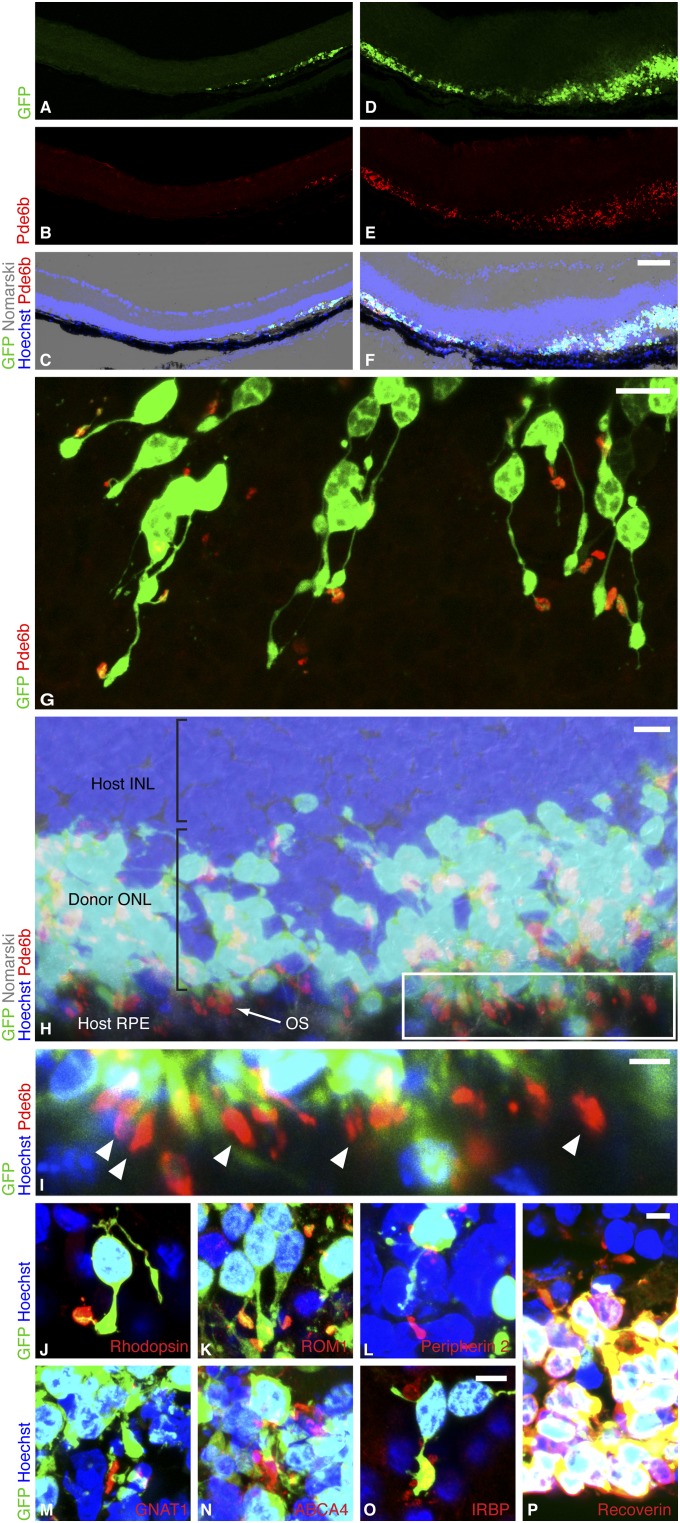
To model the clinical scenario of complete rod photoreceptor degeneration, we used C3H/HeNHsd (rd1) host mice aged 10–12 wk, which were homozygous for the retinal degeneration allele of the rod-specific cGMP phosphodiesterase β6 subunit (Pde6brd1) (4). In this fast-degenerating model of severe human RP, the death of all rods occurs by 3 wk of age, and beyond this time almost all of the ONL is lost. We used Tg(Nrl-L-EGFP) mice (5) as donors, in which green fluorescent protein (GFP) expression is restricted to postmitotic rod precursors and mature rods as a consequence of the Nrl promoter, which would therefore allow us to track donor rods after transplantation into the host. Precursors were harvested from postnatal day (P) 3–4 mice, coincident with the period of rod genesis and Nrl expression, as cells at this stage have been shown to survive transplantation (3, 6). We optimized the transplantation technique for the degenerate retina lacking the ONL and found that standardized cell transplantation could be consistently achieved in the fragile and almost transparent host retina (Fig. S1). Two weeks after transplantation, we found numerous GFP-positive donor cells appropriately interposed between the host inner nuclear layer (INL) and retinal pigment epithelium (RPE), recreating a new ONL up to 10 cells thick in places (Fig. 1 A–F). On average, 7.9 ± 6.5% (n = 8) of donor cells survived. In contrast to previous reports in which the host ONL was relatively intact (3, 7–11), we observed no significant migration of cells into the residual nuclear layers of the host. Furthermore, none of the transplanted rods that we observed adopted the normal photoreceptor profiles that have been reported in other studies where exogenous fluorescent precursors coexisted with numerous host ONL photoreceptors at the time of transplantation (3, 7–11). In a longitudinal in vivo analysis of 12 eyes, 33.3% had surviving donor cells at week 12, the last time point assessed (Fig. S1 E–L).
