Abstract
Neurons in the brains of newborns are usually connected with many other neurons through weak synapses. This early pattern of connectivity is refined through pruning of many immature connections and strengthening of the remaining ones. NMDA receptors (NMDARs) are essential for the development of excitatory synapses, but their role in synaptic refinement is controversial. Although chronic application of blockers or global knockdown of NMDARs disrupts developmental refinement in many parts of the brain, the ubiquitous presence of NMDARs makes it difficult to dissociate direct effects from indirect ones. We addressed this question in the thalamus by using genetic mosaic deletion of NMDARs. We demonstrate that pruning and strengthening of immature synapses are blocked in neurons without NMDARs, but occur normally in neighboring neurons with NMDARs. Our data support a model in which activation of NMDARs in postsynaptic neurons initiates synaptic refinement.
During early development in vertebrates, neurons in many parts of the nervous system form weak synapses with a large number of target cells (1–7). This early pattern of connectivity is refined through two processes: synapse elimination that removes many initial connections and synapse maturation whereby the remaining connections are strengthened (8, 9). This refinement of immature synapses is essential for the formation of neural circuits, and provides the basis for behavioral development. Many studies, in particular those conducted at the neuromuscular junction, in the cerebellum and visual pathways, have demonstrated that activity plays a central role in synaptic refinement (10–12). However, the mechanisms of synaptic refinement remain incompletely understood. At the neuromuscular junction, silencing of synaptic transmission disrupted the refinement process (13, 14). The role of synaptic transmission in the refinement of central synapses seems more complex. Although manipulations of presynaptic activity disrupted the refinement of connections in the visual pathway (15–17), some of the effects were independent of synaptic transmission (18, 19).
The vast majority of excitatory synapses in the brain use glutamate as neurotransmitter; synaptic transmission is usually mediated by AMPA receptors (AMPARs) and NMDA receptors (NMDARs) in postsynaptic neurons. NMDARs play an important role in the development of neural circuits. In the brains of neonates, glutamatergic synapses have few or no AMPARs, and synaptic transmission is primarily mediated by NMDARs (20–23). Activation of NMDARs by correlated activity is thought to be a key mechanism underlying synaptic refinement during development (24, 25). Consistent with this idea, chronic application of NMDAR antagonists or global knockdown of NMDARs disrupts developmental refinement of neuronal circuits in many parts of the brain (26–28). However, recent studies in the hippocampus have shown that single-cell deletion of NMDARs in newborn mice has no effect on the number or density of synaptic spines (29). These findings raised the possibility that chronic block or global knockdown of NMDARs may affect synaptic refinement through indirect mechanisms.
We directly addressed the role of NMDARs in synaptic refinement at vibrissal relay synapses in the thalamus. Tactile information from vibrissa is relayed to neurons in ventral posteromedial nucleus (VPm) of the thalamus by ascending axons from the principal trigeminal nucleus (Pr5) in the brainstem. VPm relay synapses undergo extensive refinement during the second week after birth (5). At postnatal day (P) 7, each VPm neuron is contacted by approximately six ascending axons from the brainstem; by P14, the majority of neurons receive a single ascending axon (30). The pruning of redundant inputs was accompanied by strengthening of remaining inputs. At P7, transmission at VPm relay synapses is mediated primarily by NMDARs; the number of AMPARs increases rapidly during the second week (30). To selectively examine the role of NMDARs in postsynaptic neurons, we performed mosaic deletion of NMDARs in the VPm of newborn mice. We found that pruning and synaptic strengthening were disrupted in neurons without NMDARs, but occurred normally in neighboring neurons with NMDARs. Our findings clearly demonstrate that NMDARs in postsynaptic neurons are essential for synaptic refinement.
Results
Mosaic Deletion of NMDARs in Thalamus.
