One of the major uncertainties in predicting climate change comes from a full accounting of carbon-cycle feedbacks, which roughly double physical feedbacks (1, 2). Most of this uncertainty is a result of the many pathways and time scales at which ecosystems interact with the climate system and how these will respond to change (3). The relationship between leaf nitrogen and the carbon cycle is key to many ecosystem processes because photosynthesis provides the energy and carbon-cycle molecules for growth and reproduction (4–7) and decomposition for nutrient cycling (7, 8). Ecologists have long recognized that nitrogen was the most limited nutrient for plant growth (9, 10). Quantifying changes in canopy nitrogen content provides direct information about ecosystem functioning and a method to detect and monitor changes in response to climate forcing (9, 10); thus, it has been a long-term objective for airborne and spaceborne imaging spectroscopy (11–13). Several papers have reported direct detection of canopy nitrogen from airborne imaging spectrometers (14–17). Ollinger (18) argues that selective pressure on plant competition for light, water, and nutrients should result in suites of biochemical and structural traits that integrate their functional strategies. Thus, structural traits affecting light scattering “over scales ranging from cells to canopies” (18) will be convergent with their biochemical traits. Knyazikhin et al. (19) explicitly test whether assumptions that canopy structure can be ignored in quantifying biochemical composition with a detailed analysis of the physical processes of photon scattering from leaves and plant canopies. Although there is recognition of the importance of multiple scattering (20), particularly in the near infrared, where plant compounds do not display strong absorption features (21–23), it has not been possible to quantify it at the canopy scale. The report by Knyazikhin et al. (19) is unique in being a full attempt at modeling spectral absorptions and scattering at both leaf and canopy scales (Fig. 1).
Fig. 1.
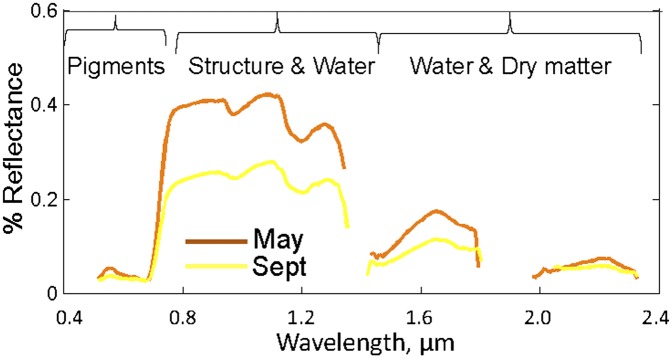
AVIRIS spectra of mixed live-oak forest showing seasonal change. Wavelength region where significant plant absorptions occur are indicated. Multiple scattering dominates the near-infrared region between 0.7 and 1.5 μm.
Given understanding of ecophysiological controls on photosynthesis that demonstrate the significance of nitrogen on controlling productivity, it is not surprising that an early goal for imaging spectroscopy (24) recognized the importance of quantifying declining photosynthetic capacity. Identification of different plant materials, especially related to photosynthetic function, was an objective of airborne imaging spectrometry since its beginnings (11, 25, 26). Early laboratory studies on estimating nitrogen content with near infrared spectroscopy provided evidence that nitrogen could be quantified through spectroscopy, but measurements were restricted to ground dry foliage (27–29). Direct detection of canopy nitrogen from remote sensing observations have been reported by others since (12, 16, 29), but results have been questioned because spectral changes generally also corresponded to changes in land cover between conifer and broadleaf forests.
The first National Aeronautics and Space Administration (NASA) Airborne Imaging Spectrometer (AIS-1), flown from 1983 to 1986, included only the 0.9- to 2.1-μm reflected infrared spectrum and the AIS-2 measured from 0.8 to 2.4 μm (11), thus the emphasis for detecting chemistry shifted from pigments to canopy water and nitrogen because their absorption features occur in the reflected infrared. Lignin content was mapped from AIS-1 data over Blackhawk Island, WI, which allowed estimates of soil nitrogen availability by correlating nitrogen mineralization with foliage lignin content (12). These results were corroborated (28, 29), although different studies identified different spectral bands as significant in multiple linear regression predictions. At this time, NASA began to address the full costs of the Earth Observing System satellite program and the High Spectral Resolution Imaging Spectrometer (HIRIS), one of the original NASA facility instruments for the Terra platform, was being considered for deselection because of its cost and uncertainty of its scientific benefits to the climate mission. However, there were concerns that high atmospheric CO2 concentrations could lead to increased C:N ratios and associated declining productivity because of higher lignin content in plant residues (30, 31). This concern about future soil nitrogen availability provided a unique climate role that only HIRIS, with its contiguous narrow spectral bands across the visible and shortwave infrared region, was capable of detecting. NASA established the Accelerated Canopy Chemistry Program (ACCP) in 1991–1992 to determine whether there was a sound theoretical and empirical basis for estimating nitrogen and lignin concentrations in ecosystem canopies from remote sensing data (13). Although NASA ultimately deselected HIRIS, this program led to numerous empirical studies over the past two decades (32) to identify nitrogen and lignin from airborne Advanced Visible Infrared Imaging Spectrometer (AVIRIS) data (e.g., refs. 13, 33, and 34). Despite concerns, the significance of structural contributions to measurements of lignin and nitrogen, predictions were never explicitly tested before Knyazikhin et al. (19).