Fig. 1.
Rod precursors transplanted into the degenerate host regenerate a new photoreceptor layer. (A–F) Two regions of rd1 host retina after subretinal transplantation of P3 Tg(Nrl-L-EGFP) rod precursors. By 2 wk, the retina had reattached and a new donor-derived ONL, marked by GFP, was formed. A–C show an area where one to three ONL rows were formed, and D–F show up to 10 rows. An internal nontransplant control region lacking an ONL is shown in the left half of A–C. (Scale bar, 75 µm.) (G) As a sign of maturation, donor-derived GFP-positive rods formed slender processes extending from the cell body and expressed phosphodiesterase β6 (Pde6b) sequestered in discrete OS. (Scale bar, 10 µm.) (H) In many regions, Pde6b-positive OS were appropriately polarized with respect to host RPE, which is the optimal configuration for rod OS maintenance. (Scale bar, 10 µm.) Boxed region of H enlarged in I shows OS (arrowheads) aligned in rows against host RPE. (Scale bar, 5 µm.) (J–N) As further evidence of maturation, rhodopsin, rod outer segment membrane protein 1 (ROM1), peripherin 2, guanine nucleotide-binding protein G(t) subunit α1 (GNAT1), and ATP-binding cassette subfamily A member 4 (ABCA4) were localized in outer segments. (O) Donor cells expressed interphotoreceptor retinoid-binding protein (IRBP), a secreted protein critical for ONL maintenance, and (P) were positive for the photoreceptor marker recoverin. (Scale bar, 5 µm for J–P.)
We asked if precursors relocated to the subretinal space of the severely degenerate retina could follow normal development and elaborate OS, thereby completing the final steps of differentiation into mature light-sensing rods. The OS is the photosensitive part of the photoreceptor and normally houses enzymes required for phototransduction and the visual cycle. In transplanted donor cells, we found widespread immunoreactivity against Pde6b, a phototransduction enzyme normally localized in the OS, which was absent in the rd1 hosts and was therefore reliably donor-cell–derived. Pde6b was consistently sequestered in a discrete OS (Fig. 1G and Table S1) that was, in many regions, elongated and appropriately orientated toward RPE (Fig. 1 H–I). This configuration is known to support the physiological function of photoreceptors as they are dependent on RPE cells. The presence of appropriately sequestered Pde6b indicated directional protein transport in the connecting cilium (12) and constituted evidence of donor rod maturation. OS were formed in 76.8 ± 18.9% (mean ± SD) of surviving donor rods (i.e., from about 6% of transplanted cells). OS were significantly more often positioned in the outer part of the donor-derived ONL against the host RPE than in the inner ONL against the host INL (63.0 ± 6.1% vs. 25.5 ± 3.5%, mean proportion ± SEM, t10 = 4.30, P = 0.002, n = 11 discrete ONL regions in four experiments). This preferential orientation suggested an interaction between these cell layers. As further evidence of maturation, donor cells expressed mature rod- and photoreceptor-specific markers (Fig. 1 J–P) 2 wk after transplantation.