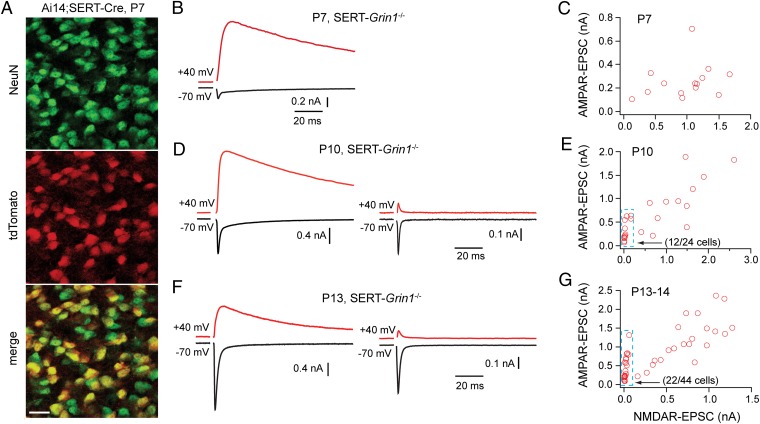
We generated mice with mosaic deletion of NMDARs in the VPm by using the BAC transgenic Cre strain Tg(Slc6a4-cre)127Gsat [serotonin transporter (SERT)-Cre] generated by GENSAT (31). By using the Rosa-tdTomato reporter (Ai14), we found that Cre recombinase activity was present as early as P2 in the cortex and thalamus of SERT-Cre mice (Fig. S1A). A mosaic pattern was observed in the VPm at P7, with approximately 50% of neurons expressing Cre recombinase (Fig. 1A); a similar pattern was observed at P14 (Fig. S1B and SI Results). In comparison, only 6% of neurons in the Pr5 were Cre-positive in SERT-Cre mice at P12 to P14 (Fig. S2 A and B and SI Results). To delete NMDARs, we generated a conditional allele (Grin12lox; Fig. S3) and a null allele (Grin1null; SI Materials and Methods) of Grin1 that encodes the essential NMDAR subunit GluN1. VPm neurons were recorded from acute slices obtained from Grin12lox/null;SERT-Cre+ or Grin12lox/2lox;SERT-Cre+ mice; we did not find any difference between these two genotypes in the efficiency or timing of NMDAR deletion, and they are collectively termed SERT-Grin1−/−.
Fig. 1.
Mosaic deletion of NMDARs in VPm neurons of SERT-Grin1−/− mice during early life. (A) Confocal images of the VPm of a SERT-Ai14 mouse at P7. Top: All VPm neurons are shown with an antibody of the neuronal marker NeuN. Middle: Cre-expressing VPm neurons in the same section as revealed by tdTomato signal. Bottom: Overlay of the two images above. (Scale bar: 20 μm.) Approximately 55% of neurons in this section were Cre-positive, and the average was 49 ± 3% for the VPm at P7 (n = 3 mice). (B) EPSCs recorded from a VPm neuron of a SERT-Grin1−/− mouse at P7. (C) Plot of maximal AMPAR-EPSCs vs. maximal NMDAR-EPSCs for VPm neurons recorded at P7. All VPm neurons recorded at P7 showed NMDAR-EPSCs (14 of 14 neurons from two 2 mice; 1.7 ± 0.2 nA). (D) EPSCs recorded from two VPm neurons of a SERT-Grin1−/− mouse at P10. Right: Neuron with no NMDAR-EPSCs. Left: Neuron with normal NMDAR-EPSCs. (E) Plot of maximal AMPAR-EPSCs vs. maximal NMDAR-EPSCs for VPm neurons recorded at P10. Fifty percent of cells (12 of 24; box with dashed blue line) had no or small NMDAR-EPSCs (<200 pA) and low NMDAR/AMPAR ratio (<0.3). (F and G) Equivalent results obtained from SERT-Grin1−/− mice at P13 to P14. Fifty percent of VPm neurons (22 of 44; box with dashed blue line) in SERT-Grin1−/− mice showed little or no NMDAR-EPSCs (<50 pA) and low NMDAR/AMPAR ratio (<0.1); these neurons were considered to be without NMDARs.
Excitatory synaptic currents (EPSCs) were evoked at VPm relay synapses by stimulation applied to the medial lemniscus. The VPm relay synapse has few GluA2-containing AMPARs (30); EPSCs recorded at +40 mV are dominated by slow NMDAR-mediated currents (i.e., NMDAR-EPSCs), whereas the fast EPSCs at −70 mV are mediated by AMPARs (i.e., AMPAR-EPSCs). VPm neurons were chosen randomly for recording. At P7, all VPm neurons in SERT-Grin1−/− mice showed NMDAR- and AMPAR-EPSCs (14 of 14 cells from two mice; Fig. 1 B and C), with amplitudes comparable with those obtained from C57BL6 (B6) mice at the same age (5). At P10, however, 50% of VPm neurons (12 of 24 cells from three mice) showed only AMPAR-EPSCs, with very small or no NMDAR-EPSCs (Fig. 1D, Right); these cells can be easily identified in the plot of AMPAR-EPSCs vs. NMDAR-EPSCs (Fig. 1E, box with dashed blue lines). Similar results were obtained at P13 to P14 in SERT-Grin1−/− mice (Fig. 1F). Fifty percent of VPm neurons at P13 to P14 (22 of 44 cells from four mice) had NMDAR-EPSCs of less than 50 pA and an NMDAR/AMPAR ratio of less than 0.1 (Fig. 1G, box with dashed blue lines); these cells were considered to be without NMDARs. These results indicate that, in the VPm of SERT-Grin1−/− mice, the mosaic deletion of NMDARs was established between P7 and P10.