Only a few leaf and canopy radiative transfer models have been developed. Only the LIBERTY (Leaf Incorporating Biochemistry Exhibiting Reflectance and Transmittance Yields) radiative transfer model, developed to estimate the optical properties of both dried and fresh conifer needles (35), specifically includes nitrogen, lignin, and cellulose. LEAFMOD, the Ganapol et al. (36) model, addressed internal leaf scattering, which they recognized must be fully modeled to define additional biochemical parameters. However, both LIBERTY and LEAFMOD have had limited distribution compared with the PROSPECT leaf optical properties model (37). After the ACCP program, nitrogen and lignin were introduced into the PROSPECT model (38, 39), but were later deleted for the more general “dry matter” because results were inconsistent (40, 41). More recently (42) the combined PROSPECT-SAIL (PROSAIL) leaf and canopy radiative transfer models have been used to predict canopy nitrogen by assuming a constant stoichiometry to chlorophyll. Bousquet et al. (43) modified PROSPECT to include the directional effects of leaf specular and diffuse reflectance, representing a start to address the 3D structure of actual canopies. Until the paper by Knyazikhin et al. (19), no one has made a thorough physically based analysis of the scattering effects of leaves and canopy structure. Such modeling efforts, combined with more rigorous measurements of the 3D structure of actual canopies, will test these models using small-footprint full-waveform light detection and ranging, and provide a path forward to achieve rigorous estimates of canopy chemistry. As Knyazikhin et al. (19) make clear, quantifying the retrieval of any biochemical information from remote sensing data is subject to leaf and canopy scattering processes, and these must be accounted for to achieve correct estimates. The paper by Knyazikhin et al. (19) quantifies the physical interactions, thus going a long way toward eventually solving these problems.
Footnotes
References
- 1.Gregory JM, Jones CD, Cadule P, Friedlingstein P. Quantifying carbon cycle feedbacks. J Clim. 2009;22(19):5232–5250. [Google Scholar]
- 2.Friedlingstein P, et al. Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison. J Clim. 2006;19(14):3337–3353. [Google Scholar]
- 3.Sacks WJ, Schimel DS, Monson RK. Coupling between carbon cycling and climate in a high-elevation, subalpine forest: A model-data fusion analysis. Oecologia. 2007;151(1):54–68. doi: 10.1007/s00442-006-0565-2. [DOI] [PubMed] [Google Scholar]
- 4.Field CB, Chapin FS, Matson PA, Mooney HA. Responses of the terrestrial ecosystems to changing atmosphere. Annu Rev Ecol Syst. 1992;23:201–235. [Google Scholar]
- 5.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 6.Kattge J, et al. TRY—A global database of plant traits. Glob Change Biol. 2011;17:2905–2935. [Google Scholar]
- 7.Reich PB. Key canopy traits drive forest productivity. Proc Biol Sci. 2012;279(1736):2128–2134. doi: 10.1098/rspb.2011.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol Lett. 2008;11(8):793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 9.LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89(2):371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- 10.Heimann M, Reichstein M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature. 2008;451(7176):289–292. doi: 10.1038/nature06591. [DOI] [PubMed] [Google Scholar]
- 11.Vane G, Goetz AFH. Terrestrial imaging spectroscopy. Remote Sens Environ. 1988;24(1):1–2. [Google Scholar]
- 12.Wessman CA, Aber JD, Peterson DL, Melillo JM. Remote sensing of canopy chemistry and nitrogen cycling in temperate forest ecosystems. Nature. 1988;335(6186):154–156. [Google Scholar]
- 13. ACCP (1994) Accelerated Canopy Chemistry Program Final Report to NASA-EOS-IWG, ed Aber J (Washington, DC) Available at http://daac.ornl.gov/ACCP/accp.html.