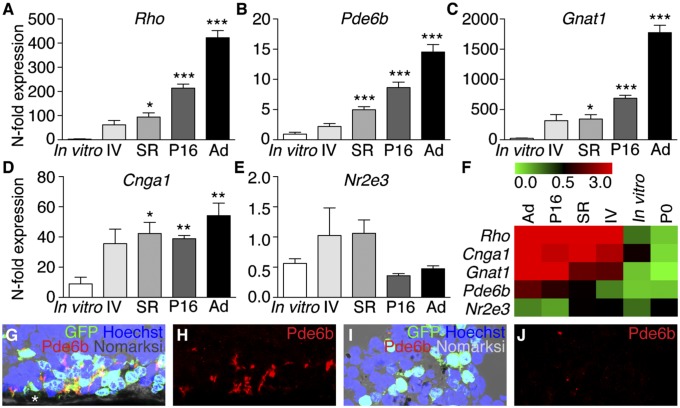
To investigate whether the degenerate subretinal niche was a permissive environment for the completion of rod precursor development, we quantified rod-specific gene expression by quantitative RT-PCR (qRT-PCR) mRNA analysis from eyes containing P2–3 precursors transplanted in vivo compared with cells maintained in culture for 2 wk. Significantly, we observed that precursors transplanted into degenerate hosts resumed developmental expression of rod-specific genes required for OS function (Pde6b, Rho, Gnat1, Cnga1) (Fig. 2 A–F and Table S2). This was not observed in precursors maintained in vitro, suggesting that the degenerate subretinal niche was able to influence OS-related gene expression beyond that which is preprogrammed at the time of harvest. This correlated with the identification of OS histologically in transplanted precursors (Fig. 2 G–J). Also, the expression of Rho, Cnga1, and Gnat1 was restored in rd1 eyes after retinal reconstruction (Fig. S2). Together, these data show that donor-derived rod cells had the capability to differentiate and form OS after being relocated from their native environment to the end-stage degenerate subretinal space.
Fig. 2.
Developmental cues support the maturation of rod precursors in the degenerate subretinal niche. (A–D) Rod-specific gene expression, measured by qRT-PCR. P2–3 Tg(Nrl-L-EGFP) retinal cells were exposed to different conditions [transplanted intravitreally (IV), transplanted subretinally (SR), maintained in vitro, or allowed to complete development in situ until P16]. Genes expressed highly in P16 and adult (Ad) Tg(Nrl-L-EGFP) eyes and vital for rod outer segment homeostasis were also highly expressed by precursors transplanted subretinally into degenerate rd1 hosts. Rho (F4,12.37 = 86.12, P = 6.5 × 10−9), Pde6b (F4,14.41 = 41.60, P = 9.3 × 10−8), Gnat1 (F4,12.41 = 87.89, P = 5.5 × 10−9), Cnga1 (F4,12.23 = 9.58, P = 0.001). *P < 0.05, **P < 0.01, ***P < 0.001 post hoc versus in vitro group, n = 5–8 per niche condition. Expression of these genes in P2–3 cells transplanted intravitreally showed a trend of being up-regulated compared with in vitro controls, but this did not reach statistical significance. Error bars, SEM. (E) Nr2e3 is a developmentally regulated rod transcription factor gene that peaks during early rod genesis around the time of transplantation and was used as an internal control. This was not up-regulated relative to in vitro controls (F4,29 = 2.47, P = 0.07), which confirmed that the increased gene expression was specific for late rod development. Data are presented as fold change in mRNA expression compared with expression in P0 retina (n = 7). (F) Heat map colors represent the expression of each gene individually in the different niches and are not comparable between genes. (G and H) Pde6b-positive OS in subretinally transplanted precursors. Asterisk, retinal pigment epithelium. (I and J) Poor OS formation after intravitreal transplantation.