Deletion of NMDARs Disrupts Elimination of Redundant Inputs.
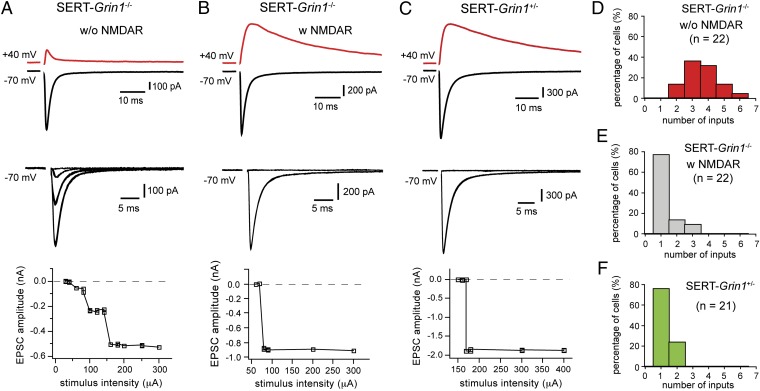
We estimated the number of ascending axons connecting with a single VPm neuron at P13 to P14. For each neuron, EPSCs were recorded at −70 mV over a wide range of stimulus intensities (20–900 μA; 100 μs); the intensity of stimulus was increased with steps of 10 or 20 μA. An EPSC increment was determined as a sudden increase in EPSC amplitude at a given intensity that is maintained at higher intensities. The number of inputs was estimated as the number of EPSC increments. We found striking differences in the number of inputs between neurons with NMDARs and those without NMDARs. Fig. 2 A and B illustrates examples from two neighboring VPm neurons recorded in the same slice obtained from a SERT-Grin1−/− mouse at P13. The cell in Fig. 2A had no NMDAR-EPSCs and showed three increments in AMPAR-EPSCs, indicating that it was connected with three axons. The neighboring cell (Fig. 2B) had normal NMDAR-EPSCs and showed an all-or-none EPSC, indicating that it was connected with a single axon. Summarized data from all cells obtained from SERT-Grin1−/− mice aged P13 to P14 are shown in Fig. 2 D and E. All neurons without NMDARs received two or more axons, with the majority (84%) of them receiving three or more axons (Fig. 2D). In contrast, 77% of VPm neurons with NMDARs were innervated by a single ascending axon (Fig. 2E). The average numbers of inputs per neuron were 3.6 ± 0.2 (n = 22) for neurons without NMDARs and 1.3 ± 0.1 (n = 22) for neurons with NMDARs (P < 0.0001, Mann–Whitney test). Because neurons with or without NMDARs also differ in Cre expression, as a control, we recorded VPm neurons from Grin12lox/wt;SERT-Cre+ (SERT-Grin1+/−) mice at P13. In these mice, approximately 50% of VPm neurons should express Cre recombinase. We found that all VPm neurons in SERT-Grin1+/− mice had normal NMDAR-EPSCs (21 of 21 cells from three mice; Fig. 2C), and 76% of the neurons received a single ascending axon (Fig. 2F), indicating that Cre expression by itself does not affect the number of inputs. We also examined excitatory synaptic transmission in Pr5 neurons; the results indicated that the vast majority of Pr5 neurons in SERT-Grin1−/− mice had normal NMDAR- and AMPAR-EPSCs (Fig. S2 C–E). To determine whether the effect of NMDAR deletion on pruning is long-lasting, we recorded from VPm neurons of SERT-Grin1−/− mice aged P16 to P17, and the results were similar to those obtained at P13 to P14 (Fig. S4). Together, these results demonstrate that NMDARs in postsynaptic neurons are essential for elimination of redundant inputs during development.
Fig. 2.
Deletion of NMDARs in VPm neurons disrupts pruning of redundant inputs. (A and B) EPSCs recorded from two neighboring VPm neurons in a SERT-Grin1−/− mouse at P13. Top: EPSCs recorded at +40 mV (in red) and −70 mV (black). Middle: EPSCs at −70 mV in response to a range of stimulus intensity. Bottom: Plots of peak amplitudes of EPSCs at −70 mV vs. stimulus intensities. (C) Equivalent results obtained from a VPm neuron in a SERT-Grin1+/− mouse at P13. (D–F) Distributions of VPm neurons receiving different numbers of ascending axons for SERT-Grin1−/− mice at P13 to P14 (D, cells without NMDARs; E, cells with NMDARs) and SERT-Cre control mice at P13 (F). The distribution of cells with NMDARs (E) is significantly different from that of cells without NMDARs (D; P < < 0.00001, χ2 test), but not from that of SERT-Grin1+/− control group (F; P = 0.11, χ2 test).