- 14.Ollinger SV, et al. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology. 2002;83(2):339–355. [Google Scholar]
- 15.Ollinger SV, et al. Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: Functional relations and potential climate feedbacks. Proc Natl Acad Sci USA. 2008;105(49):19336–19341. doi: 10.1073/pnas.0810021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin ME, et al. A generalizable method for remote sensing of canopy nitrogen across a wide range of forest ecosystems. Remote Sens Environ. 2008;112(9):3511–3519. [Google Scholar]
- 17.Asner GP, Martin RE. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens Environ. 2008;112(10):3958–3970. [Google Scholar]
- 18.Ollinger SV. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2011;189(2):375–394. doi: 10.1111/j.1469-8137.2010.03536.x. [DOI] [PubMed] [Google Scholar]
- 19.Knyazikhin Y, et al. Hyperspectral remote sensing of foliar nitrogen content. Proc Natl Acad Sci USA. 2012;110:E185–E192. doi: 10.1073/pnas.1210196109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross J. The Radiation Regime and Architecture of Plant Stands. The Hague: Springer; 1981. p. 391. [Google Scholar]
- 21.Knyazikhin Y, Marshak A, Myneni RB. Three-dimensional radiative transfer in vegetation canopies and cloud-vegetation interaction. In: Marshak A, Davis AB, editors. Three Dimensional Radiative Transfer in the Cloudy Atmosphere. Berlin: Springer; 2005. pp. 617–652. [Google Scholar]
- 22.Lewis P, Disney M. Spectral invariants and scattering across multiple scales from within-leaf to canopy. Remote Sens Environ. 2007;109(2):196–206. [Google Scholar]
- 23.Shabanov NV, Knyazikhin Y, Baret F, Myneni RB. Stochastic modeling of radiation regime in discontinuous vegetation canopies. Remote Sens Environ. 2000;74(1):125–144. [Google Scholar]
- 24.Goetz AFH, Vane G, Solomon JE, Rock BN. Imaging spectrometry for Earth remote sensing. Science. 1985;228(4704):1147–1153. doi: 10.1126/science.228.4704.1147. [DOI] [PubMed] [Google Scholar]
- 25.Goetz AFH, Rock BN, Rowan LC. Remote-sensing for exploration—An overview. Econ Geol. 1983;78(4):573–590. [Google Scholar]
- 26.Vane G, Goetz AFH. Terrestrial imaging spectroscopy current status, future trends. Remote Sens Environ. 1993;44(2-3):117–126. [Google Scholar]
- 27.Wessman CA, Aber JD, Peterson DL, Melillo JM. Foliar analysis using near infrared reflectance spectroscopy. Can J Res. 1988;18(1):6–11. [Google Scholar]
- 28.Card DH, Peterson DL, Matson PA, Aber JD. Prediction of leaf chemistry by the use of visible and near infrared reflectance spectroscopy. Remote Sens Environ. 1988;26(2):123–147. [Google Scholar]
- 29.Peterson DL, Running SW. Applications in forest science and management. In: Asrar G, editor. Theory and Applications of Optical Remote Sensing. New York: John Wiley & Sons; 1989. pp. 429–473. [Google Scholar]
- 30.Aber JD, Melillo JM. Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Can J Bot. 1982;60(11):2263–2269. [Google Scholar]
- 31.Melillo JM, Aber JD, Muratore JF. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology. 1982;63(3):621–626. [Google Scholar]
- 32.Kokaly RF, et al. Characterizing canopy biochemistry from imaging spectroscopy and its application to ecosystem studies. Remote Sens Environ. 2009;113(S):S78–S91. [Google Scholar]
- 33.Matson P, et al. Seasonal patterns and remote spectral estimation of canopy chemistry across the Oregon transect. Ecol Appl. 1994;4(2):280–298. [Google Scholar]
- 34.Martin ME, Aber JD. High spectral resolution remote sensing of forest canopy lignin, nitrogen, and ecosystem processes. Ecol Appl. 1997;7(2):431–443. [Google Scholar]
- 35.Dawson TP, Curran PJ, Plummer SE. The LIBERTY model—Modeling the effects of leaf biochemical concentration on reflectance spectra. Remote Sens Environ. 1998;65(1):50–60. [Google Scholar]
- 36.Ganapol BD, et al. LEAFMOD: A new within-leaf radiative transfer model. Remote Sens Environ. 1998;63(2):182–193. [Google Scholar]
- 37.Jacquemoud S, Baret F. PROSPECT: A model of leaf optical properties spectra. Remote Sens Environ. 2000;34(2):75–91. [Google Scholar]
- 38.Fourty T, et al. Leaf optical properties with explicit description of its biochemical composition: Direct and inverse problems. Remote Sens Environ. 1996;56(2):104–117. [Google Scholar]
- 39.Fourty T, Baret F. On spectral estimates of fresh leaf biochemistry. Int J Remote Sens. 1998;19(7):1283–1297. [Google Scholar]
- 40.Jacquemoud S, et al. Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sens Environ. 1996;56(3):194–202. [Google Scholar]
- 41.Jacquemoud S, et al. PROSPECT + SAIL: A review of use for vegetation characterization. Remote Sens Environ. 2009;113(S):S56–S66. [Google Scholar]
- 42.Blondlot A, Gate P, Poilvé H. 2005. Providing operational nitrogen recommendations to farmers using satellite imagery. Proceedings of the Fifth European Conference on Precision Agriculture, ed Stafford JV (Wageningen Academic Publishers, The Netherlands) pp. 345−352.
- 43.Bousquet L, Lachérade S, Jacquemoud S, Moya I. Leaf BRDF measurement and model for specular and diffuse component differentiation. Remote Sens Environ. 2005;98(2-3):201–211. [Google Scholar]