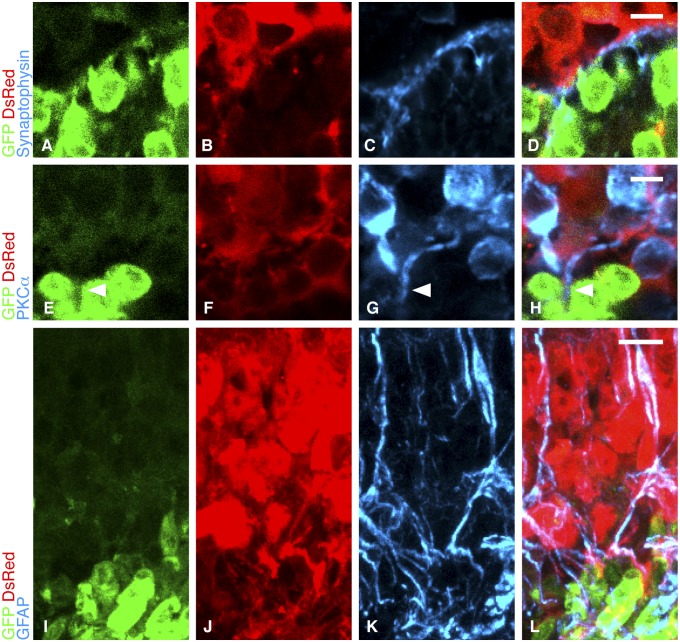
To transmit visual signals, transplanted cells would need to connect with the residual neuronal circuitry in the host. To explore donor–host connections, we used fluorescent red Tg(CAG-DsRed*MST)1Nagy/J murine hosts (13) aged 10–12 wk that were on an rd1 background. Thus, these fast-retinal-degeneration animals also ubiquitously expressed DsRed protein (Fig. S3). This enabled us to determine sites of apposition between donor (green) and host (red) cells. The normal rod synapse with the inner retina is identified by the localization of synaptophysin, which is critical for rod synaptic vesicle recycling. The terminals of donor-derived rods showed synaptophysin localization along the graft–host interface (Fig. 3 A–D). Additionally, donor-derived rods expressed the presynaptic protein bassoon (Fig. S4), a major constituent of the photoreceptor ribbon synapse essential for neurotransmitter release (14). Bipolar cells, the inner retinal second-order targets of rods, normally express the α-isoenzyme of protein kinase C (PKCα), and this was found in host INL cells at sites of contact with donor cells. Significantly, we identified host bipolar cell processes traversing the donor–host boundary to contact rods within the new ONL (Fig. 3 E–H), confirming a potential mechanism of donor–host interaction that was previously observed with retinal sheet grafts (15). These data indicate that when the ONL is absent, graft–host integration occurs not by individual cell migration among host nuclei but instead at the boundary between host and donor layers.
Fig. 3.
Integration of transplanted cells with host inner retina. (A–D) Synaptophysin (blue) expression was appropriately located between host INL marked by DsRed (red) expression and the donor-derived ONL marked by GFP (green). (E–H) Host bipolar cells, marked with PKCα (blue), extended processes (arrowheads) into the graft. (Scale bars, 5 µm for A–H.) (I–L) Host Müller cells are identified by the coexpression of DsRed (red) and GFAP (blue). Müller cell processes extend from the host INL into the graft (green). (Scale bar, 10 µm.)
Müller cells play a role in photoreceptor function (16), and, additionally in retinal degeneration, an outer retinal glial barrier may be formed that may impede the integration of transplanted cells (17). Using anti-glial fibrillary acid protein (GFAP) staining, we identified Müller cell processes extending from the host INL into the donor-derived ONL, restoring the normal integrated relationship between these two layers without a significant glial barrier forming between them (Fig. 3 I–L).