Deletion of NMDARs Disrupts Synaptic Strengthening.
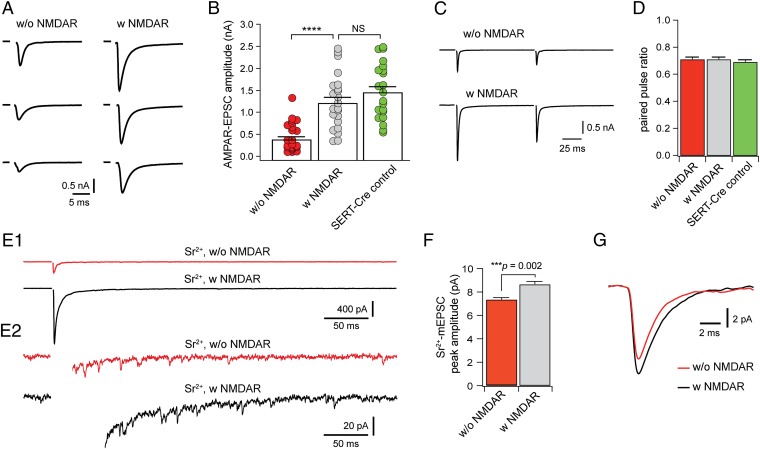
As in many other brain areas, the maturation of vibrissal relay synapses is marked by a rapid increase in the amplitude of AMPAR-EPSCs (5, 30, 32). To determine the role of NMDARs in synaptic maturation, we analyzed AMPAR-EPSCs in SERT-Grin1−/− mice. At P13 to P14, the amplitudes of maximal AMPAR-EPSCs of VPm neurons without NMDARs were significantly smaller that those with NMDARs (Fig. 3A); the mean amplitude was reduced by 64% in neurons without NMDARs (P < 0.0001; Fig. 3B). In contrast, there was no difference between neurons with NMDARs and those of SERT-Grin1+/− (SERT-Cre control) mice in AMPAR-EPSCs (P = 0.25; Fig. 3B).
Fig. 3.
Deletion of NMDARs disrupts developmental strengthening of thalamic relay synapses. (A) Maximal EPSCs at −70 mV recorded from three neurons without NMDARs (Left) and three neurons with NMDARs (Right). All six neurons were from SERT-Grin1−/− mice at P13. (B) Peak amplitudes of AMPAR-EPSCs for neurons without (red) and with NMDARs (gray) of SERT-Grin1−/− mice at P13 to P14, and for neurons of SERT-Grin1+/− control mice (green) at P13. Mean amplitudes were 0.45 ± 0.07 nA (n = 22) for neurons without NMDARs, 1.26 ± 0.13 nA (n = 22) for neurons with NMDARs, and 1.49 ± 0.14 nA (n = 21) for neurons of SERT-Grin1+/− control mice (P < 0.0001, nonparametric ANOVA). (C) Paired pulse responses recorded at −70 mV from a neuron without (Upper) and another with NMDARs (Lower) in a SERT-Grin1−/− mouse at P13. (D) Paired pulse ratio of EPSCs at −70 mV for the three groups at P13 to P14. The interpulse interval was 100 ms for all neurons. (E) 1, Evoked EPSCs recorded with 3 mM Sr2+ from a neuron without (Upper) and another with NMDARs (Lower) in a SERT-Grin1−/− mouse at P14. 2, Partial views of traces in 1 to illustrate quantal events. (F) Peak amplitude of Sr2+-mEPSCs from neurons with and without NMDARs. Mean peak amplitudes were 7.3 ± 0.2 pA (n = 15) for neurons without NMDARs and 8.6 ± 0.3 pA (n = 16) for neurons with NMDARs (P = 0.002, Mann–Whitney test). (G) Averaged Sr2+-mEPSCs from 16 neurons with (black) and 15 neurons without (red) NMDARs. Mean decay constants were 1.74 ± 0.03 ms (n = 15) for neurons without NMDARs and 2.01 ± 0.06 ms (n = 16) for neurons with NMDARs (P = 0.0004, Mann–Whitney test).
The reduction in the amplitude of AMPAR-EPSCs may be caused by changes in quantal size, the number of release sites, or release probability. There were no difference in paired pulse ratio of EPSCs between cells without NMDARs and those with NMDARs (P = 0.66, nonparametric ANOVA; Fig. 3 C and D), suggesting that release probability at the synapse was not altered by NMDAR deletion. Besides those from the Pr5, VPm neurons also receive glutamatergic inputs from corticothalamic axons. To selectively analyze quantal size at the Pr5–VPm synapse, we recorded EPSCs evoked in the presence of strontium (Sr2+). Replacing Ca2+ by 3 mM Sr2+ in the artificial cerebrospinal fluid suppressed synchronized release and induced asynchronous miniature EPSCs (Sr2+-mEPSCs; Fig. 3E, 1 and 2). Compared with those with NMDARs, cells without NMDARs had smaller Sr2+-mEPSCs with faster decay (Fig. 3 F and G), indicating that deletion of NMDARs reduced the number of AMPARs at single postsynaptic site and altered functional properties of AMPARs. We also recorded spontaneous mEPSCs in VPm neurons with or without NMDARs. Neurons without NMDARs had much fewer quantal events than those with NMDARs (Fig. S5). It is possible that many synapses on mutant neurons contained few or no AMPARs and their mEPSCs were not detected by our method.
Deletion of NMDARs Disrupts Pruning of Somatic Innervation.
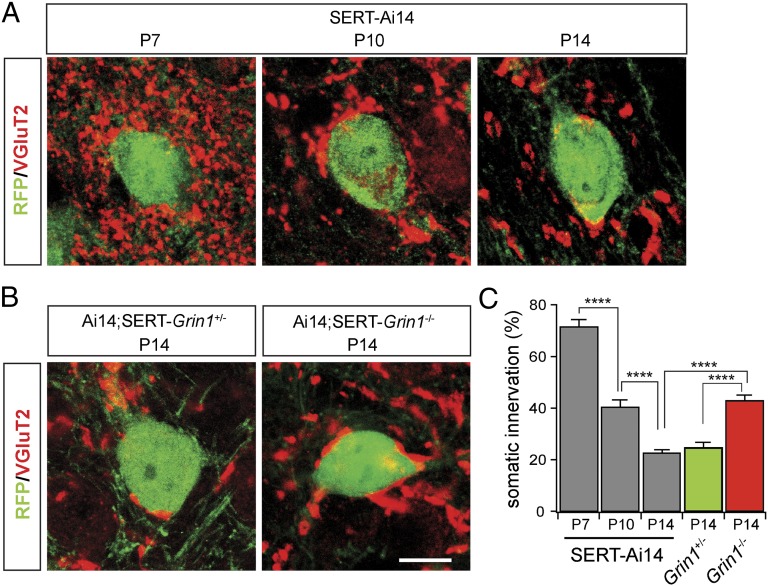
Recent studies have shown that the refinement of the climbing fiber–Purkinje cell connection in the cerebellum is associated with pruning of somatic innervation (33). Synaptic terminals of Pr5 axons in the VPm selectively express vesicular glutamate transporter 2 (VGluT2), whereas those of corticothalamic axons express only VGluT1 (34). This allowed us to visualize Pr5 axon terminals by using VGluT2 immunostaining. The Ai14 (tdTomato) reporter was used to identify Cre-positive neurons in the VPm. In Grin1 WT mice (Ai14;SERT-Cre, or SERT-Ai14), cell bodies of VPm neurons at P7 were surrounded by VGluT2-positive synaptic terminals (Fig. 4A); this somatic innervation was reduced by 40% at P10, and, by P14, only a small fraction of the cell body was contacted by VGluT2-positive terminals (Fig. 4 A and C). The pattern of VGluT2 innervation at P14 was comparable with those reported for Pr5 axonal terminals in the VPm of adult rats (35, 36). These results suggest that the refinement of vibrissal relay synapses in the VPm is associated with pruning of somatic innervation by Pr5 axons. Next we analyzed the effects of NMDAR deletion on somatic innervation in Grin1 mutant (SERT-Grin1−/−;Ai14) and littermate controls (SERT-Grin1+/−;Ai14). At P14, mutant neurons showed significantly more somatic innervation than those of control mice at the same age, but this was was comparable with those of control mice at P10 (Fig. 4 B and C). These results indicate that deletion of NMDARs disrupted pruning of somatic innervation by Pr5 axons.
Fig. 4.