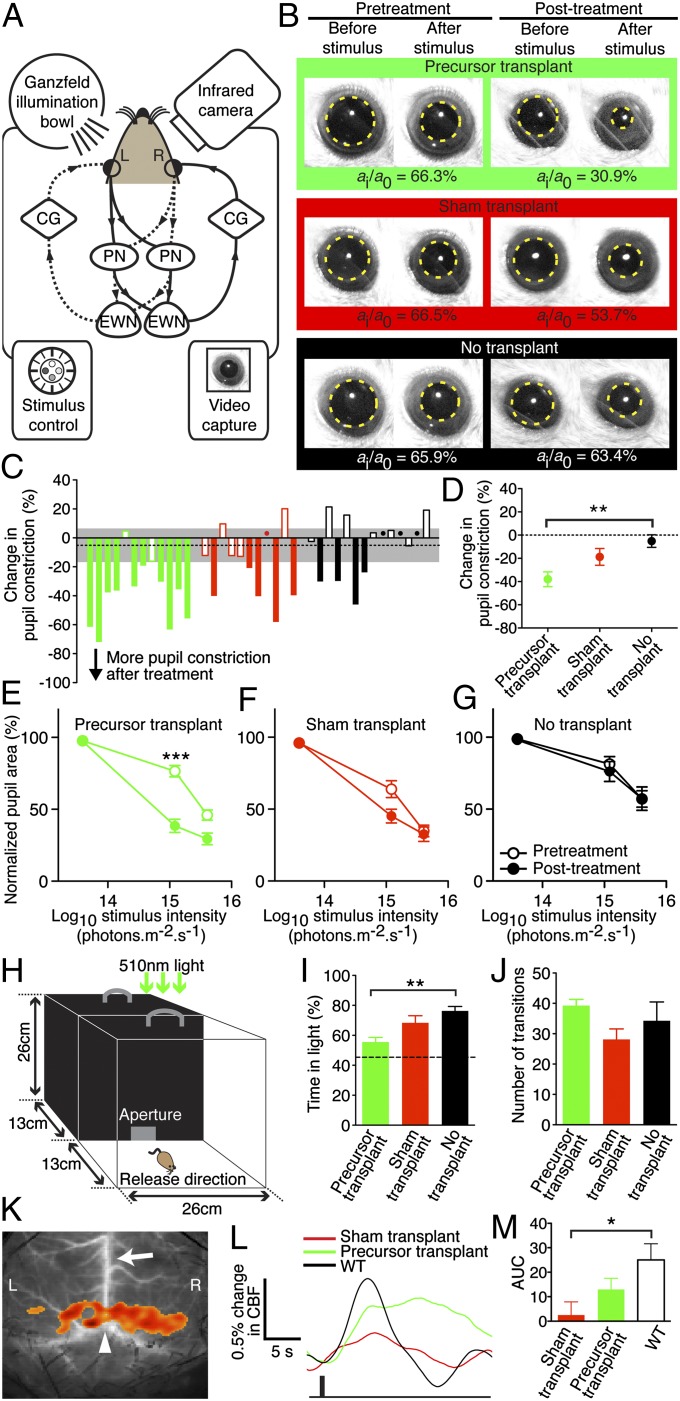
Calcium imaging of transplanted cells confirmed rod-specific mGluR8 biochemical responses (18) as previously described (3) (Fig. S5) and provided evidence of calcium homeostasis that is essential for photoreceptor maintenance. We used the pupil light response (PLR) (19), which has been validated in similar studies (3, 20–22), as a functional test that would prove not only the existence of light-sensing OS but also that efferent connections existed to central targets. We aimed to test for an improvement above the zero baseline in these mice that were structurally and functionally devoid of rods at the time of intervention and therefore measured the PLR before and after treatment to compare outcomes pairwise in the same animal. To avoid influences such as transplantation-related inflammation, we stimulated the transplanted eye and measured the consensual PLR (19) (Fig. 4A) in the unoperated eye after precursor cell transplantation (n = 12). To identify specifically the effects of transplanted rods, the sham group (n = 11) received retinal cells harvested from rod-free adult (>6 wk old) Tg(CAG-DsRed*MST)1Nagy/J rd1 donor mice that would control for the potential effects of other neuronal (cones, bipolar cells) and nonneuronal cells (Müller cells) present in the treatment precursor cell suspension. A nontransplant group (n = 14) was included as natural history controls. In vivo fluorescence imaging with confocal scanning laser ophthalmoscopy performed before PLR analysis confirmed transplanted cell survival (Fig. S1 C and D). The PLR in the treatment group improved significantly at the median intensity of 1.21 × 1015 photons⋅cm−2⋅s−1, constricting down from 76.5 ± 3.9% before transplantation to 38.5 ± 4.6% after (mean normalized pupil area ± SEM; t11 = 5.95, P = 0.000097; n = 12) whereas no statistically significant improvement was found in other groups (Fig. 4 B–G). We investigated light-mediated behavior by adapting a published protocol (23), using dim 510-nm light (150 nW⋅cm−2⋅s−1, ∼10 lx) to minimize melanopsin stimulation (Fig. 4H). Precursor transplantation into rd1 mice restored light-mediated activity toward the mean wild-type (WT) level (Fig. 4 I–J and Movies S1 and S2). Furthermore, laser speckle imaging (24) showed blood flow changes in the visual cortices of precursor-transplanted rd1 mice that were not seen in sham-treated controls (Fig. 4 K–M). In all, these data are consistent with a significant recovery of visual function by photoreceptor layer reconstruction after host rod loss.