Deletion of NMDARs disrupts pruning of somatic innervation during development. (A) Confocal images of VPm neurons and Pr5 axonal terminals in Ai14;SERT-Cre (SERT-Ai14) mice at P7, P10, and P14. Cre-expressing neurons were visualized with the RFP antibody (green); corticothalamic axons were also labeled with the RFP antibody. Pr5 axonal terminals were visualized with the VGluT2 antibody (red). (B) Confocal images of VPm neurons and Pr5 axonal terminals in SERT-Grin1+/−;Ai14 and SERT-Grin1−/−;Ai14 mice at P14. (Scale bar: 10 μm; A and B use the same scale bar.) (C) comparison among the five groups of somatic innervation by VGluT2-positive terminals. Somatic innervation was quantified as the fraction (as a percentage) of the cell body perimeter in contact with VGluT2-positive terminals. In SERT-Ai14 mice, there was a progressive reduction of somatic innervation by VGluT2-positive terminals (71.6 ± 2.8%, n = 12 for P7; 40.2 ± 2.8%, n = 13 for P10; 22.4 ± 1.5%, n = 10 for P14; P < 0.0005, P7 vs. P10, P10 vs. P14). There was no difference between SERT-Grin1+/− control mice and those in SERT-Ai14 mice (24.7 ± 2.3%, n = 32 for SERT-Grin1+/− at P14; P = 0.43 vs. SERT-Ai14 at P14); somatic innervation was significantly higher in Cre-positive neurons of SERT-Grin1−/− mice at P14 than those in SERT-Grin1+/− or SERT-Ai14 mice at the same age (42.9 ± 2.3%, n = 34 for SERT-Grin1−/−; P < 0.0001 vs. SERT-Grin1+/− or SERT-Ai14).
Discussion
NMDARs are implicated in many aspects of neural circuit development. Here we report the use of a genetic mosaic analysis on pruning and strengthening of glutamatergic synapses during early life. We find that pruning and strengthening of thalamic relay synapses are disrupted in neurons without NMDARs, but occur normally in neighboring neurons with NMDARs. These findings demonstrate a direct and essential role of NMDARs in the refinement of glutamatergic synapses in the brain, and support a model in which activation of NMDARs in postsynaptic neurons initiates synaptic refinement.
Although NMDARs are thought to play a critical role in synaptic refinement, evidence available so far came from studies that used chronic application of antagonists (26, 27) or constitutive receptor knockdown (28). Because of the ubiquitous presence of NMDARs and their wide range of functions, those interventions may affect synaptic refinement through indirect mechanisms. Indeed, chronic blockade of NMDARs in the cerebellum disrupted the refinement of the climbing fiber–Purkinje cell connection despite the lack of NMDARs at this synapse (37, 38). Two recent studies used single-cell deletion of NMDARs. Mosaic deletion of GluN2B disrupted dendrite patterning in the hippocampus and cortex (39). In the hippocampus, neurons without GluN2B had more primary dendrites than neighboring neurons with GluN2B, presumably because of a failure in pruning supernumerary dendrites in neurons without GluN2B. However, such effect was not observed in another study that used single-cell deletion of GluN2B or GluN1 in the hippocampus (29). One possibility for this discrepancy is the difference in the time of deletion between these two studies, as the former used the embryonic nestin Cre driver (39), whereas the latter used viral transfection in newborn mice for Cre expression (29). However, both studies found significant reductions in spine density in hippocampal neurons without GluN2B, which is inconsistent with a role of the receptor in pruning of synapses.
Our results demonstrate that NMDARs are directly implicated in pruning of redundant glutamatergic inputs and in strengthening the remaining ones. Taking advantage of the mosaic deletion in the thalamus of SERT-Grin1−/− mice, we showed that neurons without NMDARs displayed developmental arrest, whereas neighboring neurons with NMDARs underwent normal synaptic refinement. Besides the thalamus, SERT-Cre mice also show Cre expression in other brain regions, including the brainstem and cortex. Therefore, it is important to consider whether deletion of NMDARs in other parts of the brain is implicated in the effects that we found at the vibrissal relay synapse in the thalamus. One possibility is that disruption of synaptic refinement in VPm neurons is caused by the deletion of NMDARs in Pr5 neurons. This seems unlikely. In contrast to the VPm, in which approximately 50% of neurons were Cre-positive, only 6% of Pr5 neurons in SERT-Cre mice expressed Cre recombinase, and excitatory transmission was not affected in Pr5 neurons in SERT-Grin1−/− mice. Another consideration is the deletion of NMDARs in corticothalamic neurons. Our expression data indicate that the majority of neurons in layer VI of the neocortex are Cre-positive in SERT-Cre mice. Activities of corticothalamic neurons are likely to be altered in SERT-Grin1−/− mice. In the cerebellum, the refinement of climbing fiber–Purkinje cell synapse is disrupted by changes of parallel fiber inputs (40, 41). Therefore, it is possible that a reduction of corticothalamic inputs disrupts the refinement of thalamic relay synapses. However, a reduction of corticothalamic inputs would equally affect all thalamic relay neurons in the VPm. The fact that VPm neurons with NMDARs show normal number of inputs indicates that the refinement of VPm relay synapses is not affected by the deletion of NMDARs in the cortex of SERT-Grin1−/− mice. Together, our results in the VPm provide strong evidence that NMDARs in postsynaptic neurons are directly implicated in synaptic refinement. The vibrissal relay synapse in the thalamus bears strong resemblance to other sensory relay synapses with regard to functional maturation (2, 22, 42); it is likely that NMDARs play a similar role at many other glutamatergic synapses in the brain.
The role of NMDARs in synaptic strengthening has been extensively investigated. Most studies support the model in which activation of NMDARs promotes the recruitment of AMPARs to immature synapses (25, 43). However, single-cell deletion of NMDARs in the hippocampus led to an up-regulation of AMPAR-EPSCs in CA1 neurons by increasing the number of functional synapses (29, 44). This is in contrast with our results in the thalamus, in which deletion of NMDARs blocked the up-regulation of AMPARs at the synapse. One possibility for this discrepancy is the differences in AMPAR composition between these two synapses. The Schaffer collateral–CA1 synapse contains receptors made of mostly GluA1 and GluA2 subunits (45), whereas the vibrissal relay synapse in the thalamus expresses receptors made of mostly GluA3 and GluA4 subunits (30). As the trafficking of AMPARs is regulated through subunit-specific mechanisms (46, 47), a difference in subunit composition could result in different responses to NMDAR deletion.
In contrast to the large change in the amplitude of AMPAR-EPSCs, there was only a small reduction in quantal size in mutant neurons. The effective quantal content, calculated by using the peak amplitude of AMPAR-EPSCs and quantal size, was reduced by approximately 60% in mutant neurons. On the contrary, somatic innervation is significantly higher (by approximately 70%) in mutant neurons. There was no change in paired pulse ratio, indicating that release probability was not altered. Together, these findings suggest that the majority of synapses on the soma of mutant neurons have few or no AMPARs.
Compared with synaptic strengthening, the mechanisms by which NMDARs regulate pruning are largely unknown. Studies on synaptic plasticity suggest that NMDARs may regulate pruning through mechanisms reminiscent of long-term depression (48, 49). However, blocking of synaptic strengthening through deletion of AMPARs had no effect on spine number or dendritic structure of hippocampal neurons (45), or on pruning of vibrissal relay synapses in the thalamus (30). The fact that AMPARs are not required for pruning suggest that signaling through NMDARs may be sufficient to cause local changes at the synapse, and to alter retrograde signaling at selective synapses. Consistent with a recent study in the cerebellum (33), we found extensive pruning of synapses on the soma of VPm neurons during the refinement period. Most interestingly, deletion of NMDARs disrupts pruning of somatic synapses, suggesting that signaling through NMDARs initiates pruning of somatic innervation of VPm neurons.
In conclusion, our results demonstrate that NMDARs are directly implicated in developmental refinement of whisker relay synapses in the thalamus. Pruning of redundant inputs and strengthening of remaining ones require the presence of NMDARs in the postsynaptic neuron. These findings can be related to the development of excitatory synapses in many parts of the brain.
Materials and Methods
Mice.
A mouse strain carrying Grin1 conditional allele was generated by using ES cells obtained from the European Mouse Mutant Cell Repository. The SERT-Cre transgenic strain was obtained from the Mutant Mouse Regional Resource Centers. All other strains were obtained from the Jackson Laboratory Repository. Further details are provided in SI Materials and Methods.
Slice Preparation.
Brain slices were prepared from mice aged P7 through P17. Further details are provided in SI Materials and Methods.
Electrophysiology.
Whole-cell patch-clamp recordings were made in acute brain slices at 32 to 34 °C. Data were analyzed by using AxoGraph and IgorPro. Further details are provided in SI Materials and Methods.
Immunostaining, Image Acquisition and Analysis.
Immunostaining was performed on sections 50 to 60 μm thick by using standard methods. Confocal images were taken with a Leica SP5 microscope, and analyzed with ImageJ. Further details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sue Ackerman, Rob Burgess, Da-Ting Lin, and Wayne Frankel for comments; and Hao Wang and Joseph Zak for participating in preliminary studies. This work was supported by National Institutes of Health Grant NS064013 (to Z.-w.Z.). The Jackson Laboratory Cell Biology and Microinjection Services were subsidized by a National Cancer Institute Core Grant P30 CA034196-27.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212971110/-/DCSupplemental.