Fig. 4.
Rod precursor transplantation restores visual function to eyes with complete rod degeneration. (A) Retinal illumination results in signal transmission to both pretectal nuclei (PN) and Edinger–Westphal nuclei (EWN). Impulses are relayed via the ciliary ganglia (CG) and both pupils constrict. Solid and dashed lines show reflex arcs triggered by left (L) and right (R) eye stimulation, respectively. (B) Representative pupil images. (C) Overview of the change in pupil constriction from before to after treatment in all mice at 1.21 × 1015 photons⋅cm−2⋅s−1. Green, red, and black denote precursor, sham, and no transplant controls, respectively. Dots indicate values close to zero and dashed line indicates the mean change with 95% confidence interval (gray) of the non-transplanted controls. The PLR was measured in the same eye 3 d before and 2 wk after subretinal transplantation of 3 × 105 cells. PLR was expressed as normalized pupil area as previously described (3). (D) Precursor transplantation improved pupil constriction, compared with no transplant controls (F2,34 = 7.264, P = 0.002, n = 11–14 each; **P = 0.001 post hoc). (E) Paired analysis before versus after treatment showed that at the median light stimulus intensity, the PLR improved significantly in precursor-treated animals (***t11 = 5.95, P < 0.0001, n = 12). (F and G) The PLR did not improve after sham transplantation (n = 11) or in untreated controls (n = 14) at any stimulus intensity. There were no significant differences between groups at baseline (P = 0.12). (H) Light-mediated behavior was measured under dim 510-nm illumination (150 nW⋅cm−2⋅s−1). (I) Precursor-transplanted mice spent less time in the lit compartment than rd1 controls (F2,20 = 7.608, P = 0.003, n = 7–8 each; **P = 0.003 post hoc). Dashed line indicates mean WT level. (J) Anxiety-related behavior (transitions between compartments) was similar across groups. (K) Cortical activation map in WT showing increased cortical blood flow (CBF) over L and R visual cortices; arrow, superior sagittal sinus; arrowhead, lambda. (L) Averaged L and R CBF time series from all mice after a 4-Hz (1-s) stimulus (black bar). (M) Precursor transplantation led to a higher CBF change than sham treatment compared with WT (F2,16 = 4.108, P = 0.04, n = 5–7 each; *P = 0.01). AUC, area under curve (0–7 s). Error bars, SEM.
Previous reports examining rod precursor integration into a preexisting ONL showed peak efficacy with early postnatal cells (3, 25). Conversely, a recent study suggested that adult donor rods could be transplanted (6), and adult rods have been shown to exert a trophic effect on host cone cells (26). Hence we examined functional effects in degenerate retinae using older donors. Adult (P30–P60) and late-developing (P14) donor cells survived after subretinal transplantation, but did not form a functional ONL (Fig. S6). We compared host cone morphology and opsin levels between P3 precursor-treated and sham-treated rd1 mice, but no differences were observed at 2 wk (t10 = 0.50, P = 0.6, n = 6 in each group) (Fig. S7). Collectively, these observations are in keeping with a visual improvement arising directly from transplanted rod precursors, rather than indirectly through host cone rescue.
Discussion
The data show that a functional photoreceptor layer of rods may be recreated de novo by the transplantation of rod precursor cells. In advanced disease typical of the human situation featuring a degenerated host ONL, transplanted cells were able to re-form an outer retinal layer in the correct anatomical location. The cells resumed their developmental program to polarize appropriately and form mature light-sensing outer segments, and synaptic integration at the donor–host interface allowed the transmission of light-evoked responses to the brain.
The development of a discrete OS with sequestered phototransduction enzymes represents a final stage of rod differentiation that has yet to be observed consistently in ES or induced pluripotent stem cell derivatives transplanted into other models or in ES cell-derived optic cups in culture (2, 7, 27–29) (Fig. S8A). We have shown that cells at the Nrl-expressing stage have the capacity to resume normal OS development when relocated to the terminally degenerate niche, and so the data reflect the replacement cell therapy paradigm in introducing new light-sensing components into the retina. The formation of elongated and enzyme-localizing outer segments, in many cases apposed to RPE (Fig. S8 B–D), supports the hypothesis that the improvement in visual function is due to the physiological function of repopulated photoreceptors.