References
- 1.Balice-Gordon RJ, Chua CK, Nelson CC, Lichtman JW. Gradual loss of synaptic cartels precedes axon withdrawal at developing neuromuscular junctions. Neuron. 1993;11(5):801–815. doi: 10.1016/0896-6273(93)90110-d. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28(3):955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38(5):785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6(3):282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- 5.Arsenault D, Zhang ZW. Developmental remodelling of the lemniscal synapse in the ventral basal thalamus of the mouse. J Physiol. 2006;573(pt 1):121–132. doi: 10.1113/jphysiol.2006.106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu T, Trussell LO. Development and elimination of endbulb synapses in the chick cochlear nucleus. J Neurosci. 2007;27(4):808–817. doi: 10.1523/JNEUROSCI.4871-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapia JC, et al. Pervasive synaptic branch removal in the mammalian neuromuscular system at birth. Neuron. 2012;74(5):816–829. doi: 10.1016/j.neuron.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 9.Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25(2):269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 11.Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19(2):154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- 13.Misgeld T, et al. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36(4):635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 14.An MC, et al. Acetylcholine negatively regulates development of the neuromuscular junction through distinct cellular mechanisms. Proc Natl Acad Sci USA. 2010;107(23):10702–10707. doi: 10.1073/pnas.1004956107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33(3):357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- 17.Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52(2):247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicol X, et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10(3):340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- 19.Koch SM, et al. Pathway-specific genetic attenuation of glutamate release alters select features of competition-based visual circuit refinement. Neuron. 2011;71(2):235–242. doi: 10.1016/j.neuron.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375(6529):325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274(5289):972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 22.Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18(2):269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 23.Petralia RS, et al. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2(1):31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LI, Tao HW, Poo M. Visual input induces long-term potentiation of developing retinotectal synapses. Nat Neurosci. 2000;3(7):708–715. doi: 10.1038/76665. [DOI] [PubMed] [Google Scholar]
- 25.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 26.Simon DK, Prusky GT, O’Leary DD, Constantine-Paton M. N-methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci USA. 1992;89(22):10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colonnese MT, Constantine-Paton M. Chronic NMDA receptor blockade from birth increases the sprouting capacity of ipsilateral retinocollicular axons without disrupting their early segregation. J Neurosci. 2001;21(5):1557–1568. doi: 10.1523/JNEUROSCI.21-05-01557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasato T, et al. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19(6):1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 29.Gray JA, et al. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71(6):1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Liu H, Zhang ZW. Elimination of redundant synaptic inputs in the absence of synaptic strengthening. J Neurosci. 2011;31(46):16675–16684. doi: 10.1523/JNEUROSCI.4569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27(37):9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Liu H, Storm DR, Zhang ZW. Adenylate cyclase 1 promotes strengthening and experience-dependent plasticity of whisker relay synapses in the thalamus. J Physiol. 2011;589(pt 23):5649–5662. doi: 10.1113/jphysiol.2011.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009;63(1):106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507(2):1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- 35.Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. Eur J Neurosci. 1994;6(3):429–453. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 36.Veinante P, Deschênes M. Single- and multi-whisker channels in the ascending projections from the principal trigeminal nucleus in the rat. J Neurosci. 1999;19(12):5085–5095. doi: 10.1523/JNEUROSCI.19-12-05085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabacchi S, Bailly Y, Delhaye-Bouchaud N, Mariani J. Involvement of the N-methyl D-aspartate (NMDA) receptor in synapse elimination during cerebellar development. Science. 1992;256(5065):1823–1825. doi: 10.1126/science.1352066. [DOI] [PubMed] [Google Scholar]
- 38.Kakizawa S, Yamasaki M, Watanabe M, Kano M. Critical period for activity-dependent synapse elimination in developing cerebellum. J Neurosci. 2000;20(13):4954–4961. doi: 10.1523/JNEUROSCI.20-13-04954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62(2):205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kano M, et al. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18(1):71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto K, et al. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21(24):9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu W, Constantine-Paton M. Eye opening rapidly induces synaptic potentiation and refinement. Neuron. 2004;43(2):237–249. doi: 10.1016/j.neuron.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25(11):578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 44.Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci USA. 2008;105(14):5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62(2):254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3(11):1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 47.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 48.Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21(2):347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395(6697):37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.