The potential for photoreceptor replacement to be translated into humans with complete outer retinal degeneration has not been previously addressed. We used a severe and rapidly degenerating murine model, the rd1 mouse, in order to effectively model the anatomy in end-stage human retinal degenerations. Models used in previous studies included wild-type (3, 8, 9, 25, 27, 30–33), rd9 (10), Rho−/− (3, 28, 33), Crx−/− (7), Crb1rd8/rd8 (8, 31, 33), Prph2rd2/rd2 (31), Gucy2e−/− (8), and Gnat1−/− (11) mice. These hosts featured the relative anatomical integrity of the native ONL at the time of precursor transplantation, compared with the end-stage ONL loss in the rd1 mouse. In this regard, the rd1 host used in this study was an appropriate model to study the typical situation in advanced human RP, where the need for cell replacement arises when visual symptoms develop due to near-complete outer retinal cell loss. Outer neural retinal replacement would also likely be required as one component of a cell replacement approach for the treatment of advanced age-related macular degeneration. Our data indicate that donor cell survival, maturation, and integration are possible despite the reorganization that is known to occur in advanced degeneration (17).
A critical issue relating to stem cell treatments is whether the restorative effects are mediated by differentiation of the immature cells into a specific cell type or, alternatively, from donor–host cell fusion. Stem and progenitor cells that generate neurons have been shown to generate fused cells (34). In theory, the presence of preexisting host rods at the time of stem or precursor cell transplantation may allow for the fusion of fluorescence-labeled donor cells with host cells and give rise to the appearance of integrated and well-formed donor-derived cells residing among host cells (11). Our experimental design using rodless hosts excluded the possibility of immature donor rod precursors fusing with host rods. The morphological results presented here, including the misaligned OS (Fig. 1G) and the absence of long inner fiber formation (Fig. 3 A–H), may reflect the outcomes to be expected following cell transfer in the absence of coexisting host ONL cells.
The use of the consensual PLR provided evidence not only that the reformed ONL was capable of detecting light, but also that signals were being transmitted to central nervous system targets. There is a complementary contribution from intrinsically photosensitive retinal ganglion cells to the PLR, especially at high irradiances with long stimuli (35). It was important in this study to assess the functional contribution of the newly introduced rods in the treatment group. Consequently, we used a short (0.1 ms) stimulus and measured the PLR at a relatively short time after stimulus offset (SI Materials and Methods) to better isolate the high-amplification rod photoreceptor contribution to the measured PLR. This approach has been validated as a measure of photoreceptor function in studies of human and mouse visual dysfunction due to Rpe65 mutations (36). The temporal characteristic of the cortical hemodynamic response after precursor transplantation deviates from the WT response; this may be due to alterations in neurovascular coupling mechanisms following reduced neuronal activity associated with loss of visual input before ONL reconstruction.
In summary, transplanted rod precursors may mature and recreate a new functional photoreceptor layer in the degenerate outer retina. These data support the potential use of photoreceptor precursors for the treatment of human blindness due to advanced RP and related conditions.
Materials and Methods
Animals were maintained in the animal facility at the University of Oxford. All experiments were conducted according to the UK Home Office guidelines on the Animal (Scientific Procedures) Act of 1986 and were approved by the University of Oxford Animal Ethics Committee. Donor cells were harvested from P3–P4, P14, P30, or P60 Tg(Nrl-L-EGFP) mice (kind gift of Anand Swaroop, National Eye Institute, Bethesda, MD). For sham transplants, retinal cells were harvested from Tg(CAG-DsRed*MST)1Nagy/J mice (Jackson Laboratories) older than 6 wk, using the Papain Dissociation System (Worthington Biochemical) according to the manufacturer’s instructions. Cells were diluted to a final concentration of 2 × 105 cells/µL DMEM for transplantation. Approximately 3 × 105 cells were transplanted in every case. Further experimental details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Anand Swaroop for supplying Tg(Nrl-E-GFP) mice and Shankar Srivinas, Stuart Peirson, Carina Pothecary, Wayne Davies, Stephen Hughes, and Qisheng You for their kind assistance. This research was supported by the National Institute for Health Research Biomedical Research Centres at Oxford University Hospitals NHS Trust and Moorfields Eye Hospital, the UK Medical Research Council, the Wellcome Trust, the Health Foundation, the Royal College of Surgeons of Edinburgh, the Royal Society, Fight for Sight, the Lanvern Foundation, the Special Trustees of Moorfields Eye Hospital, and the Oxford Stem Cell Institute. M.S.S. was supported by the Singapore National Medical Research Council and the Department of Ophthalmology, National University Hospital, Singapore.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119416110/-/DCSupplemental.
References
- 1.Zrenner E, et al. Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Soc. 2011;278(1711):1489–1497. doi: 10.1098/rspb.2010.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 3.MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 4.Bowes C, et al. Retinal degeneration in the rd mouse is caused by a defect in the β subunit of rod cGMP-phosphodiesterase. Nature. 1990;347(6294):4. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 5.Akimoto M, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci USA. 2006;103(10):3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gust J, Reh TA. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci. 2011;52(8):5266–5272. doi: 10.1167/iovs.10-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4(1):73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakowski J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet. 2010;19(23):4545–4559. doi: 10.1093/hmg/ddq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West EL, et al. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28(11):1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J, et al. XIAP therapy increases survival of transplanted rod precursors in a degenerating host retina. Invest Ophthalmol Vis Sci. 2011;52(3):1567–1572. doi: 10.1167/iovs.10-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson RA, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfrum U, Schmitt A. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil Cytoskeleton. 2000;46(2):95–107. doi: 10.1002/1097-0169(200006)46:2<95::AID-CM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 13.Vintersten K, et al. Mouse in red: Red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40(4):241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 14.Dick O, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37(5):775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 15.Kwan ASL, Wang S, Lund RD. Photoreceptor layer reconstruction in a rodent model of retinal degeneration. Exp Neurol. 1999;159(1):21–33. doi: 10.1006/exnr.1999.7157. [DOI] [PubMed] [Google Scholar]
- 16.Newman E, Reichenbach A. The Müller cell: A functional element of the retina. Trends Neurosci. 1996;19(8):307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 17.Jones BW, Marc RE. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005;81(2):123–137. doi: 10.1016/j.exer.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Koulen P, Kuhn R, Wässle H, Brandstätter JH. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc Natl Acad Sci USA. 1999;96(17):9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain RZ, et al. Direct and consensual murine pupillary reflex metrics: Establishing normative values. Auton Neurosci. 2009;151(2):164–167. doi: 10.1016/j.autneu.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grozdanic SD, et al. Morphological integration and functional assessment of transplanted neural progenitor cells in healthy and acute ischemic rat eyes. Exp Eye Res. 2006;82(4):597–607. doi: 10.1016/j.exer.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Caporale N, et al. LiGluR restores visual responses in rodent models of inherited blindness. Mol Ther. 2011;19(7):1212–1219. doi: 10.1038/mt.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radel JD, Kustra DJ, Das S, Elton S, Lund RD. The pupillary light response: Assessment of function mediated by intracranial retinal transplants. Neuroscience. 1995;68(3):909–924. doi: 10.1016/0306-4522(95)00192-l. [DOI] [PubMed] [Google Scholar]
- 23.Thompson S, et al. Light aversion in mice depends on nonimage-forming irradiance detection. Behav Neurosci. 2010;124(6):821–827. doi: 10.1037/a0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21(3):195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Bartsch U, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86(4):691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118(6):807–811. doi: 10.1001/archopht.118.6.807. [DOI] [PubMed] [Google Scholar]
- 27.Lamba DA, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker BA, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS ONE. 2011;6(4):e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29(6):972–980. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West EL, et al. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86(4):601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakowski J, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29(9):1391–1404. doi: 10.1002/stem.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberle D, Schubert S, Postel K, Corbeil D, Ader M. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci. 2011;52(9):6462–6471. doi: 10.1167/iovs.11-7399. [DOI] [PubMed] [Google Scholar]
- 33.Pearson RA, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19(4):487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen KA, et al. Fusion of neural stem cells in culture. Exp Neurol. 2006;198(1):129–135. doi: 10.1016/j.expneurol.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299(5604):245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 36.Aleman TS, et al. Impairment of the transient pupillary light reflex in Rpe65(-/-) mice and humans with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45(4):1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.